Abstract
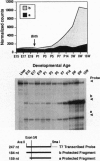
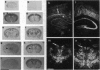
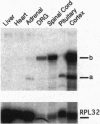
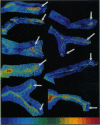
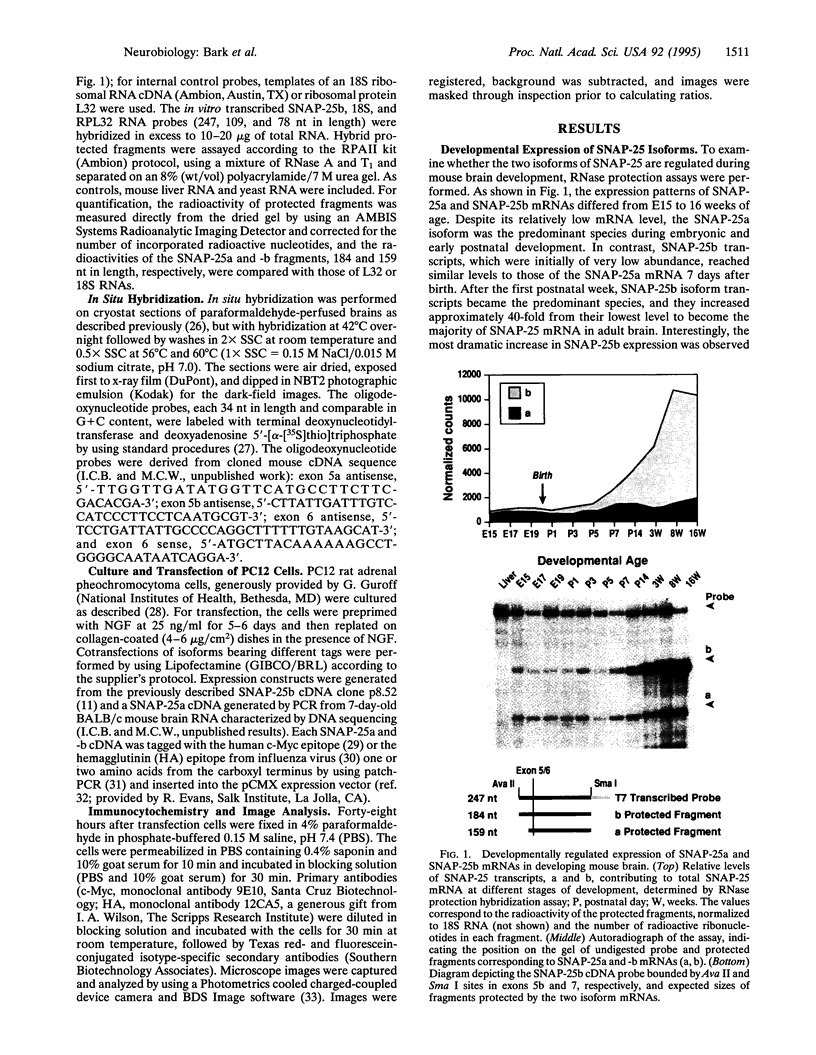
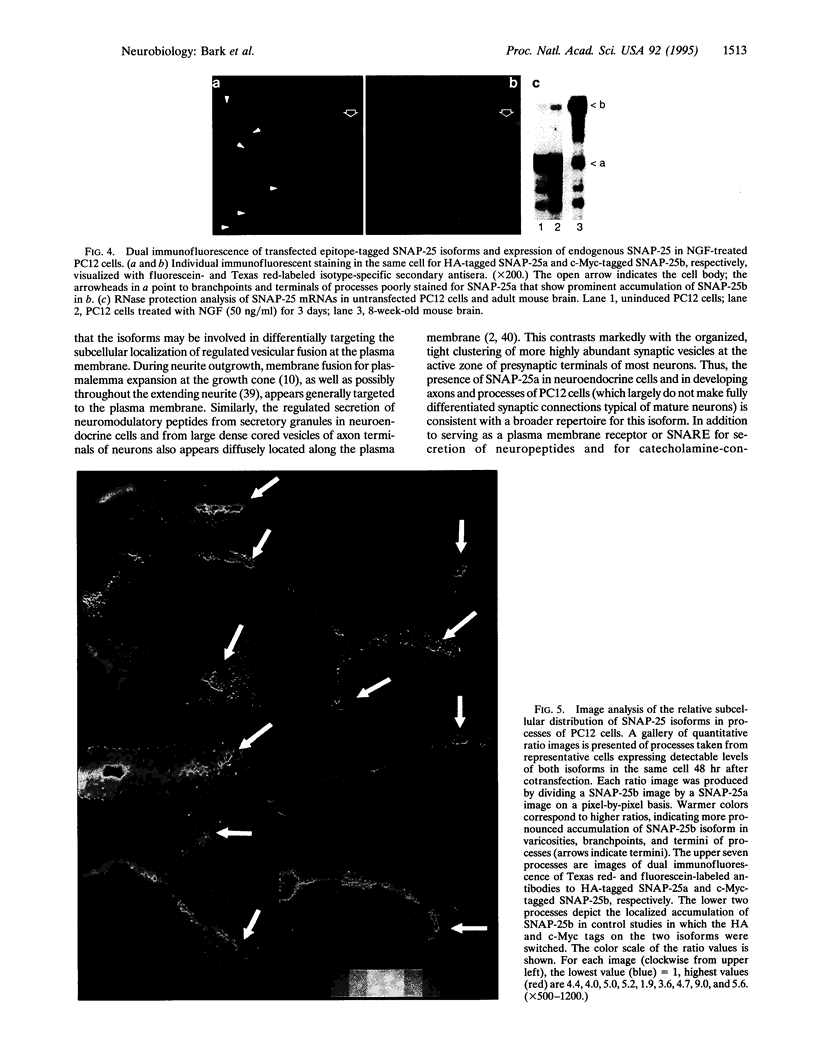
The presynaptic plasma membrane protein SNAP-25 (synaptosome-associated protein of 25 kDa) has been implicated as one of several neural-specific components that direct constitutive fusion mechanisms to the regulated vesicle trafficking and exocytosis of neurotransmitter release. There exist two alternatively spliced isoforms of SNAP-25, a and b, which differ in a putative membrane-interacting domain. We show that these two isoforms have distinct quantitative and anatomical patterns of expression during brain development, in neurons, and in neuroendocrine cells and that the proteins localize differently in neurites of transfected PC12 pheochromocytoma cells. These findings indicate that alternative isoforms of SNAP-25 may play distinct roles in vesicular fusion events required for membrane addition during axonal outgrowth and for release of neuromodulatory peptides and neurotransmitters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967 Dec;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Bark I. C. Structure of the chicken gene for SNAP-25 reveals duplicated exon encoding distinct isoforms of the protein. J Mol Biol. 1993 Sep 5;233(1):67–76. doi: 10.1006/jmbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- Bark I. C., Wilson M. C. Human cDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25. Gene. 1994 Feb 25;139(2):291–292. doi: 10.1016/0378-1119(94)90773-0. [DOI] [PubMed] [Google Scholar]
- Bark I. C., Wilson M. C. Regulated vesicular fusion in neurons: snapping together the details. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4621–4624. doi: 10.1073/pnas.91.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy. Methods Cell Biol. 1989;30:157–192. doi: 10.1016/s0091-679x(08)60979-6. [DOI] [PubMed] [Google Scholar]
- Calakos N., Bennett M. K., Peterson K. E., Scheller R. H. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994 Feb 25;263(5150):1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Grenningloh G., Pich E. M. Nerve-terminal proteins: to fuse to learn. Trends Neurosci. 1994 Sep;17(9):368–373. doi: 10.1016/0166-2236(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Larhammar D., Blomqvist A., Sanna P. P., Milner R. J., Wilson M. C. Expression of a conserved cell-type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J. W., Hess E. J., Hart R. A., Kesslak J. P., Cotman C. W., Wilson M. C. Lesions of hippocampal circuitry define synaptosomal-associated protein-25 (SNAP-25) as a novel presynaptic marker. Neuroscience. 1990;38(2):515–525. doi: 10.1016/0306-4522(90)90047-8. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Slater T. M., Wilson M. C., Skene J. H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992 Dec;12(12):4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa H. P., Saisu H., Ishizuka T., Sekine Y., Tsugita A., Odani S., Abe T. A complex of rab3A, SNAP-25, VAMP/synaptobrevin-2 and syntaxins in brain presynaptic terminals. FEBS Lett. 1993 Sep 13;330(2):236–240. doi: 10.1016/0014-5793(93)80281-x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991 Dec;7(6):867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Lockerbie R. O., Miller V. E., Pfenninger K. H. Regulated plasmalemmal expansion in nerve growth cones. J Cell Biol. 1991 Mar;112(6):1215–1227. doi: 10.1083/jcb.112.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy A., Liu W. S., Baitinger C., Willard M. B. The major 35S-methionine-labeled rapidly transported protein (superprotein) is identical to SNAP-25, a protein of synaptic terminals. J Neurosci. 1991 Nov;11(11):3412–3421. doi: 10.1523/JNEUROSCI.11-11-03412.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor V. M., Shamotienko O., Grishin E., Betz H. On the structure of the 'synaptosecretosome'. Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 1993 Jul 12;326(1-3):255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A., Catsicas M., Staple J. K., Jones K. A., Ayala G., Knowles J., Grenningloh G., Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993 Jul 29;364(6436):445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler G. A., Polli J. W., Wilson M. C., Billingsley M. L. Developmental expression of the 25-kDa synaptosomal-associated protein (SNAP-25) in rat brain. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5247–5251. doi: 10.1073/pnas.88.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S. C., Braun J. E., Calakos N., Ting A. E., Bennett M. K., Scheller R. H. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994 Aug;13(2):353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Popov S., Brown A., Poo M. M. Forward plasma membrane flow in growing nerve processes. Science. 1993 Jan 8;259(5092):244–246. doi: 10.1126/science.7678471. [DOI] [PubMed] [Google Scholar]
- Risinger C., Blomqvist A. G., Lundell I., Lambertsson A., Nässel D., Pieribone V. A., Brodin L., Larhammar D. Evolutionary conservation of synaptosome-associated protein 25 kDa (SNAP-25) shown by Drosophila and Torpedo cDNA clones. J Biol Chem. 1993 Nov 15;268(32):24408–24414. [PubMed] [Google Scholar]
- Roth D., Burgoyne R. D. SNAP-25 is present in a SNARE complex in adrenal chromaffin cells. FEBS Lett. 1994 Sep 5;351(2):207–210. doi: 10.1016/0014-5793(94)00833-7. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994 Mar 1;4(3):220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Sanna P. P., Bloom F. E., Wilson M. C. Dibutyryl-cAMP induces SNAP-25 translocation into the neurites in PC12. Brain Res Dev Brain Res. 1991 Mar 18;59(1):104–108. doi: 10.1016/0165-3806(91)90035-h. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Rossetto O., Catsicas S., Polverino de Laureto P., DasGupta B. R., Benfenati F., Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem. 1993 Nov 15;268(32):23784–23787. [PubMed] [Google Scholar]
- Squinto S. P., Aldrich T. H., Lindsay R. M., Morrissey D. M., Panayotatos N., Bianco S. M., Furth M. E., Yancopoulos G. D. Identification of functional receptors for ciliary neurotrophic factor on neuronal cell lines and primary neurons. Neuron. 1990 Dec;5(6):757–766. doi: 10.1016/0896-6273(90)90334-c. [DOI] [PubMed] [Google Scholar]
- Stecher B., Gratzl M., Ahnert-Hilger G. Reductive chain separation of botulinum A toxin--a prerequisite to its inhibitory action on exocytosis in chromaffin cells. FEBS Lett. 1989 May 8;248(1-2):23–27. doi: 10.1016/0014-5793(89)80424-7. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Thureson-Klein A. K., Klein R. L. Exocytosis from neuronal large dense-cored vesicles. Int Rev Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]