Abstract
Background
The impact of a healthy lifestyle on risk of heart failure (HF) is not well known.
Objective
To evaluate the effect of a combination of lifestyle factors on incident HF, and further investigate whether weighting each lifestyle factor has additional impact.
Methods
Participants were 84,537 post-menopausal women from the Women's Health Initiative Observational Study, free of self-reported HF at baseline. A healthy lifestyle score (HL-score) was created, where women received 1 point for each healthy criterion met: high-scoring Alternative Healthy Eating Index, physically active, healthy body mass index, and currently not smoking. A weighted score (wHL-score) was also created where each lifestyle factor was weighted according to its independent magnitude of effect on HF. Incident hospitalized HF was determined by trained adjudicators using standardized methodology.
Results
There were 1,826 HF cases over a mean follow-up of 11 years. HL-score was strongly associated with risk of HF (multivariable-adjusted HR [95% CI] = 0.49 [0.38,0.62], 0.36 [0.28,0.46], 0.24 [0.19,0.31], and 0.23 [0.17,0.30] for HL-score of 1,2,3,4 vs 0, respectively]. The HL-score and wHL-score were similarly associated with HF risk (HR [95% CI] = 0.46 [0.41,0.52] for HL-score and 0.48 [0.42,0.55] for wHL-score, comparing the highest tertile to the lowest). The HL-Score was also strongly associated with HF risk among women without antecedent coronary heart disease, diabetes, or hypertension.
Conclusions
An increasingly healthy lifestyle was associated with decreasing HF risk among post-menopausal women, even in the absence of antecedent coronary heart disease, hypertension, and diabetes. Weighting the lifestyle factors had minimal impact.
Keywords: heart failure, lifestyle, cardiovascular diseases, risk factors, primary prevention
Heart failure (HF) is a major public health concern, characterized by a high prevalence, poor clinical outcomes, and significant health care costs (1). HF primary prevention through lifestyle approaches may be more effective and less costly than secondary or tertiary prevention efforts. A healthy lifestyle, often characterized by a combination of prudent diet, regular exercise, healthy weight, and not smoking, is related to a lower risk of atherosclerotic cardiovascular diseases, such as coronary heart disease (CHD) (2) and stroke (3). Few studies, however, have focused on a healthy lifestyle in relation to HF. Healthy lifestyle factors were individually and collectively associated with a lower risk of HF among white males in the Physician's Health Study I (4), and among men and women from a large and homogenous Finnish sample (5).
Post-menopausal women and African Americans experience a greater burden of HF (6-8), and they are predicted to make up a greater proportion of future HF cases in the United States (9,10). Therefore, examining the impact of a healthy lifestyle on HF risk in these groups is of particular interest. In addition, it has been proposed that the association of healthy lifestyle factors with HF risk may largely accounted for by the development of interim CHD (4,11), as well as interim hypertension and diabetes (11-13). However, we were particularly interested in whether an association between healthy lifestyle and HF risk would be present among women without development of any of these conditions prior to HF development. For example, HF in women is less associated with CHD as compared with men (14); thus, other mechanisms are of interest. Lastly, most prior studies investigating a combination of lifestyle factors weighted each lifestyle factor equally (2-5,15). This approach assumes that each lifestyle factor has the same magnitude of effect on the outcome, and this may lead to misclassification when combining lifestyle factors.
Accordingly, we examined whether a healthy lifestyle, as captured by a combination of high dietary quality, physical activity, healthy BMI, and not currently smoking, is associated with risk of HF in a diverse prospective cohort of post-menopausal women from the Women's Health Initiative (WHI) Observational Study, and we further assessed the additional impact of weighting each lifestyle factor according to its independent magnitude of effect on HF. We further examined the association of healthy lifestyle with HF in women with versus without antecedent CHD, African American versus non-Hispanic White women, and among women without antecedent CHD, hypertension, or diabetes.
Methods
Study Sample
The WHI Observational Study recruited women from 40 U.S. clinical centers from 1993 to 1998. The WHI Observational Study comprised a sample of post-menopausal women (ages 50-79 at baseline) who were in overall good health and were either unwilling or ineligible to be WHI clinical trials participants (16-18). This study was approved by each center's Institutional Review Board, and the subjects provided informed consent. Women were excluded from the current analyses if they: reported a history of HF at baseline (n=897); were missing information on lifestyle factors (n=3,110); had energy intake < 600 or > 5000 kcal/day (n=3,571); or were underweight (BMI ≤ 18.5 kg/m2) at baseline (due to potential for preclinical disease; n=1,050). Those excluded were more likely (p < 0.05) to be non-white, from the South, divorced, separated or, widowed, to have a history of hypertension or CHD at baseline, and to have lower levels of education.
Measures
We considered four lifestyle variables: diet quality as measured by the Alternative Healthy Eating Index (AHEI), physical activity, body mass index (BMI), and smoking. Data used to generate the AHEI were derived from the semi-quantitative WHI-food frequency questionnaire (19). The AHEI is a composite numerical measure of dietary quality, based on foods and nutrients predictive of chronic disease risk (20,21) and consisting of 11 dietary components, with each scored based on a 10-point scale (0 points = least healthy; 10 points = most healthy) (12). Women also reported on their physical activity. For analyses, physical activity was categorized as inactive (e.g., no report of moderate or vigorous physical activity); somewhat active (i.e., less active than recommendations (22): < 150 minutes/week of moderate physical activity, or < 75 minutes/week of vigorous physical activity, or equivalent combination); and active (e.g., meeting physical activity recommendations (22): ≥ 150 minutes of moderate physical activity/week or ≥ 75 minutes of vigorous physical activity or an equivalent combination). Smoking status was categorized as a current, former, or never smoker. BMI was calculated from weight and height measures obtained at clinical examinations using a calibrated stadiometer, and categorized as normal weight (18.5 ≤ BMI <25 kg/m2), overweight (25 ≤ BMI ≤ 30 kg/m2), and obese (BMI > 30 kg/m2).
At baseline, women reported on socio-demographic factors and medical history including CHD (includes cardiac arrest, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, angina, and myocardial infarction), diabetes, and hypertension. Race/ethnicity was self-reported as American Indian or Alaskan Native, Asian or Pacific Islander, black or African American, Hispanic/Latino, white (not of Hispanic origin), or other.
Incident hospitalized HF was ascertained yearly in WHI by medical record abstraction of self-report hospitalizations and classified by trained adjudicators using standardized methodology. Specifically, hospitalized HF requiring and/or occurring during hospitalization required physician diagnosis of new-onset or worsened congestive HF on the reported hospital admission and 1 or more of the following 4 criteria: HF diagnosed by a physician and receiving medical treatment for HF; symptoms plus documentation in the current medical record of a history of an imaging procedure showing impaired left ventricular systolic or diastolic function, pulmonary edema/congestion on chest x-ray on the current admission, dilated ventricle(s) or “poor” left ventricular or right ventricular function by echocardiography, radionuclide ventriculography, or other contrast ventriculography; or evidence of LV diastolic dysfunction. This method was found to have high (79%) agreement rate (kappa) comparing central adjudicated HF to local adjudication (23,24).
Statistical Analyses
To create a healthy lifestyles score (HL-score) for the present analyses, each lifestyle factor was dichotomized as ‘healthy’ versus ‘unhealthy’ as follows: high-scoring AHEI (quintiles 4 and 5) versus low-scoring AHEI (quintiles 1,2, 3), physically active versus somewhat active or inactive, normal BMI (18.5 ≤ BMI < 25 kg/m2) versus overweight or obese (BMI ≥ 25 kg/m2), and not a current smoker versus current smoker. Women then received 1 point for every healthy criterion met, and points were summed to obtain a HL-score ranging from 0 (least healthy) to 4 (most healthy).
A weighted HL-score (wHL-score) also was created, where each dichotomous lifestyle factor was first weighted according to its independent magnitude of effect (e.g., beta coefficient adjusted for the other dichotomous lifestyle factors) on HF risk. Weighted points attained by each individual were summed to obtain a wHL-score, which ranged from 0 (least healthy) to 1.55 (most healthy). To compare the HL-scores with the wHL-scores, both were analyzed as tertiles (given that the scores can only take on a certain number of values [e.g. 0,1,2,3,or 4 for the HL-score], they could not be partitioned into quintiles).
We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Person-time was calculated from baseline-interview until development of HF, death, or date of last contact. The proportional hazards assumption, assessed with models including log(time)-by-covariate interaction terms, was not violated.
Analyses were initially adjusted for age and race/ethnicity, with further adjustment for education, marital status, U.S. region, and antecedent: CHD, hypertension, and diabetes (‘antecedent’ referring to occurrence at any point prior to censoring time, whether reported on at baseline or developed during follow-up). Additional analyses stratified on absence versus presence of antecedent CHD, African-American women versus non-Hispanic white women, as well as restricting analyses to the subgroup of women without antecedent CHD, hypertension, or diabetes.
Partial population attributable risk (PAR) calculations (25) estimated the proportion of HF that would hypothetically be prevented if all women were in the healthiest category of the lifestyle factors or the HL-score. All Statistical analyses were conducted using SAS software version 9 (SAS Institute Inc., Cary, NC).
Results
The final analytic sample included 84,537 women, with a mean (SD) age of 63.5 (7.3) years at baseline. General baseline characteristics are presented in Table 1. Approximately 41% of participants had normal BMI, 44% were physically active, 94% were current non-smokers, and 40% had a high-scoring AHEI. Approximately 14% of women met all 4 of these healthy lifestyle criteria. There were 1,826 documented HF cases during a mean follow-up of 11 years.
Table 1. Baseline Characteristics According to the Healthy Lifestyle Score, Women's Health Initiative Observational Study.
| Healthy lifestyle score | |||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1 | 2 | 3 | 4 | |
| (n =1,529) | (n =19,389) | (n =27,366) | (n =24,271) | (n =11,982) | |
| Age in years, mean (SD) | 60.5 (6.9) | 63.2(7.3) | 63.7 (7.3) | 63.8 (7.3) | 63.6 (7.4) |
| African American, % | 18.8 | 12.5 | 8.0 | 4.5 | 2.0 |
| Hispanic/Latino, % | 5.1 | 5.1 | 3.9 | 2.6 | 1.6 |
| Non-Hispanic white, % | 73.2 | 79.1 | 84.1 | 88.2 | 90.8 |
| Less than High school, % | 10.1 | 8.1 | 5.1 | 2.7 | 1.1 |
| Never married, % | 5.6 | 4.7 | 4.7 | 4.5 | 4.3 |
| Divorced/separated, % | 27.7 | 17.3 | 15.3 | 14.2 | 13.6 |
| history of diabetes, % | 6.5 | 6.6 | 4.2 | 2.5 | 1.1 |
| History of hypertension, % | 37.3 | 43.6 | 35.4 | 27.9 | 19.9 |
| History of CHD*, % | 7.8 | 6.8 | 5.7 | 4.7 | 3.6 |
| BMI (kg/m2), mean (SD) | 30.8 (5.5) | 31.3 (6.0) | 28.1 (5.4) | 25.5 (4.5) | 22.4 (1.6) |
| AHEI score, mean (SD) | 45.1 (7.8) | 47.2 (7.5) | 53.1 (9.4) | 60.2 (9.7) | 67.4 (6.4) |
abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; CHD, coronary heart disease
Self-reported CHD includes cardiac arrest, CABG, PTCA, angina, and MI
Categories of a given variable (e.g. race) may not necessarily add up to 100%, given that not all categories were included
After multivariable adjustment, each individual lifestyle factor was independently associated with risk of HF in an inverse and graded manner (Table 2), with the strongest associations observed for BMI and smoking. The percentage of partial PAR (95% CI) was highest for AHEI [27% (15%, 39%)], followed by BMI [20% (10%, 29%)], cigarette smoking [16% (10%, 23%)], and physical activity [12% (4%, 20%)].
Table 2. Risk of Heart Failure in Relation to Individual Lifestyle Factors, Women's Health Initiative Observational Study.
| No. of cases | Person-years | Crude Incidence rate/1000 person-years | Model 1* HR (95% CI) | P value for trendł | Model 2† HR (95% CI) | P value for trend‡ | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| AHEI | |||||||
| Quintile 1 | 499 | 178,032 | 2.80 | Referent | <0.001 | Referent | 0.001 |
| Quintile 2 | 411 | 183,884 | 2.24 | 0.83 (0.73,0.95) | 0.85 (0.74,0.97) | ||
| Quintile 3 | 350 | 188,254 | 1.86 | 0.76 (0.66,0.87) | 0.81 (0.70,0.93) | ||
| Quintile 4 | 321 | 191,496 | 1.68 | 0.72 (0.62,0.83) | 0.76 (0.65,0.88) | ||
| Quintile 5 | 245 | 194,954 | 1.26 | 0.60 (0.51,0.70) | 0.61 (0.52,0.73) | ||
| Physical Activity | |||||||
| Inactive | 386 | 117,311 | 3.29 | Referent | 0.008 | Referent | 0.06 |
| Somewhat active | 614 | 285,095 | 2.15 | 0.77 (0.67,0.87) | 0.77 (0.67,0.87) | ||
| Active | 826 | 534,214 | 1.55 | 0.66 (0.58,0.75) | 0.69 (0.61,0.79) | ||
| BMI | |||||||
| BMI ≥ 30 kg/m2 | 752 | 223,128 | 3.37 | Referent | 0.008 | Referent | 0.1 |
| 25 ≤ BMI ≤ 30 kg/m2 | 557 | 322,941 | 1.72 | 0.50 (0.45,0.56) | 0.56 (0.50,0.63) | ||
| 18.5 ≤ BMI < 25 kg/m2 | 517 | 390,552 | 1.32 | 0.43 (0.38,0.48) | 0.51 (0.45,0.57) | ||
| Smoking | |||||||
| Current smoker | 174 | 52,182 | 3.33 | Referent | <0.001 | Referent | <0.001 |
| Past smoker | 820 | 407,776 | 2.01 | 0.52 (0.44,0.62) | 0.56 (0.47,0.66) | ||
| Never smoker | 832 | 476,663 | 1.75 | 0.42 (0.35,0.49) | 0.46 (0.39,0.55) | ||
abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index
Model 1 adjusted for age, race/ethnicity, and all other healthy lifestyle factors
Model 2 adjusted for model 1 covariates, marital status, education, U.S. region, and antecedent: coronary heart disease, treated diabetes, and hypertension
test for linear trend
A strong inverse and graded association was observed between the HL-score and HF risk (Table 3). According to PAR% calculations, 35% (28%,43%) of HF could theoretically be prevented if all women met all 4 healthy lifestyle criteria. When comparing the HL-score and the wHL-score, differences between the 2 were minimal (Figure 1). Comparing the highest versus lowest tertile, a multi-variable adjusted HR (95% CI) of 0.46 (0.41,0.52) was observed for the regular HL-score and 0.48 (0.42,0.55) for the wHL-score. Results were very similar when time-dependent lifestyle factors were used to create the HL-score and the wHL-score.
Table 3. Risk of Heart Failure in Relation to the Healthy Lifestyle Score, Women's Health Initiative Observational Study.
| Healthy lifestyle score | No. of cases | Person-years | Crude Incidence rate/1000 person-years | Age and race-adjusted HR (95% CI) | Multivariable-adjusted* HR (95% CI) | P value for trend |
|---|---|---|---|---|---|---|
| 0 | 74 | 15,099 | 4.90 | Referent | Referent | < 0.001 |
| 1 | 614 | 204,287 | 3.01 | 0.45 (0.35,0.57) | 0.49 (0.38,0.62) | |
| 2 | 620 | 300,462 | 2.06 | 0.30 (0.24,0.38) | 0.36 (0.28,0.46) | |
| 3 | 365 | 276,654 | 1.32 | 0.19 (0.15,0.25) | 0.24 (0.19,0.31) | |
| 4 | 153 | 140,121 | 1.09 | 0.17 (0.13,0.22) | 0.23 (0.17,0.30) |
Adjusted for age, race/ethnicity, marital status, education, U.S. region, and antecedent: coronary heart disease, treated diabetes, and hypertension
Figure 1. Comparison of Non-weighted and Weighted Healthy Lifestyle Score in relation to Heart Failure Risk.
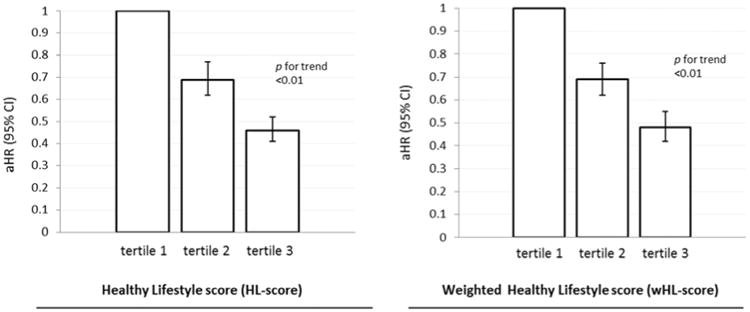
Risk of heart failure in relation to tertiles of the healthy lifestyle score and weighted healthy lifestyle score, Women's Health Initiative Observational Study.
HRs adjusted for age, race, marital status, US region, education, and antecedent: CHD, hypertension and diabetes.
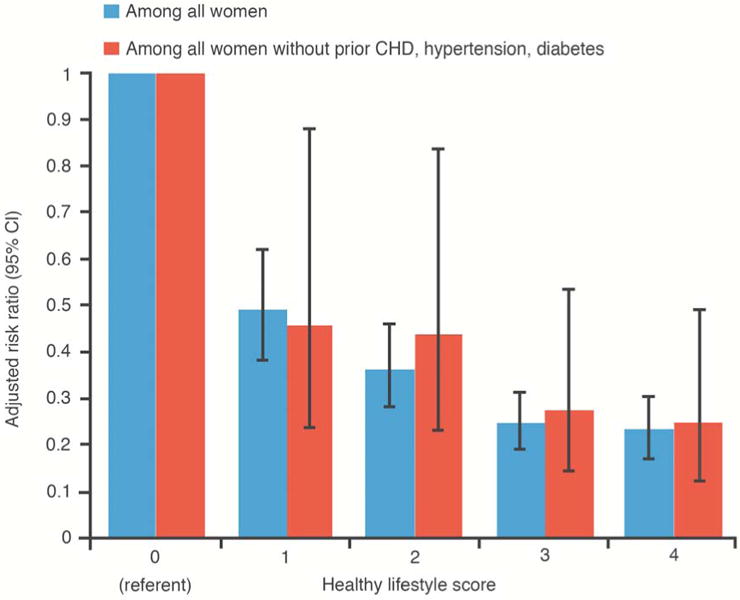
In stratified analyses, the HL-score was strongly related to HF risk in a graded manner in both women with and without antecedent CHD (Figure 2), and this was similarly observed among the subgroup of women without antecedent CHD, hypertension, and diabetes. (Figure 3). Associations of HL-score with HF risk were similar when comparing African Americans to non-Hispanic whites (Figure 4).
Figure 2. Healthy Lifestyle and Heart Failure Risk in Women Without vs. With Antecedent CHD.
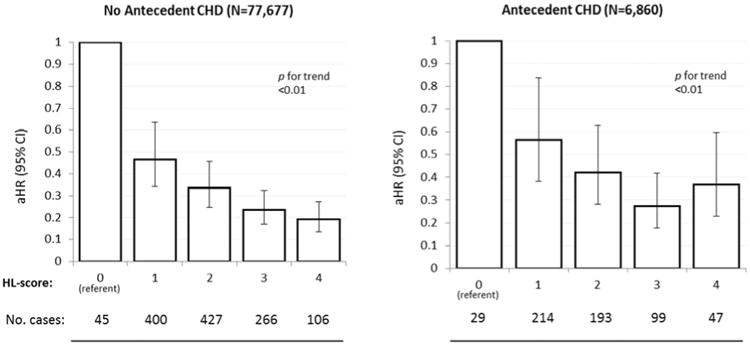
Risk of heart failure in relation to the healthy lifestyle score among women without versus with antecedent CHD, Women's Health Initiative Observational Study. Abbreviations: CHD, coronary heart disease; HL-score, healthy lifestyle score. HRs adjusted for age, race, marital status, US region, education, and antecedent hypertension or diabetes.
Figure 3. Healthy Lifestyle and Heart Failure Risk in Women Without Antecedent CHD, Hypertension, or Diabetes.
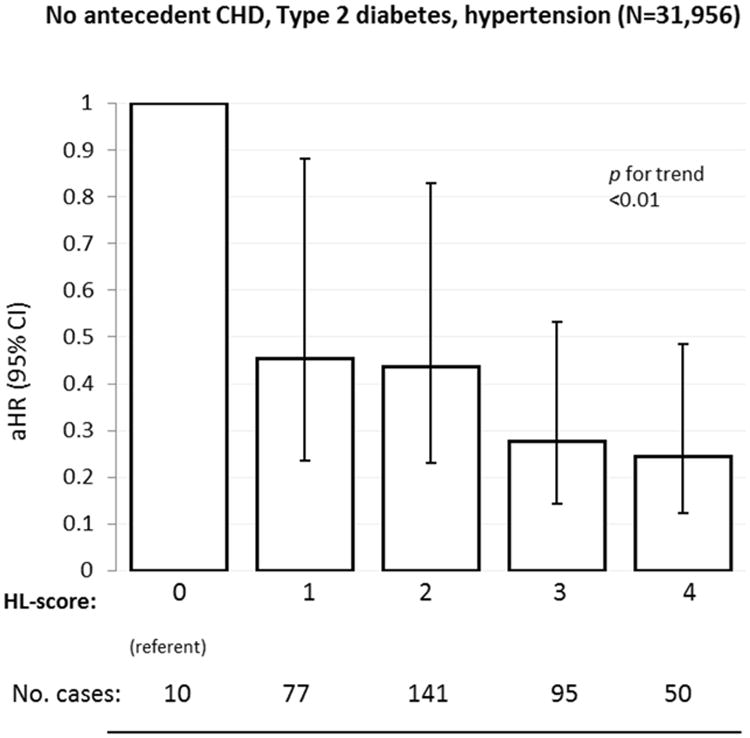
Risk of heart failure in relation to the healthy lifestyle score among women without antecedent CHD, hypertension, and diabetes, Women's Health Initiative Observational Study.
Abbreviations: HL-score, healthy lifestyle score. HRs adjusted for age, race, marital status, US region, and education.
Figure 4. Healthy Lifestyle and Heart Failure Risk in African-Americans vs. Non-Hispanic Whites.
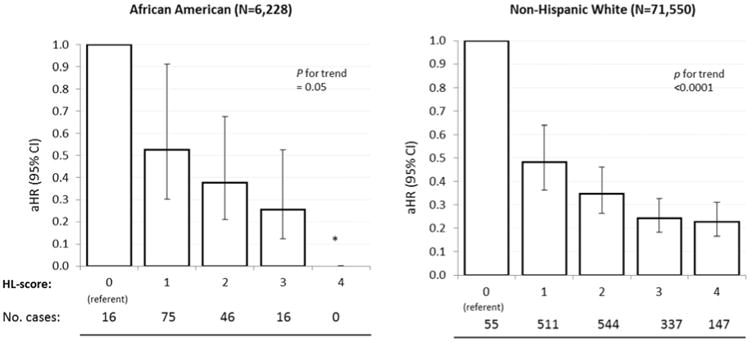
Risk of heart failure in relation to the healthy lifestyle score among african americans versus non-hispanic whites, Women's Health Initiative Observational Study. Abbreviations: HL-score, healthy lifestyle score. HRs adjusted for age, race, marital status, US region, education, and antecedent hypertension and diabetes. *HR could not be calculated due to 0 heart failure cases in this category.
Discussion
In this large and diverse prospective study of post-menopausal women, an increasingly healthy lifestyle was associated with a progressively decreasing HF risk. Weighting each lifestyle factor according to its magnitude of effect on HF did not have a notable impact on the associations observed. Associations of healthy lifestyle with HF risk were also strong and graded among women without antecedent CHD, hypertension, or diabetes (Central Illustration), and similarly so among African Americans.
Central Illustration. Healthy Lifestyle and Risk of Heart Failure in the Women's Health Initiative.
An overall healthy lifestyle is strongly associated with lower risk of heart failure among post-menopausal, even in the absence of coronary heart disease (CHD), hypertension, or diabetes.
Smoking is currently well-known as a strong and independent risk factor for HF (26). BMI also has been shown to be strongly and independently related to HF incidence in women (11,27). Excess weight gain may increase risk of HF through increased blood pressure (28,29), insulin resistance (30,31), and elevated cholesterol levels (32), or through mechanisms that involve inflammation (33,34), sleep apnea (35-37), or kidney disease (38-40).
Fewer studies focused on dietary pattern in relation to HF. The AHEI was recently shown to be strongly related to HF (41) and overall CVD risk (21), more so than other dietary indices (20,21). The AHEI-2010 explicitly emphasizes high intakes of whole grains, poly-unsaturated fatty acids, nuts, legumes, and low intakes of sugar-sweetened beverages, red and processed meats, and trans-fats.(12) High levels of physical activity also have been related to reduced risk of HF in previous studies and may act through beneficial effects on body weight, hypertension, diabetes, and CHD (42-45).
Given that certain lifestyle factors show stronger associations with risk of disease than others, simply adding the lifestyle factors when combining them may lead to misclassification due to heterogeneous people having the same HL-score. However, we found that the relative weighting of lifestyle factors did not have any impact on associations of healthy lifestyle with HF risk, suggesting that adopting an overall healthy lifestyle where these healthy lifestyle habits are integrated is optimal.
A large proportion of HF incidence is accounted for by antecedent CHD (46,47), and lifestyle factors are thought to increase risk of HF largely through development of CHD as an intermediate endpoint (4,11). In our analyses, however, the association between healthy lifestyle and HF risk was strong and graded, even among women without antecedent CHD. These findings are consistent with previous studies showing that smoking, BMI, and physical activity are strongly associated with HF risk independent of the presence or absence of CHD or other atherogenic risk factors for HF (11,13,26). Thus, healthy lifestyle factors may act on HF through other mechanisms. For example, obesity may impact HF risk through renal insufficiency (38-40). In addition, HF in the elderly, and particularly among elderly women, is more commonly characterized by impaired diastolic function but preserved left ventricular (LV) systolic function, and the vast majority of HF patients with preserved systolic function do not have a defined myocardial disease but rather a clinically significant impairment of diastolic function (47,48). Even in HF characterized by LV systolic dysfunction, one-third of cases are not defined by underlying CHD, but rather by non-ischemic causes (48). Similarly, the association of healthy lifestyle with risk of HF risk remained strong even after further exclusion of women with antecedent hypertension and diabetes. Finally, healthy lifestyle was strongly related to risk of HF among both African Americans and non-Hispanic whites.
Prior literature
Two prior studies examined a combination of lifestyle factors in relation to HF. Normal body weight, not smoking, regular exercise, moderate alcohol intake, and consumption of fruits, vegetables and breakfast cereals were individually and collectively associated with lower lifetime risk of HF among male physicians. Lifetime risk of HF was 21% and 10% in men with 0 versus ≥ 4 healthy lifestyle factors, respectively (4). Smoking, BMI, physical activity, and vegetable intake also were individually and collectively associated with risk of HF in both men and women from a large, homogenous Finnish sample (5), with an inverse association observed between number of healthy lifestyle factors and HF risk.
Strengths and Limitations
Strengths of this study include the large sample size, prospective design, and the racial, socioeconomic, and geographic diversity within WHI. Our investigation was the first, to our knowledge, to report on the association of healthy lifestyle with risk of HF among a diverse group of US women. In addition, we took into account the relative magnitude of impact of each lifestyle factor. Finally, a thorough follow-up of HF cases using comprehensive criteria and standardized methodology used in WHI ensures satisfactory case ascertainment (23).
Several limitations should also be noted. The ascertainment of HF was based on initial self-report, and this may have led to some HF cases being missed. Similarly, hospitalization was used to identify incident HF, and exclusion of outpatient diagnoses of HF may have underestimated mild or transient cases of HF.
Conclusions
Heart failure remains a costly disease, with approximately two-thirds of costs reported to come from hospitalizations (9). Despite improved medical and surgical management, mortality after onset of heart failure remains high (49,50). In parallel, the prevalence of healthy habits (with the exception of smoking abstinence) is generally low among middle-aged and older women in the United States (51). We observed that an overall healthy lifestyle was strongly protective against HF among post-menopausal women, even in the absence of antecedent CHD, hypertension, and diabetes. This suggests that a healthy lifestyle is beneficial for protection against HF over and above its benefits in lowering risk for these clinical intermediates. Evidence from randomized trials suggest that multi-component lifestyle interventions, where several healthy lifestyle habits are simultaneously promoted, are successful in increasing healthful habits and decreasing cardiovascular disease risk (52-55)—in some cases, even more so than clinical treatment (56). Therefore, prevention strategies that place a higher emphasis on the combination and integration of healthy lifestyle habits may be of most benefit.
Perspectives.
Competency in Medical Knowledge
In the Women's Health Initiative Observational Study, a healthy lifestyle was associated with a substantially lower risk of developing heart failure, even among women without coronary disease, hypertension or diabetes.
Translational Outlook
Further research is required to determine whether a comprehensive lifestyle modification approach can be effectively implemented in routine clinical practice and at the population level, in order to reduce the incidence of heart failure in both men and women.
Acknowledgments
WHI investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Funding sources: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Support for Dr. Waring provided by NIH grants KL2TR000160 and U01HL105268.
Abbreviations
- AHEI
Alternative Healthy Eating Index
- BMI
body mass index
- CHD
coronary heart disease
- HF
heart failure
- HL-score
healthy lifestyle score
- wHL-score
weighted healthy lifestyle score
Footnotes
Disclosures: No conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941–55. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 3.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Tuomilehto J, Jousilahti P, et al. Lifestyle factors in relation to heart failure among finnish men and women. Circ Heart Fail. 2011;4:607–12. doi: 10.1161/CIRCHEARTFAILURE.111.962589. [DOI] [PubMed] [Google Scholar]
- 6.Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. American heart journal. 1999;137:919–27. doi: 10.1016/s0002-8703(99)70417-5. [DOI] [PubMed] [Google Scholar]
- 7.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women's Health Initiative. Circulation. 2012;126:688–96. doi: 10.1161/CIRCULATIONAHA.111.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–93. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieminen MS, Harjola VP, Hochadel M, et al. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. Eur J Heart Fail. 2008;10:140–8. doi: 10.1016/j.ejheart.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 15.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 20.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 21.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–7. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health & Human Services. 2008 Physical Activity Guidelines for Americans. 2008 [Google Scholar]
- 23.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 24.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–8. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 25.Wand H, Spiegelman D, Law M, Jalaludin B, Kaldor J, Maher L. Estimating population attributable risk for hepatitis C seroconversion in injecting drug users in Australia: implications for prevention policy and planning. Addiction. 2009;104:2049–56. doi: 10.1111/j.1360-0443.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 26.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 27.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 28.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240:1607–10. doi: 10.1001/jama.240.15.1607. [DOI] [PubMed] [Google Scholar]
- 29.Stamler J. Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol. 1991;1:347–62. doi: 10.1016/1047-2797(91)90045-e. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–73. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 32.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 33.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 34.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–11. [PubMed] [Google Scholar]
- 36.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 37.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American journal of respiratory and critical care medicine. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 38.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. 2003;92:682–6. doi: 10.1016/s0002-9149(03)00822-1. [DOI] [PubMed] [Google Scholar]
- 39.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 40.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am. 2004;88:1273–94. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Belin RJ, Greenland P, Allison M, et al. Diet quality and the risk of cardiovascular disease: the Women's Health Initiative (WHI) Am J Clin Nutr. 2011;94:49–57. doi: 10.3945/ajcn.110.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 43.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–8. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 44.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Intern Med. 2004;164:31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292:1188–94. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 46.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 47.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 48.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 49.Gillum RF. Epidemiology of heart failure in the United States. American heart journal. 1993;126:1042–7. doi: 10.1016/0002-8703(93)90738-u. [DOI] [PubMed] [Google Scholar]
- 50.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 51.Schoenborn CA, Adams PE. Health behaviors of adults: United States. Vital Health Stat. 2005-2007;10:1–132. [PubMed] [Google Scholar]
- 52.Hayashi T, Farrell MA, Chaput LA, Rocha DA, Hernandez M. Lifestyle intervention, behavioral changes, and improvement in cardiovascular risk profiles in the California WISEWOMAN project. Journal of women's health. 2010;19:1129–38. doi: 10.1089/jwh.2009.1631. [DOI] [PubMed] [Google Scholar]
- 53.Keyserling TC, Samuel Hodge CD, Jilcott SB, et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: the North Carolina enhanced WISEWOMAN project. Preventive medicine. 2008;46:499–510. doi: 10.1016/j.ypmed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Khare MM, Carpenter RA, Huber R, et al. Lifestyle intervention and cardiovascular risk reduction in the Illinois WISEWOMAN Program. Journal of women's health. 2012;21:294–301. doi: 10.1089/jwh.2011.2926. [DOI] [PubMed] [Google Scholar]
- 55.Stoddard AM, Palombo R, Troped PJ, Sorensen G, Will JC. Cardiovascular disease risk reduction: the Massachusetts WISEWOMAN project. Journal of women's health. 2004;13:539–46. doi: 10.1089/1540999041281106. [DOI] [PubMed] [Google Scholar]
- 56.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]


