Abstract
Connecting Peptide, or C-peptide, is a product of the insulin prohormone, and is released with and in amounts equimolar to those of insulin. While it was once thought that C-peptide was biologically inert and had little biological significance beyond its role in the proper folding of insulin, it is now known that C-peptide binds specifically to the cell membranes of a variety of tissues and initiates specific intracellular signaling cascades that are pertussis toxin sensitive. Although it is now clear that C-peptide is a biologically active molecule, controversy still remains as to the physiological significance of the peptide. Interestingly, C-peptide appears to reverse the deleterious effects of high glucose in some tissues, including the kidney, the peripheral nerves, and the vasculature. C-peptide is thus a potential therapeutic agent for the treatment of diabetes-associated long-term complications. This review addresses the possible physiologically relevant roles of C-peptide in both normal and disease states and discusses the effects of the peptide on sensory nerve, renal, and vascular function. Furthermore, we highlight the intracellular effects of the peptide and present novel strategies for the determination of the C-peptide receptor(s). Finally, a hypothesis is offered concerning the relationship between C-peptide and the development of microvascular complications of diabetes.
Keywords: C-peptide, diabetes, diabetes-associated complications
in the course of insulin biosynthesis, as described in 1967 (100), proinsulin is cleaved into insulin and C-peptide. The two are stored in the secretory granules of the pancreatic β-cells and eventually released together in equimolar amounts. C-peptide has an important role in facilitating the correct folding of insulin and formation of its disulfide bridges. Following its discovery, several unsuccessful attempts were made to detect insulin-like effects of C-peptide (33, 40), gradually leading to the view that C-peptide was without physiological effects. Interest focused instead on its ability to serve as a biomarker of β-cell activity. As such, C-peptide has been a valuable tool in elucidating the pathophysiology of type 1 and type 2 diabetes.
C-peptide is a 31-amino acid peptide. It is negatively charged and not reported to show an ordered tertiary structure under physiological conditions (37). The amino acid sequence of C-peptide shows considerable variability between species, in contrast to the well-preserved molecular structure of insulin. However, in mammals the eight residues at positions 1, 3, 6, 11, 12, 21, 27, and 31 of C-peptide are conserved or vary in only one species (37). Of these, Glu27 and Gln31 have been ascribed special importance for interaction between C-peptide and cell membranes (80). A similar structural variability exists for relaxin (4), and the possibility may be considered that both relaxin and C-peptide have taken on hormonal roles late in the course of evolution by affecting specific cellular processes needed only in higher mammals.
The plasma concentration of C-peptide in the overnight-fasted state is 0.3–0.6 nM in healthy subjects, and postprandial levels may rise to 1–3 nM. Higher levels are observed in overweight individuals (78). Its biological half-life is ∼30 min in healthy individuals and longer in subjects with type 2 diabetes (22). Unlike insulin, C-peptide escapes hepatic retention and is eventually catabolized primarily by the renal cortex (35, 68), with only a small fraction being excreted in the urine.
Indirect evidence of biological effects of C-peptide has long been recognized. Thus, it is a clinical observation that subjects with type 1 diabetes who retain low plasma concentrations of C-peptide are less prone to develop microvascular complications than those in whom β-cell function has ceased completely (98, 116). In addition, pancreas or β-cell transplantation, which results in restoration of endogenous insulin and C-peptide concentrations, is accompanied by significant amelioration of diabetes-induced abnormalities, both structural and functional, of the peripheral nerves and the kidneys (23, 103).
In the early 1990s, the possibility that C-peptide might exert direct physiological effects of its own was reevaluated. A series of studies were undertaken involving administration of the peptide to patients with type 1 diabetes, who lack C-peptide. It soon became apparent that replacement of C-peptide in physiological concentrations resulted in significant improvements of several diabetes-induced functional abnormalities (47–50). These findings prompted a renewed interest in C-peptide physiology, and during the past 20 years a steadily increasing number of reports on new aspects of C-peptide physiology have emerged. The information available today includes studies of the peptide's interaction with cell membranes and its intracellular signaling properties (39, 113). In vivo studies in animal models of type 1 diabetes have defined a beneficial influence of C-peptide on diabetes-induced functional and structural abnormalities of the kidney, peripheral nerves, and the central nervous system. In addition, several clinical studies describing positive effects of C-peptide replacement therapy on nerve and kidney function in type 1 diabetic patients have been reported (for a review of in vivo animal and human studies, see Ref. 110). The wealth of information now available supports the hypothesis that C-peptide, contrary to previous views, does exert important physiological effects. These effects and their underlying mechanisms of action are discussed in this review.
C-Peptide and Nerve Impairment in Type 1 Diabetes
Diabetic neuropathy.
Diabetic neuropathy is the most common long-term complication of diabetes. It usually manifests as sensory loss in the extremities with severe clinical implications, potentially leading to foot ulceration and limb amputation. There may also be signs of autonomic dysfunction involving problems with gastric emptying, urinary bladder dysfunction, or erectile dysfunction. Several studies in animal models of diabetes and in patients with type 1 diabetes have demonstrated beneficial effects of C-peptide replacement on both peripheral and autonomic nerve function in diabetes.
Animal studies.
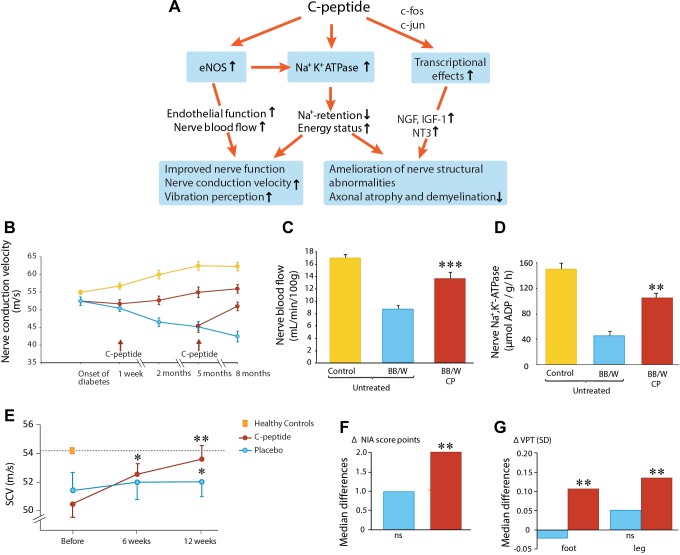
Positive effects of C-peptide on peripheral nerve function have been demonstrated in two animal models of type 1 diabetes; an overview of C-peptide effects is shown in Fig. 1A. C-peptide administration in BB/Wor rats, with spontaneous development of type 1-like diabetes, prevented the development of nerve conduction velocity (NCV) deficits when administration of the peptide was started 1 wk after the onset of diabetes (Fig. 1B) (97). In addition, C-peptide elicited an increase in NCV, partially correcting the NCV deficit, when treatment was commenced at 5 mo after onset of diabetes when nerve abnormalities had become established. Diabetes-induced nerve structural abnormalities were also prevented or reversed by C-peptide administration, as indicated by amelioration of paranodal swelling, axonal atrophy, and demyelination (97, 117). The C-peptide concentrations reached in these studies were in the low physiological concentration range (0.5–0.7 nM). Similarly, in streptozotocin (STZ)-diabetic rats receiving C-peptide in replacement doses for 2 wk starting at 6 wk after induction of diabetes, the peptide gave rise to 80% and 60% corrections of the sensory (saphenous) and motor (sciatic) NCV deficits, respectively (15). The C-peptide-induced improvement in NCV was found to be concentration dependent (117), and a scrambled C-peptide, a control peptide with the same 31 residues but assembled in random order, was without effect.
Fig. 1.
C-peptide and diabetic neuropathy. A: schematic overview of C-peptide's effects on nerve function and structure in diabetes. eNOS, endothelial NO synthase. B: effects of C-peptide (red) or placebo (blue) administration for up to 8 mo on nerve conduction velocity (NCV) in diabetic rats. Yellow symbols indicate NCV development in healthy nondiabetic animals; data from Ref. 97. C: sciatic nerve blood flow in healty (yellow), untreated diabetic (blue), and C-peptide-treated (red) diabetic BB/W rats; data from Ref. 15. D: Na+,K+-ATPase activity of the sciatic nerve in healthy (yellow), untreated diabetic (blue), and C-peptide-treated diabetic rats; data from Ref. 97. E: effects of C-peptide in replacement dose (red) or placebo (blue) for 3 mo on sensory nerve conduction velocity (SCV) in type 1 diabetes subjects with early-stage nerve impairment (Ref. 19). F: influence of C-peptide (red) or placebo (blue) administration for 6 mo on neuropathy impairment score (NIA) (Ref. 20). G: vibration perception in subjects with type 1 diabetes and manifest neuropathy; data from Ref. 20.
The mechanism behind the beneficial effects of C-peptide on nerve function and structure involves several factors. Direct measurements of nerve blood flow have shown that endoneurial blood flow is substantially reduced in diabetes (15, 101). Administration of C-peptide in replacement doses resulted in a marked improvement of the endoneurial perfusion deficit (Fig. 1C), possibly a result of C-peptide-elicited stimulation of endothelial nitric oxide synthase (eNOS) and augmented NO availability (112). C-peptide effects on both endoneurial blood flow and NCV were abrogated by an eNOS blocker, indicating that C-peptide in physiological concentrations improves nerve function in type 1 diabetes via a NO-sensitive neurovascular mechanism, mediating dilation of the vasa nervorum.
Decreased Na+,K+-ATPase activity in peripheral nerve tissue is also a characteristic abnormality in type 1 diabetes (44). It is associated with inactivation of Na+ channels, intra-axonal sodium accumulation, and swelling of the paranodal region during the early phase of the disorder. C-peptide in physiological concentrations prevents or partially corrects the diabetes-induced reduction in nerve Na+,K+-ATPase activity in diabetes (Fig. 1D) (44, 97), thereby contributing to diminished Na+ retention and partial correction of nerve structural abnormalities, paranodal swelling in particular.
Painful neuropathy is a debilitating consequence of diabetes, which is at least partly due to damage to the unmyelinated and the small myelinated nociceptive fibers (17). Degeneration of these fibers initially leads to high firing frequencies and spinal sensitization, which is experienced as hyperalgesia. Replacement of C-peptide from the onset of diabetes in rats completely prevents thermal hyperalgesia as well as degeneration and loss of unmyelinated fibers (53). These findings are accompanied by improved regulation via the transcription factors c-fos and c-jun of gene expression of several neurotropic factors, e.g., NGF, NT3, and IGF-I, and their receptors. Experimental evidence thus supports the notion that C-peptide may be useful in alleviating painful neuropathy.
Clinical studies.
The beneficial effects of C-peptide on impaired nerve function observed in animal models of diabetes have been confirmed in clinical studies. A double-blind placebo-controlled study in patients with type 1 diabetes with early-stage neuropathy showed that C-peptide replacement over a 3-mo period resulted in a gradual increase in sural NCV, reaching 2.7 m/s, amounting to 80% correction of the initial nerve conduction deficit (Fig. 1E) as well as improvements in vibration perception (19). These results have subsequently been confirmed and extended in a larger study involving patients with clinically manifested neuropathy and type 1 diabetes (20). Six months of C-peptide replacement resulted in improvements in sensory (sural) NCV, clinical scores of neuropathy impairment (Fig. 1F), and vibration perception (Fig. 1G). Levels of glycemic control were similar in C-peptide- and placebo-treated patients and unchanged during the study.
Abnormal cardiac autonomic nerve function is associated with cardiac arrhythmias and sudden death. The condition may be evaluated as reduced heart rate variability (HRV) during deep breathing, a measurement that primarily reflects vagal nerve function. In patients with type 1 diabetes, a 2-h infusion of C-peptide is reported to significantly increase HRV, whereas no change was seen after saline infusion (48). The heart rate brake index after a tilting manoeuver was also improved after C-peptide infusion. A similar, albeit less-marked improvement was seen after 3 mo of C-peptide administration in patients with type 1 diabetes (47). Finally, it should be mentioned that in a study of type 1 diabetes and manifest neuropathy (20), subjects with erectile dysfunction at baseline reported significant improvement after C-peptide treatment for 6 mo, an effect that may possibly be ascribed to improved parasympathetic tone.
In summary, the available experimental and clinical evidence demonstrates that C-peptide, by stimulating endoneurial eNOS, increasing nerve blood flow, augmenting nerve Na+,K+-ATPase, and stimulating nerve trophic factors exerts beneficial effects on the mechanisms underlying nerve dysfunction in type 1 diabetes (Fig. 1A).
Effects of C-Peptide in the Diabetic Kidney
Diabetic nephropathy.
Diabetes is the single leading cause of end-stage renal disease (ESRD) in the Western world and is associated with increased cardiovascular risk, high morbidity, and mortality (82). Despite the improvements in clinical management and treatment of ESRD, the prevalence of diabetic nephropathy (i.e., ESRD due to diabetes) is likely to continue to rise due to the increasing prevalence of diabetes and obesity worldwide. As a result, diabetic nephropathy will remain a significant economic and health burden unless novel treatments can be identified.
Both clinically and experimentally, diabetic nephropathy is initially characterized by glomerular hyperfiltration [i.e., increase in the glomerular filtration rate (GFR), glomerular hypertrophy, and microalbuminuria (defined as the presence of 30–300 mg/day albumin in the urine) (3, 82)]. Although characteristic of only a few experimental models, around 70% of human diabetic nephropathy is associated with elevated mean arterial pressure (MAP), which, along with poor glycemic control, further exacerbates the disease progression to proteinuria, nodular glomerulosclerosis, tubulointerstitial fibrosis, and a decline in GFR, eventually leading to ESRD (82). Unfortunately, experimental models rarely develop ESRD due to diabetes, making them mainly appropriate for the study of early diabetic nephropathy. Although the exact mechanisms that lead to the development of diabetic nephropathy are still under investigation, it is commonly thought to result from the cumulative interaction among multiple metabolic and hemodynamic factors which activate common intracellular signaling pathways, which in turn trigger the production of a number of locally active growth factors and peptides, leading to renal disease (9).
The conventional strategies for the treatment of diabetic nephropathy include glycemic control, blood pressure lowering, and blockade of the renin-angiotensin system as a means of blood pressure control, but also direct preservation of renal function. However, despite overall improvements in treatment, diabetic nephropathy remains associated with high morbidity and mortality, indicating the need for the development of novel treatments, and in recent years, C-peptide has started to emerge as a potential novel therapeutic treatment for type 1 diabetes-associated end-organ complications, including diabetic nephropathy (43, 47, 74, 88, 99). This part of the review will focus on describing the mechanisms by which C-peptide is thought to protect the diabetic kidney.
C-peptide prevents the development of diabetic kidney disease.
Several studies have shown that acute and long-term infusions of C-peptide reduce hallmarks of early diabetic nephropathy, namely glomerular hyperfiltration, glomerular hypertrophy, and microalbuminuria. For example, in STZ-induced diabetic rats maintained diabetic for 2 wk with daily insulin injections, treatment with C-peptide as a bolus intravenous injection of 6 nmol/kg followed by a continuous infusion at a dose of 30 nmol·kg−1·h−1 for 1 h prevents glomerular hyperfiltration (43). Infusion of C-peptide over 2 wk in STZ-induced diabetic rats in the absence of insulin also prevents glomerular hyperfiltration as well as glomerular hypertrophy and microlabuminuria (87). Similar beneficial effects following treatment with several different doses of either full-length C-peptide or with its active COOH-terminal fragment EVARQ were observed in other studies using STZ-induced diabetic rats (43, 74, 88, 99). Effects of C-peptide have also been studied in humans during early stages of diabetic nephropathy. A randomized study was performed in 21 normotensive patients with microalbuminuria that received, along with their regular insulin regimen, either subcutaneous injections of C-peptide (600 nmol/day) or placebo over 3 mo. Patients receiving C-peptide showed a decline in urine albumin excretion compared with baseline in a time-dependent manner, but no effects on GFR were observed (47). The lack of an effect on GFR could probably be explained by the fact that, unlike the STZ-induced diabetic rat, which develops glomerular hyperfiltration within days of induction of diabetes, patients included in this particular study had normal GFR at baseline. Thus, these data suggest that C-peptide has no effect on GFR when GFR is normal. Cumulatively, experimental and clinical observations clearly demonstrate a beneficial effect of C-peptide in early prevention of diabetic nephropathy; however, many questions remain unanswered. 1) In addition to preventing, can C-peptide also reverse or slow the progression of functional and structural changes associated with more advanced stages of renal disease due to diabetes? 2) Since the vast majority of studies examining the longer-term (>2 wk) effects of C-peptide to date have been performed in the presence of insulin, it is unclear whether the beneficial effects of C-peptide may be dependent on the presence of insulin.
Does C-peptide attenuate or reverse the progression of diabetic kidney disease?
To test the hypothesis that C-peptide attenuates, rather than just prevents, the progression of diabetic renal disease, a study was performed in male STZ-induced diabetic rats whose diabetes was left untreated for 8 wk. During that time, all animals developed microalbuminuria and glomerular hyperfiltration. Following the initial 8 wk, the animals were randomized to receive either vehicle (0.9% saline) or C-peptide (50 pmol·kg−1·min−1) for 4 wk in the presence of low doses (2–4 U every 3 days) of insulin, mimicking moderately controlled type 1 diabetes. Similarly to previous studies looking at the ability of C-peptide to prevent renal functional changes, this study found that treatment with C-peptide in the face of already elevated GFR was able to attenuate further progression of glomerular hyperfiltration (24). Although the study did not specifically examine the mechanisms behind this observation, it was suggested, based on previous reports in STZ-induced diabetic rats and alloxan-induced type 1 diabetic mice, that the mechanism by which C-peptide normalizes GFR is via reducing afferent arteriolar diameter and inhibiting tubular sodium reabsorption (72, 73).
In addition to developing glomerular hyperfiltration, after 8 wk of untreated diabetes STZ-induced diabetic rats also exhibit increases in urine albumin excretion, i.e., microalbuminuria. Treatment with C-peptide after 8 wk of untreated diabetes attenuated microalbuminuria. While it is conceivable that C-peptide reduces albuminuria simply via reducing GFR, evidence suggests that C-peptide directly decreases glomerular permeability. A study performed in an experimental model of chronic renal disease, namely the Dahl salt-sensitive (DSS) rat fed a 2% NaCl diet for 4 wk, showed that treatment with C-peptide was associated with a higher reflection coefficient of albumin, indicating a less permeable glomerular filtration barrier (90). Furthermore, this study showed that glomeruli from animals treated with C-peptide had a higher abundance of podocin (an integral component of the slit diaphragm) than vehicle-treated animals, suggesting that one of the mechanisms by which C-peptide may also attenuate albuminuria is by protecting the integrity of the slit diaphragm (90).
Other than attenuating glomerular hyperfiltration and microalbuminuria, treatment with C-peptide after 8 wk of untreated diabetes also attenuated glomerular hypertrophy, renal cortical inflammation, and apoptosis and preserved the renal microvascular architecture (24). Others have shown additional mechanisms by which C-peptide exerts its renoprotective effects in type 1 diabetes, including increasing renal Na+,K+-ATPase activity (72) and eNOS expression and NO production (112), inhibiting tumor necrosis factor (TNF)-α-induced apoptosis (2), and attenuating transforming growth factor (TGF)-β-induced epithelial-to-mesenchymal transition (38). These studies clearly show that C-peptide is biologically active and exerts its beneficial effects in the diabetic kidney through several different mechanisms.
Are the effects of C-peptide independent of insulin and glucose lowering?
Given that many of the studies examining the effects of C-peptide have been performed in experimental models or humans with type 1 (i.e., insulin-dependent) diabetes, it is not surprising that they needed to be conducted in the presence of insulin. However, this brings up the question whether the observed beneficial effects of C-peptide may in fact be insulin dependent. To answer this question, we performed a study in STZ-induced diabetic rats in which either vehicle or C-peptide was administered for 2 wk following 4 wk of untreated diabetes in the absence of insulin. Similarly to the effects in diabetic rats receiving low doses of insulin, treatment with C-peptide in diabetic rats not receiving insulin at all also attenuated glomerular hyperfiltration and microalbuminuria (24), suggesting that C-peptide, when administered over 2 wk, is renoprotective independent of insulin. Other studies looking at the acute effects of C-peptide on the kidney also reported beneficial effects in the absence of insulin (71–73, 87, 98). A study looking at chronic effects of C-peptide in STZ-induced diabetic kidney showed that, regardless of whether it was administered in the presence or absence of insulin, C-peptide reduced blood glucose and HbA1c levels (24). However, acute infusion of C-peptide in nondiabetic rats does not reduce blood glucose (unpublished observations), suggesting that C-peptide may only lower blood glucose over a longer period of time. Although these findings are consistent with one previous study showing augmented whole body glucose disposal rate following treatment with C-peptide (59), most studies either do not report the effects on blood glucose or show no effect (81, 86–88). Indeed, clinical trials indicate that treatment with C-peptide for 3 or 6 mo does not alter HbA1C levels (19, 20, 47). Possible explanations for these disparate observations include differences in the length of the treatment (acute vs. chronic), basal blood glucose levels (administration of C-peptide at the time of initiation of diabetes vs. following a period of uncontrolled diabetes), and strain of animals used. If indeed C-peptide has the ability to regulate glucose levels, it would indicate that it has insulin-like properties and may potentially be effective in the treatment of chronic hyperglycemia. Alternatively, it has been proposed that C-peptide disaggregates the insulin hexamers in insulin solutions and increases the availability of the bioactive insulin monomer (92). However, further studies are warranted to examine the precise mechanisms and conditions under which C-peptide may lower blood glucose levels. It should also be noted that although our studies examined the renoprotective effects of C-peptide in the presence of low doses of insulin (mimicking moderately controlled diabetes), we have not examined the effects of chronic administration of C-peptide in the face of high doses of insulin and, thus, controlled diabetes. However, our studies performed in nondiabetic models of chronic kidney disease (described below) suggest that C-peptide is renoprotective even under conditions of physiological levels of insulin.
To date, studies examining the renoprotective effects of C-peptide have focused solely on experimental models or humans with type 1 diabetes, but its role in type 2 diabetes or nondiabetic renal disease remains largely unexplored. A study in patients with type 2 diabetes reported that higher baseline levels of C-peptide are associated with a reduced risk of incident microvascular complications (8), indicating that treatment with C-peptide in these patients may potentially afford renoprotection. In light of the observations and from our own studies demonstrating that C-peptide exerts renoprotective effects independently of blood glucose lowering, we asked whether C-peptide has equal beneficial effects in experimental models of nondiabetic (i.e., normoglycemic) renal disease. For this purpose, we used the DSS rat, which when fed a 2% NaCl diet over 4 wk develops chronic, nondiabetic renal disease. These studies showed that treatment with C-peptide reduces albuminuria, glomerular permeability, and renal inflammation without affecting blood glucose levels or GFR (90). These observations further support the notion that C-peptide has no effect on GFR if GFR is normal, that C-peptide is renoprotective independent of blood glucose regulation, that C-peptide is renoprotective in the presence of physiological levels of insulin, and, most importantly, that C-peptide may have potential benefit in the treatment of both diabetic and non-diabetic renal disease.
Renoprotective effects of C-peptide: conclusions.
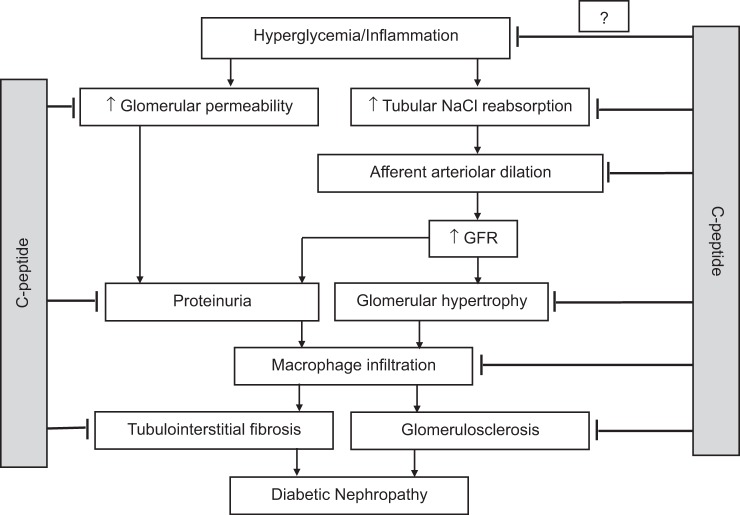
Accumulating evidence suggests that C-peptide is renoprotective in type 1 diabetes via preventing and attenuating the progression of glomerular hyperfiltration, hypertrophy, and permeability, reducing afferent arteriolar diameter, microalbuminuria and renal inflammation, and inhibiting tubular sodium reabsorption etc (Fig. 2). Studies from our laboratory have shown that C-peptide may also have blood glucose-lowering effects (24); however, its renoprotective effects appear to be independent of glucose regulation. Finally, whereas most studies to date have focused on C-peptide as a therapeutic treatment for diabetic nephropathy associated with type 1 diabetes, emerging evidence suggests that its beneficial effects may extend to renal disease in type 2 diabetes as well as chronic, nondiabetic renal disease.
Fig. 2.
Simplified model of the pathogenesis of diabetic nephropathy. C-peptide is renoprotective in type 1 diabetes via inhibiting tubular sodium reabsorption, reducing afferent arteriolar diameter and glomerular permeability, preventing and attenuating the progression of glomerular hyperfiltration, hypertrophy and microalbuminuria, renal inflammation, glomerulosclerosis, and tubulointerstitial fibrosis. GFR, glomerular filtration rate. Studies from our laboratory have also shown that C-peptide may also have blood glucose-lowering effects; however, it appears that C-peptide exerts its renoprotective effects independently of blood glucose regulation.
Anti Inflammatory Effects of C-Peptide
C-peptide acts as an endogenous antioxidant in cells exposed to stressful conditions.
Increasing evidence suggests that a major biological activity of C-peptide in the periphery is its anti-inflammatory and antiapoptotic effects on vascular endothelial cells of different tissues as well as in neurons and kidney tubular cells affected by the deleterious effects of high glucose or TNF-α (2, 5, 13, 61, 90, 95). Results from small clinical trials in which C-peptide was administered, together with insulin, to type 1 diabetic subjects suffering from nephropathy and neuropathy demonstrated C-peptide efficacy in amelioration of these complications, suggesting that maintenance of physiological levels of C-peptide prevents or reverses the development of diabetic complications (67, 110). In support of the idea of a protective role of C-peptide is a study in which the association between fasting plasma C-peptide values and microvascular complications was evaluated retrospectively in a large clinic-based cohort of type 1 diabetic patients. The study shows that patients who have a fasting C-peptide level of >0.06 nM have a lower risk for microvascular complications independent of glycemia, disease duration, hypertension, and other risk factors for cardiovascular disease (76). Thus, preserving a small residual β-cell function, and therefore a residual C-peptide secretion, protects against the development of diabetic microvascular complications.
Diabetes is characterized by the presence of a generalized inflammatory response with activation of circulating monocytes, oxidative stress, and plasma elevation of several inflammatory cytokines. The vascular endothelium represents a likely target of this inflammatory response, which induces activation of endothelial cells, alters endothelial function, and promotes monocyte adherence eventually leading to apparent vascular damage in the later stages of diabetes. The mechanism(s) by which C-peptide employs its cytoprotective effects on the endothelium is not entirely understood, although it has been reported that C-peptide can alter activation of different signaling pathways that subsequently modulate or shut down inflammatory responses. Increasing evidence demonstrates that exposure to physiological concentrations of C-peptide decreases high-glucose-induced expression of several key adhesion molecules, including intercellular adhesion molecule (ICAM)-1, vascular cellular adhesion molecule (VCAM)-1, and P-selectin, that allow attachment of leukocytes to endothelial cells. Firm adhesion and rolling of leukocytes to the endothelial wall require the presence of the chemokines interleukin (IL)-8 and monocyte chemoattractant protein (MCP)-1, whose secretion is also reduced by C-peptide application in high-glucose-exposed endothelial cells (64, 91, 115). Thus, C-peptide modulates recruitment of leukocytes to the vascular wall, one of the first events in atherosclerosis plaque formation, and prevents endothelial dysfunction (64, 91, 115). In addition to decreasing leukocyte-endothelial cell interaction, recent data suggest that C-peptide may also play a role in endothelial cell proliferation. The autologous saphenous vein is routinely used for revascularization procedures undertaken secondary to coronary artery disease; however, post-bypass graft occlusions are common because of intimal hyperplasia. An investigation into insulin's and C-peptide's roles in modulation of human saphenous vein neointima formation revealed that C-peptide increased endothelial cell number by about 40%, thus indicating that C-peptide may promote re-endothelialization and thus limiting neointimal development (70). C-peptide has also been shown to reduce systemic inflammatory responses that are deleterious to the endothelium by lowering circulating levels of the proinflammatory cytokines IL-1, IL-6, macrophage inflammatory protein (MIP)-1α, and TNF-α, effects that most likely occur through inhibition of activated monocytes (11, 34, 108). Vascular smooth muscle cells are yet another cell type that plays a critical role in vascular disease and atherosclerotic plaque formation. In high-glucose circumstances, vascular smooth muscle cells proliferate and migrate from the media to the subendothelial space, thus contributing to early atherosclerotic lesions. While several studies show that physiological levels of C-peptide inhibit high-glucose-induced proliferation of vascular smooth muscle cells (14, 55, 70), one other report reveals increased cellular proliferation upon stimulation with C-peptide, thus suggesting a proatherogenic effect (111). Although the data are conflicting, these findings underscore a role of C-peptide in vascular smooth muscle cell function. Source of smooth muscle cells, C-peptide concentration, and glucose concentration all need to be considered, and further studies need to be pursued to delineate the exact role of C-peptide in vascular smooth muscle cell proliferation.
It has now become increasingly evident that several mediators of the apoptotic process, including members of the caspase family, are affected by C-peptide treatment. C-peptide reduces levels and activity of activated caspase-3 while increasing levels of the antiapoptotic molecule Bcl-2 in high-glucose-exposed endothelial cells (13). Similar antiapoptotic activities of C-peptide are reported in human neuroblastoma SH-SY5Y cells in the hippocampus of type 1 diabetes rats and in TNF-α-exposed kidney proximal tubular cells (2, 61, 95). Other mechanisms by which C-peptide protects endothelial cells from high-glucose-induced apoptosis involve inhibition of the proapoptotic enzyme transglutaminase-2 (6) and activation of the major energy-sensing enzyme adenosine monophosphate-activated protein kinase (AMPK)α (6), restoring mitochondrial function and physiological reactive oxygen species (ROS) production (16).
A focal point potentially unifying these C-peptide bioactivities and suggesting an underlying mechanism is C-peptide's ability to reduce excessive intracellular ROS production (5, 13, 107) and many of its downstream effects, including activation of the nuclear factor (NF)-κB pathway, a major signaling pathway mediating inflammatory responses in many different cell types (64, 96). Physiological levels of ROS play important signaling roles and are necessary for the maintenance of cellular functions (16). However, excessive and prolonged intracellular ROS accumulation is a deleterious event leading to cellular injury. A major site of ROS production in glucose-exposed endothelial cells is activation of plasma membrane NAD(P)H oxidase. NAD(P)H oxidase includes membrane-bound elements (p22phox and gp91phox), and the cytosolic adapter proteins p47phox and p67phox, which are recruited to the membrane during stimulation to help form a catalytically active oxidase. Recruitment of p47phox and p67phox to the plasma membrane requires the presence of RAC-1, a small GTP-binding protein, which assembles with the cytosolic proteins to regulate NAD(P)H oxidase activity (30, 41). There is evidence that C-peptide inhibits high-glucose-induced NAD(P)H oxidase-dependent ROS generation in endothelial cells by preventing translocation of RAC-1 from the cytoplasm to the membrane (13), an effect also reported in the aorta from STZ-induced diabetic mice exposed to C-peptide (5). An inhibitory effect of C-peptide on mitochondrial ROS generation has also been reported in glucose-exposed murine endothelial cells (6, 107). By reducing excessive ROS accumulation in endothelial cells exposed to high glucose or other stressful agents, C-peptide inhibits the generation of a cascade of deleterious inflammatory responses that eventually results in cellular death. The specific intracellular pathways by which C-peptide achieves its anti-inflammatory effects in target cells after binding to cellular membranes and localization to early endosomes are largely unknown (66). As discussed below, evidence for a putative G protein-coupled receptor (GPCR) for C-peptide, GPR146, has been recently reported for different cell types (113). The identification of GPR146 as a potential C-peptide receptor provides a platform to elucidate C-peptide anti-inflammatory signaling pathway components.
C-peptide leveraging of antioxidant protection through a β-cell autocrine mechanism.
A critically important question is whether C-peptide displays any biological activity on the pancreatic β-cells that secrete it, in an autocrine fashion. Since C-peptide has been shown to reduce oxidative stress in different cell types, one possibility is that C-peptide is an autocrine hormone that protects against β-cell apoptosis by lowering β-cell ROS levels otherwise elevated by conditions linked to diabetes.
Oxidative stress results from a persistent imbalance between an excessive production of ROS and a limited capacity to detoxify these reactive intermediates. While a transient increase in ROS is thought to accompany glucose-stimulated insulin secretion (GSIS) (7, 26, 77), abnormally prolonged generation of ROS causes oxidative stress and leads to impairment of β-cell secretory function and apoptosis (27, 60). The primary cellular enzymatic antioxidant defenses are conducted by superoxide dismutase (SOD), which catalyzes the conversion of superoxide radicals into hydrogen peroxide (H2O2), and by catalase and glutathione peroxidase (Gpx), both of which eliminate H2O2 (58). In pancreatic β-cells, expression of these protective enzymes is low (21, 105) but can be induced under conditions of chronic hyperglycemia (57) and by exposure to insulinotropic agents in wild-type murine islets and cell lines (29, 54). The importance of antioxidant enzymes in the protection against the toxicity of excessive ROS production is confirmed by studies in which their overexpression in β-cells or exposure to antioxidant enzyme mimetics protect against ROS toxicity, improve GSIS, and prevent NF-κB activation (12, 57, 71, 89, 104, 106). Interestingly, perturbed NF-κB activity in β-cells impairs GSIS (75), suggesting that too much, as well as too little, of NF-κB activation at the wrong time might be deleterious.
Oxidative stress is a major factor causing β-cell death in both type 1 and type 2 diabetes (21, 85). In type 1 diabetes, exposure to inflammatory cytokines secreted by infiltrating immune cells in the pancreatic islets during autoimmune responses generates an excess of intracellular ROS, which leads to β-cell stress and apoptosis (18, 52, 69). In type 2 diabetes, several factors, such as hyperglycemia, elevated circulating cytokines, and free fatty acid, trigger intracellular ROS accumulation leading to loss of functional β-cell mass and apoptosis in the late stage of the disease (85). Since β-cells are among the most ROS-sensitive cells in the body (58, 105), a critically important hypothesis is that C-peptide represents a native antioxidant whose anti-inflammatory activity extends to β-cells when exposed to stressful conditions. Like insulin, C-peptide is secreted dynamically in response to ever-changing glycemia governed by meals and by liver glucose mobilization via the glucagon-insulin axis during fasting. However, C-peptide does not undergo hepatic retention and circulates at a concentration approximately tenfold higher than that of insulin (79). In the portal circulation, for example, fasting C-peptide concentration was measured to be 1.4 nM, whereas glucose-stimulated C-peptide rose to 6.2 nM (42). The same study sampled the systemic circulation, where fasting C-peptide concentration was 1 nM and rose to 2.5 nM after glucose (42). C-peptide concentrations at the β-cells of the islet of Langherans are unknown but are likely to be equal to or higher than the portal concentrations because the interstitial volume at the site of C-peptide release is very small. Because C-peptide naturally confers antioxidant protection, loss of C-peptide's pulsatile secretion or impairment of its postprandial secretion consequent to β-cell stress could lead to cycles of loss in β-cell functional mass, contributing to the progression to diabetes. Evidence for impairment in GSIS, and therefore in C-peptide secretion, has been reported in the early stages of type 1 diabetes before β-cell destruction has occurred (102). A similar situation occurs in type 2 diabetes, with a loss of pulsatile insulin and, therefore, C-peptide secretion, identified as a possible factor in the pathogenesis of the disease (109).
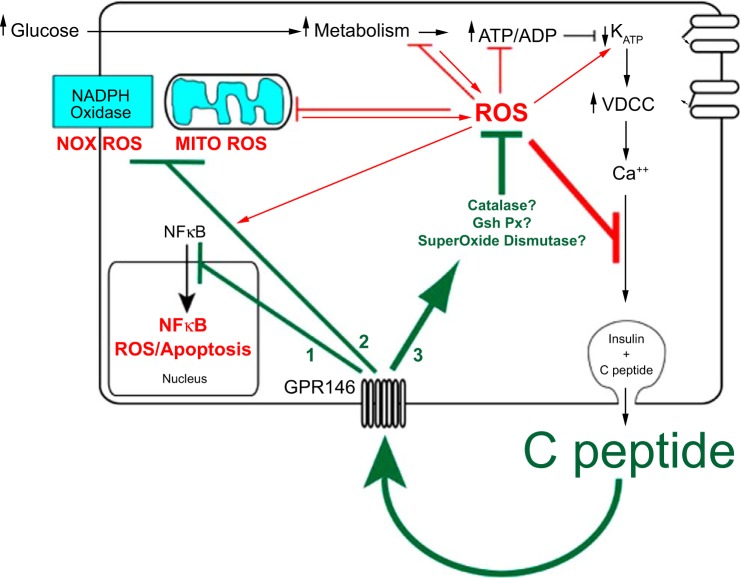
The question of whether C-peptide displays autocrine effects on stressed β-cells is still open, as only a few studies have tested for cytoprotective effects of C-peptide on islets or β-cells. Bugliani et al. (10) showed using human islets in normal conditions that overnight exposure to 14 nM C-peptide reduced apoptosis by increasing levels of the antiapoptotic protein Bcl-2. Whether the attenuation of apoptosis was specific to, or included, β-cells is unknown. Also, it is unknown whether C-peptide's protective effects extend to abnormal conditions of β-cells, such as when β-cells are exposed to high glucose and/or to inflammatory cytokines associated with diabetes. To start unraveling the question of a possible role of C-peptide in decreasing cellular stress in compromised β-cells, INS-1 β-cells were exposed to either 5.5 or 22 mM glucose for 3 h, the latter in the presence or absence of C-peptide. It was observed that C-peptide reduced high-glucose-induced ROS to baseline levels measured in low glucose. The beneficial effect of C-peptide extended to INS-1 β-cells exposed to the model ROS compound H2O2. A key observation was that INS-1 β-cells undergoing secretory blockade in the presence of the KATP channel opener diazoxide exhibited increased ROS when challenged with H2O2, and this increase was reversed by application of exogenous C-peptide. Importantly, the antidiabetic sulfonylurea glibenclamide, which induces endogenous C-peptide secretion, also reversed the diazoxide increase in ROS. Similarly, extracellular potassium, which depolarizes the cells otherwise hyperpolarized by diazoxide and leads to secretion of C-peptide, caused a concentration-dependent reduction in H2O2-induced ROS to levels measured with diazoxide absent (65). These results extend previous results (10) by specifying a significant bioactivity of C-peptide directly on the β-cells and supports an autocrine mechanism in which C-peptide acts on INS-1 β-cells by lowering intracellular ROS however generated. The underlying cellular mechanisms of C-peptide's anti-inflammatory activity in β-cells are currently under investigation. We propose a model in which C-peptide acts by potentiating cellular antioxidant defenses of the β-cells by increasing expression and activity levels of several antioxidant enzymes (Fig. 3). C-peptide might also act preemptively by decreasing intracellular ROS generation by reducing or inhibiting activity of the plasma membrane NAD(P)H oxidase enzyme or by improving mitochondrial respiration or by lowering both ROS sources in the β-cell. Regardless of the specific mechanism, the outcome of C-peptide's bioactivity on β-cells is to reduce oxidative stress and the downstream deleterious chain of effects, thus improving β-cell cellular survival and function (Fig. 3). The implications of the C-peptide autocrine model implies that C-peptide can protect the same cells that secrete it and insulin, which might be the centerpiece of why type 1 diabetes patients with low but residual C-peptide have better outcomes with diabetes complications. Thus, the downspiral of oxidative stress in these type 1 diabetic patients comes down to an equilibrium in which the remaining β-cells can make enough C-peptide to protect themselves from complete destruction and thereby provide residual C-peptide to protect other cells of the body. Whereas it remains to be fully studied, higher residual insulin secretion is also likely to be beneficial in diabetic states. Our model predicts that with higher residual C-peptide secretion there necessarily will be higher residual insulin secretion, the latter having the beneficial tendency of normalizing hyperglycemia, a key component of β-cell preservation by the autocrine effect of residual C-peptide levels.
Fig. 3.
Autocrine C-peptide model underlying β-cell adaptation to oxidative stress. The centerpiece of functional β-cell mass is insulin secretion, which is cosecreted in equimolar amounts with C-peptide. Autocrine action of C-peptide, which protects functional β-cell mass, should therefore provide a buffer of protection for its own secretion as well as that of insulin. In addition to binding to its receptor GPR146 for such autocrine actions, C-peptide is predicted to regulate at least 3 distinct pathways in the β-cell: 1) deactivation of the NF-κB pathway, which protects against apoptosis; 2) inhibition of pathways that generate ROS including the plasmalemma NADPH oxidase and the mitochondrial electron transport chain; 3) activation of pathways that catalyze the degradation of ROS by activating antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase. In the absence of these autocrine actions of C-peptide, more prolonged and higher accumulation of ROS and apoptosis would accelerate loss of β-cell functional mass. This suggests a tipping point in loss of functional β-cell mass where the loss in C-peptide secretion and its autocrine protection results in a downward spiral of both secretion and protection, ultimately leading to few if any β-cells.
Searching for a C-Peptide Receptor
Evidence for a C-peptide receptor.
Since C-peptide was thought for many years to be a biological byproduct of insulin biosynthesis, the idea that C-peptide had a specific membrane receptor was not seriously considered. However, once C-peptide was hypothesized to be a biologically active molecule, the search for the receptor(s) of C-peptide progressed in earnest, as it is difficult to ascertain physiological relevance without knowledge of the receptor. The first essential evidence for a membrane-bound receptor was the demonstration that C-peptide could bind to human cell membranes, including skin fibroblasts, endothelial cells, and kidney tubule cells (36, 80, 84). C-peptide binding was not displaced by insulin, IGF-I, or IGF-II, indicating that C-peptide likely does not directly or physically interact with the insulin receptor (84). However, binding of full-length C-peptide was displaced by excess amount of the C-peptide C-terminal pentapeptide (EGSLQ), indicating that the COOH-terminal portion of the peptide may exert most of C-peptide's biological activity (80). Interestingly, the binding dynamics of C-peptide indicated that the peptide may bind to a complex of proteins on the cell surface rather than to a single receptor (80, 84).
The second series of experiments indicating that C-peptide signaled through a specific receptor or receptors was the finding that C-peptide initiated multiple specific intracellular signaling cascades (39) (Fig. 3). In erythrocytes, C-peptide has been shown to activate the Na+,K+-ATPase (25), resulting in enhanced deformability and perhaps functionality of erythrocytes (32, 56), such as by enhancing release of ATP from these cells (83). Interestingly, the beneficial effects of C-peptide on erythrocytes appear to require coexposure of C-peptide and insulin (83), although the signaling mechanisms underlying this interaction have not yet been elucidated. In kidney, C-peptide initiates multiple signaling cascades, including activation of phosphatidylinositol 3-kinase (PI3K), leading to enhanced NF-κB activity and protection against TNF-α-induced apoptosis (2). In addition, C-peptide has been shown to stimulate extracellular signal-related kinase (ERK) activity, intracellular calcium accumulation, and protein kinase C (PKC) activity (1, 118, 119). C-peptide has been shown to be internalized by human endothelial cells (66) and to reduce endothelial dysfunction via the NF-κB pathway (64). Additionally, C-peptide-induced p38 MAPK activation was shown to stimulate the production of c-Jun NH2-terminal kinase (JNK) in lung endothelial cells (28). Recent studies suggest that C-peptide protects against high-glucose-induced ROS formation through the activation of AMPK in endothelial cells (6). C-peptide also has been shown to modulate insulin receptor substrate (IRS)-1 phosphorylation (31), which could have important implications for insulin signaling and provides further evidence for a functional interaction between C-peptide- and insulin-initiated signaling cascades. These experiments showed not only that C-peptide was capable of exerting specific cellular activities, but also that C-peptide likely was interacting with a GPCR, since most of the intracellular signaling cascades observed following exposure to C-peptide are usually associated with the activation of a GPCR.
Importantly, several groups have shown that the cellular actions of C-peptide are pertussis toxin sensitive, indicating that C-peptide interacts with a GPCR coupled to Gαi or Gαo (1, 2). Furthermore, specific binding of C-peptide to human cell membranes also was disrupted by treatment with pertussis toxin (84). These critical experiments provided direction to the search for the C-peptide receptor(s) toward the identification of a GPCR or family of GPCRs responsible for C-peptide-induced signaling.
Strategy for identification of the C-peptide receptor.
Because C-peptide initiates multiple signaling cascades that are typically associated with the activation of a GPCR, and because many of the cellular actions of C-peptide are pertussis toxin sensitive, we hypothesized that the C-peptide receptor was a GPCR and, in particular, an orphan GPCR. To identify candidate C-peptide receptors, we used the Deductive Ligand-Receptor Matching Strategy developed by our laboratory to identify the receptor for the neuropeptide neuronostatin (114). We first screened three cell lines found to respond to C-peptide, including the human erythroleukemia cell line TF-1, the human embryonic kidney cell line HEK 293, and the human gastric tumor cell line KATOIII, for the expression of all known orphan GPCRs, as catalogued by IUPHAR (93). We found that 24 of the ∼136 orphan GPCRs were expressed by TF-1, HEK 293, and KATOIII cells. To narrow this pool of candidates, we utilized bioinformatic databases to evaluate the sequence homology, proposed function, and known distribution of the 24 receptor candidates. By a process of elimination, we formulated a short list of nine top candidates. We then proceeded to knock down individual receptors using siRNA in KATOIII cells and assessed continued responsiveness to C-peptide. KATOIII cells were used as a cell model because this cell line is rapidly growing, is amenable to gene silencing technologies, and consistently responds with a significant increase in c-Fos expression when exposed to C-peptide. If knockdown of a receptor in KATOIII cells resulted in an inability of C-peptide to elicit an increase in c-Fos mRNA production, then that receptor was considered a good candidate for the C-peptide receptor.
Using this strategy, we identified an orphan GPCR, GPR146, which is essential for C-peptide signaling in KATOIII cells (113). We therefore focused on the role of GPR146 in C-peptide signaling and found that stimulation of KATOIII cells with C-peptide led to internalization of GPR146. Furthermore, we found that KATOIII cells exposed to C-peptide exhibited punctate colocalization between C-peptide and GPR146, and the number of colocalized objects increased as a function of time. These data suggest that GPR146 is a good candidate for the C-peptide receptor. However, further experimentation is required to confirm the physical interaction between C-peptide and GPR146. Confirmatory experiments must include binding studies, coimmunoprecipitation, and pull-down assays combined with protein sequencing and mass spectrometry analysis. These confirmatory experiments are especially important given that several lines of evidence suggest that C-peptide signals through a receptor complex, perhaps including a receptor-activated modulating protein (RAMP) (51) or an integrin (94), rather than a single receptor.
Evidence against a C-peptide receptor.
Despite the evidence that C-peptide interacts with a specific cell surface receptor, and in particular with a GPCR, a few studies suggest that C-peptide exerts its actions through alternative mechanisms. For example, C-peptide was shown to interact physically and functionally with α-enolase, a glycolytic enzyme that can localize to the cell membrane (45). Additionally, C-peptide was shown to internalize and localize to the cytosol of HEK 293 and Swiss 3T3 cells (63) and to interact with the intracellular protein, protein tyrosine phosphatase 1B (PTP1B) (46). C-peptide likewise has been visualized in the nucleus of mesangial cells (62), suggesting that C-peptide may exert some of its actions via an intracellular mechanism. In addition, one group speculated that C-peptide could act via direct nonchiral interaction with the cell membrane, in similarity with amphiopathic bacterial peptides (44). Further studies are necessary to fully elucidate the cellular site(s) of C-peptide's actions.
C-Peptide Signaling and GPR146: Implications for the Physiological Relevance of C-Peptide
C-peptide has been shown to initiate multiple signaling cascades and appears to interact with GPR146 to exert at least some of those cellular actions. But the question remains as to what can be gleaned from these data in terms of the physiological relevance of C-peptide. Although C-peptide-initiated signaling cascades are varied, knowledge of these cellular activities can be used to develop hypotheses concerning C-peptide's role in normal physiology. For example, as noted above, C-peptide has been shown to modulate IRS-1 phosphorylation (31, 110) as well as Akt activation (110), both of which are associated with insulin receptor signaling. Given that several lines of evidence suggest that C-peptide and insulin interact to influence the biology of multiple tissue systems (83, 110), it could be hypothesized that one of C-peptide's roles is to act as a counterbalance or dampener of insulin signaling or, alternatively, as an enhancer of insulin's actions, depending on the ratio of the two peptides, as has been speculated previously (31). Future studies investigating the molecular mechanisms underlying the functional interaction between insulin and C-peptide could reveal important insights into glucose homeostasis and the pathogenesis of diabetes and its complications.
C-peptide and the Pathogenesis of Microvascular Complications of Diabetes
Brownlee (9) recently presented a “unifying hypothesis” that seeks to explain the biochemical mechanisms behind the development of microvascular complications of diabetes. The hypothesis, now widely accepted, focuses on the fact that the metabolic abnormalities of diabetes, primarily the elevated blood glucose levels and the lack of insulin, cause formation of ROS in vascular endothelial cells, generating oxidative stress. The increased ROS formation leads to a series of downstream metabolic disturbances such as stimulation of glucose metabolism along the polyol pathway, augmented intracellular formation of advanced glycation end products and their receptors, activation of PKC isoforms, and increased activity of the hexosamine pathway. These metabolic changes, in turn, give rise to suppression of endothelial eNOS and Na+,K+-ATPase activities of endothelial cells and several tissues as well as upregulation of proinflammatory genes via NF-κB-mediated mechanisms, resulting in augmented formation of cytokines, chemokines, cell adhesion molecules, VEGF, and TGF-β. This panoply of detrimental events results in the gradual development of microvascular circulatory abnormalities, impairment of endothelial function, and endothelial cell apoptosis, cornerstones of what is clinically known as microcirculatory complications.
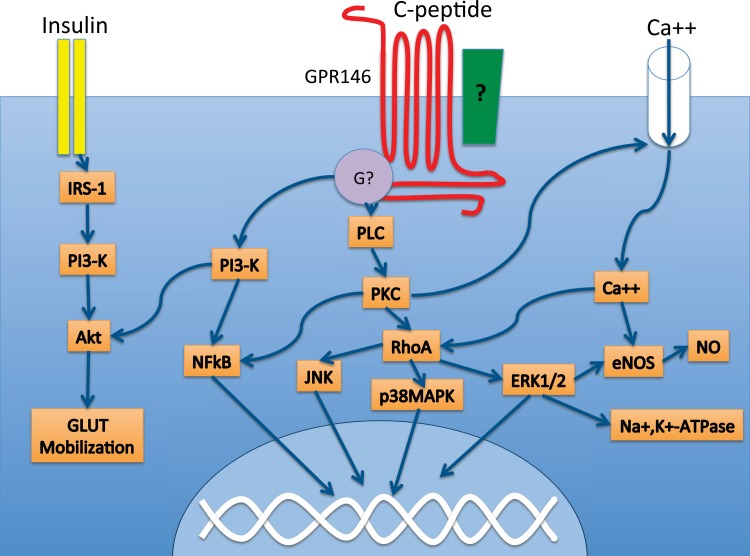
From what has been described above, it is apparent that C-peptide has the capacity to influence the development of diabetic complications via the following mechanisms. A direct inhibitory effect of C-peptide on endothelial cell ROS formation is now documented from several laboratories (5, 13, 107). Stimulation and increased expression of both eNOS and Na+,K+-ATPase activities in several tissues is also well documented (25, 44, 72, 97, 112), and an inhibitory effect on NF-κB results in diminished diabetes-mediated expression of cytokines, chemokines, and cell adhesion molecules (12, 57, 71, 89, 104, 106). Finally, C-peptide activates antiapoptotic mechanisms by inhibiting TG-2 and caspase-3 (13). Overall, C-peptide may protect against the deleterious effects of hyperglycemia by improving microcirculation, alleviating endothelial dysfunction, and inhibiting endothelial apoptosis; see Fig. 4 for an overview.
Fig. 4.
C-peptide-initiated signaling cascades. C-peptide is associated with the regulation of several signaling cascades, including phospholipase C (PLC) and the NF-κB pathway. These intracellular signaling events are likely mediated by a G protein-coupled receptor such as GPR146, which has been shown to be necessary for C-peptide signaling in KATOIII cells. GPR146 interacts with an as yet unknown G protein, which could be either Gαi or Gαo, since several of the cellular actions of C-peptide were shown to be pertussis toxin sensitive. GPR146 may interact physically with additional proteins on the cell membrane, such as an integrin (green box). C-peptide and insulin appear to functionally interact, particularly at the level of Akt. Akt, protein kinase B; Ca++, calcium ion; eNOS, endothelial NO synthase; ERK1/2, extracellular signal-regulated kinase; G?, G protein; GLUT, glucose transporter; IRS-1, insulin receptor substrate 1; JNK, c-Jun NH2-terminal kinase; Na+,K+-ATPase, sodium/potassium ATPase; NO, nitric oxide; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; PKC, protein kinase C; PLC, phospholipase C; PI3-K, phosphotidylinositol 3-kinase; p38 MAPK, mitogen-activated protein kinase; RhoA, Ras homolog gene family, member A.
It can also be considered that a normal physiological role of C-peptide may be to prevent or diminish the formation of ROS and other oxidant species that accompany the modest elevations of blood glucose levels that results from carbohydrate meals. If so, it becomes apparent that patients with diabetes, especially type 1, are particularly vulnerable. They both lack C-peptide and are exposed to excessive levels of glucose.
In summary, it is apparent that C-peptide, in combination with regular insulin therapy, may serve as an endogenous antioxidant and retard or prevent the generation of hyperglycemia-mediated oxidative stress. In addition, the peptide is capable of directly retarding the deleterious effects of several of the downstream metabolic abnormalities resulting from hyperglycemia, thereby further emphasizing the potential therapeutic benefits from C-peptide therapy.
Unanswered Questions and Future Directions
There is now much evidence indicating that C-peptide is a biologically active molecule that initiates intracellular signaling cascades through interaction with a specific membrane-bound receptor or receptor complex and that C-peptide signaling results in protection against hyperglycemia-associated microvascular dysfunction. However, several unanswered questions remain. First, full identification of the C-peptide signaling mechanism and the receptor or receptor complex needs to be identified in order to enable the positioning of C-peptide as an endogenous peptide hormone. Moreover, several lines of evidence suggest that C-peptide and insulin functionally interact (110), but the nature and necessity of that interaction across multiple tissue beds require further investigation. Although the renoprotective effects of C-peptide appear to be independent of insulin (24), the ability of C-peptide to enhance low-oxygen-induced ATP release from erythrocytes is dependent on coadministration with insulin (83), and indeed it appears that exposure to C-peptide alone is actually detrimental to erythrocyte function (83). However, there is no physiological situation in which cells are exposed to C-peptide in the absence of insulin, although in the setting of type 1 diabetes or in advanced type 2 diabetes patients are exposed to insulin in the absence of C-peptide. Second, although it is now recognized that C-peptide has therapeutic potential for the treatment of diabetes complications, the physiological significance of C-peptide in normal physiology remains hypothetical. Regardless of the answers to these questions, it is clear that the therapeutic potential of C-peptide must be further explored. Thus far, C-peptide replacement in type 1 diabetic patients has yielded positive results (110), but the utility of C-peptide for the treatment of type 2 diabetes-associated microvascular dysfunction must be considered as well. The development of C-peptide sensitizers or high-affinity agonists would be an important next step, and, given the increasingly high prevalence of type 2 diabetes worldwide, it would be most timely.
DISCLOSURES
J Wahren is employed by Cebix Inc., Karolinska Science Park, Stockholm, Sweden.
AUTHOR CONTRIBUTIONS
Author contributions: G.Y., C.M.-B., P.L., and J.W. prepared figures; G.Y., C.M.-B., P.L., and J.W. drafted manuscript; G.Y., C.M.-B., P.L., and J.W. edited and revised manuscript; G.Y., C.M.-B., P.L., and J.W. approved final version of manuscript.
REFERENCES
- 1.Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signaling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol 17: 986–995, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Alpers CE, Hudkins KL. Mouse models of diabetic nephropathy. Curr Opin Nephrol Hypertens 20: 278–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev 93: 405–480, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt MP, Lim YC, Hwang J, Na S, Kim YM, Ha KS. C-peptide prevents hyperglycemia-induced endothelial apoptosis through inhibition of reactive oxygen species-mediated transglutaminase 2 activation. Diabetes 62: 243–253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt MP, Lim YC, Kim YM, Ha KS. C-peptide activates AMPKα and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes 62: 3851–3862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 278: 9796–9801, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bo S, Gentile L, Castiglione A, Prandi V, Canil S, Ghigo E, Ciccone G. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: a retrospective cohort study after a 14-year follow-up. Eur J Endocrinol 167: 173–180, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Bugliani M, Torri S, Lupi R, Del Guerra S, Grupillo M, Del Chiaro M, Mosca F, Boggi U, Del Prato S, Marchetti P. Effects of C-peptide on isolated human pancreatic islet cells. Diabetes Metab Res Rev 23: 215–219, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chima RS, Maltese G, Lamontagne T, Piraino G, Denenberg A, O'Connor M, Zingarelli B. C-peptide ameliorates kidney injury following hemorrhagic shock. Shock 35: 524–529, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou DH, Bodycombe NE, Carrinski HA, Lewis TA, Clemons PA, Schreiber SL, Wagner BK. Small-molecule suppressors of cytokine-induced beta-cell apoptosis. ACS Chem Biol 5: 729–734, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cifarelli V, Geng X, Styche A, Lakomy M, Trucco M, Luppi P. C-peptide reduces high glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation. Diabetologia 54: 2702–2712, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Cifarelli V, Luppi P, Tse HM, He J, Piganelli J, Trucco M. Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis 201: 248–257, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Cotter MA, Ekberg K, Wahren J, Cameron NE. Effects of proinsulin C-peptide in experimental diabetic neuropathy: vascular actions and modulation by nitric oxide synthase inhibition. Diabetes 52: 1812–1817, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck PJ, Lambert EH, O'Brien PC. Pain in peripheral neuropathy related to rate and kind of fiber degeneration. Neurology 26: 466–471, 1976. [DOI] [PubMed] [Google Scholar]
- 18.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44: 2115–2133, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindstrom P, Wahren J. Amelioration of sensory nerve dysfunction by C-Peptide in patients with type 1 diabetes. Diabetes 52: 536–541, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Ekberg K, Brismar T, Johansson BL, Lindstrom P, Juntti-Berggren L, Norrby A, Berne C, Arnqvist HJ, Bolinder J, Wahren J. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care 30: 71–76, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Faber OK, Hagen C, Binder C.Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Invest 62: 197–203, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorina P, Folli F, Zerbini G.Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol 14: 2150–2158, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Flynn ER, Lee J, Hutchens ZM, Jr, Chade AR, Maric-Bilkan C. C-peptide preserves the renal microvascular architecture in the streptozotocin-induced diabetic rat. J Diabetes Complications 27: 538–547, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forst T, De La Tour DD, Kunt T, Pfutzner A, Goitom K, Pohlmann T, Schneider S, Johansson BL, Wahren J, Lobig M, Engelbach M, Beyer J, Vague P. Effects of proinsulin C-peptide on nitric oxide, microvascular blood flow and erythrocyte Na+,K+-ATPase activity in diabetes mellitus type 1. Clin Sci (London) 98: 283–290, 2000. [PubMed] [Google Scholar]
- 26.Freeman H, Shimomura K, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a link between insulin secretion, glucose metabolism and oxidative stress. Biochem Soc Trans 34: 806–810, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto S, Mukai E, Inagaki N. Role of endogenous ROS production in impaired metabolism-secretion coupling of diabetic pancreatic β cells. Prog Biophys Mol Biol 107: 304–31, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Furuya DT, Ishii T, Kamikawa A, Shimada K, Machado UF, Saito M, Kimura K. Proinsulin C-peptide induces c-Jun N-terminal kinase 1 expression in LEII mouse lung capillary endothelial cells. Jpn J Vet Res 57: 163–167, 2009. [PubMed] [Google Scholar]
- 29.Gier B, Krippeit-Drews P, Sheiko T, Aguilar-Bryan L, Bryan J, Düfer M, Drews G. Suppression of KATP channel activity protects murine pancreatic β-cells against oxidative stress. J Clin Invest 119: 3246–3256, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol 285: C723–C734, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Grunberger G, Qiang X, Li Z, Mathews ST, Sbrissa D, Shisheva A, Sima AA. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia 44: 1247–1257, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Hach T, Forst T, Kunt T, Ekberg K, Pfutzner A, Wahren J. C-peptide and its C-terminal fragments improve erythrocyte deformability in type 1 diabets patients. Exp Diabetes Res 2008: 730594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagen C, Faber OK, Binder C, Alberti K. Lack of metabolic effect of C-peptide in normal subjects and juvenile diabetic patients. Acta Endocrinol 85: 29, 1977. [Google Scholar]
- 34.Haidet J, Cifarelli V, Trucco M, Luppi P. C-peptide reduces pro-inflammatory cytokine secretion in LPS-stimulated U937 monocytes in condition of hyperglycemia. Inflamm Res 61: 27–35, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen JH, Tronier B, Bulow JB. Kinetics of circulating endogenous insulin, C-peptide, and proinsulin in fasting nondiabetic man. Metabolism 36: 463–468, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Henriksson M, Pramanik A, Shafqat J, Zhong Z, Tally M, Ekberg K, Wahren J, Rigler R, Jahansson J, Jornvall H. Specific binding of proinsulin C-peptide to intact and to detergent-solubilized human skin fibroblasts. Biochem Biophys Res Commun 280: 423–427, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Henriksson M, Shafqat J, Liepinsh E.Unordered structured of proinsulin C-peptide in aqueous solution and in the presence of lipid vesicles. Cell Mol Life Sci 57: 337–342, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hills CE, Al-Rasheed N, Willars GB, Brunskill NJ. C-peptide reverses TGF-β1-induced changes in renal proximal tubular cells: implications for treatment of diabetic nephropathy. Am J Physiol Renal Physiol 296: F614–F621, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hills CE, Brunskill NJ. C-peptide and its intracellular signaling. Rev Diabet Stud 6: 138–147, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogwerf BJ, Bantle JP, Gaenslen HE.Infusion of synthetic human C-peptide does not affect plasma glucose, serum insulin, or plasma glucagon in healthy subjects. Metabolism 35: 122–125, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Hordij KPL. Regulation of NADPH oxidases. The role of Racc proteins. Circ Res 98: 453–462, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-Peptide concentrations in human portal and peripheral blood. J Clin Invest 55: 1278–1283, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose-response study. Naunyn Schmiedebergs Arch Pharmacol 365: 67–73, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD, Di Cera E, Williamson JR. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277: 563–566, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Ishii T, Fukano K, Shimada K, Kamikawa A, Okamatsu-Ogura Y, Terao A, Yoshida T, Saito M, Kimura K. Proinsulin C-peptide activates alpha-enolase: implications for C-peptide-cell membrane interaction. J Biochem 152: 53–62, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Jagerbrink T, Lindahl E, Shafqat J, Jornvall H. Proinsulin C-peptide interaction with protein tyrosine phosphatase 1B demonstrated with a labeling reaction. Biochem Biophys Res Comm 387: 31–35, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabet Med 17: 181–189, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Johansson BL, Borg K, Fernqvist-Forbes E, Odergren T, Remahl S, Wahren J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia 39: 687–695, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Johansson BL, Kernell A, Sjöberg S, Wahren J. Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab 77: 976–981, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Johansson BL, Sjöberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 35: 121–128, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Kadmiel M, Fritz-Six KL, Caron KM. Understanding RAMPs through genetically engineered mouse models. Adv Exp Med Biol 744: 49–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaminitz A, Stein J, Yaniv I, Askenasy N. The vicious cycle of apoptotic beta-cell death in type 1 diabetes. Immunol Cell Biol 85: 582–589, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Kamiya H, Zhang W, Sima AA. C-peptide prevents nociceptive sensory neuropathy in type 1 diabetes. Ann Neurol 56: 827–835, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Kimoto K, Suzuki K, Kizaki T, Hitomi Y, Ishida H, Katsuta H, Itoh E, Ookawara T, Suzuki K, Honke K, Ohno H. Gliclazide protects pancreatic beta-cells from damage by hydrogen peroxide. Biochem Biophys Res Commun 303: 112–119, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi Y, Naruse K, Hamada Y, Nakashima E, Kato K, Akiyama N, Kamiya H, Watarai A, Nakae M, Oiso Y, Nakamura J. Human proinsulin C-peptide prevents proliferation of rat aortic smooth muscle cells cultured in high-glucose conditions. Diabetologia 48: 2396–401, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Kunt T, Schneider S, Pfutzner A, Goitum K, Engelbach M, Schauf B, Beyer J, Forst T. The effect of human proinsulin C-peptide on erythrocyte deformability in patients with Type 1 diabetes mellitus. Diabetologia 42: 465–471, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, Groff A, Ferran C, Bonner-Weir S, Sharma A, Weir GC. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes 51: 413–423, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Oshida Y, Kusunoki M, Yamanouchi K, Johansson BL, Wahren J, Sato Y. Rat C peptide I and II stimulate glucose utilization in STZ-induced diabetic rats. Diabetologia 42: 958–964, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Li N, Brun T, Cnop M, Cunha DA, Eizirik DL, Maechler P. Transient oxidative stress damages mitochondrial machinery inducing persistent beta-cell dysfunction. J Biol Chem 284: 23602–23612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li ZG, Zhang W, Sima AA. C-peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev 19: 375–385, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Zhao M, Li B, Qi J. Dynamic localization and functional implications of C-peptide might for suppression of iNOS in high glucose-simtulated rat mesangial cells. Mol Cell Endocrinol 381: 255–260, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Lindahl E, Nyman U, Melles E, Sigmundsson K, Stahlberg M, Wahren J, Obrink B, Safqat J, Joseph B, Jornvall H. Cellular internatilzation of proinsulin C-peptide. Cell Mol Life Sci 64: 479–486, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced 27 endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia 51: 1534–1543, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Luppi P, Drain P. Autocrine C-peptide mechanism underlying INS1 β-cell adaptation to oxidative stress. Diabetes Metab Res Rev In Press, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Luppi P, Geng X, Cifarelli V, Drain P, Trucco M. 1 C-peptide is internalised in human endothelial and 2 vascular smooth muscle cells via early endosomes. Diabetologia 252: 2218–2228, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Luppi P, Kallas Å, Wahren J. Can C-peptide mediated anti-inflammatory effects retard the development of microvascular complications of type 1 diabetes? Diabetes Metab Res Rev 29: 357–362, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Melles E, Jornvall H, Tryggvason S.Degradation of proinsulin C-peptide in kidney and placenta extracts by a specific endoprotease activity. Cell Mol Life Sci 61: 2979–2982, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Melloul D. Role of NF-κB in beta-cell death. Biochem Soc Trans 36: 334–339, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Mughal RS, Scragg JL, Lister P, Warburton P, Riches K, O'Regan DJ, Ball SG, Turner NA, Porter KE. Cellular mechanisms by which proinsulin C-peptide prevents insulin-induced neointima formation in human saphenous vein. Diabetologia 53: 1761–1771, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol 297: F1265–F1272, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nordquist L, Lai EY, Sjoquist M, Patzak A, Persson AE. Proinsulin C-peptide constricts glomerular afferent arterioles in diabetic mice. A potential renoprotective mechanism. Am J Physiol Regul Integr Comp Physiol 294: R836–R841, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Nordquist L, Moe E, Sjoquist M. The C-peptide fragment EVARQ reduces glomerular hyperfiltration in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 23: 400–405, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Norlin S, Ahlgren U, Edlund H. Nuclear factor-(kappa)B activity in (beta)-cells is required for glucose-stimulated insulin secretion. Diabetes 54: 125–132, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, Segre O, Grassi G, Cavallo-Perin P, Bruno G. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 32: 301–305, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 81: 442–448, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polonsky K, O'Meara N. Secretion and metabolism of insulin, proinsulin and C-peptide. In: Endocrinology. DeGroot L, Jameson J. eds. Philadelphia, PA: WB Saunders, 2001, p. 697–711. [Google Scholar]
- 80.Pramanik A, Ekberg K, Zhong Z, et al. C-peptide binding to human cell membranes: importance of Glu27. Biochem Biophys Res Commun 284: 94–98, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, Vague P, Tsimaratos M. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes Metab 32: 223–228, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol 17: S90–S97, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Richards JP, Stephenson AH, Ellsworth ML, Sprague RS. Synergistic effects of C-peptide and insulin on low O2-induced ATP release from human erythrocytes. Am J Physiol Regul Integr Comp Physiol 305: R1331–R1336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rigler R, Pramanik A, Jonasson P, Kratz G, Jansson OT, Nygren PA, Stahl S, Ekberg K, Jahansson BL, Uhlen S, Uhlen M, Jornvall H, Wahren J. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci USA 96: 13318–13323, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic β-cells in diabetes. J Biol Chem 279: 42351–42354, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjoquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant 20: 532–538, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int 60: 1258–1265, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Samnegard B, Jacobson SH, Johansson BL, Ekberg K, Isaksson B, Wahren J, Sjoquist M. C-peptide and captopril are equally effective in lowering glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19: 1385–1391, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Sasaki M, Fujimoto S, Sato Y, Nishi Y, Mukai E, Yamano G, Sato H, Tahara Y, Ogura K, Nagashima K, Inagaki N. Reduction of reactive oxygen species ameliorates metabolism-secretion coupling in islets of diabetic GK rats by suppressing lactate overproduction. Diabetes 62: 1996–2003, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sawyer RT, Flynn ER, Hutchens ZM, Jr, Williams JM, Garrett MR, Maric-Bilkan C. Renoprotective effects of C-peptide in the Dahl salt-sensitive rat. Am J Physiol Renal Physiol 303: F893–F899, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J 14: 2357–2364, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Shafqat J, Melles E, Sigmundsson K, Johansson BL, Ekbergy K, Alvelius G, Henriksson M, Johansson J, Wahren J, Jornvall H. Proinsulin C-peptide elicits disaggregation of insulin resulting in enhanced physiological insulin effects. Cell Mol Life Sci 63: 1805–1811, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharman JL, Benson HE, Pawson AJ, Lukito V, Pmamhanga CP, Bombail V, Davenport AP, Peters JA, Spedding M, Harmar AJ; NCIUPHAR. IUPHAR-DB: updated database content and new features. Nucleic Acids Res 41: D1083–D1088, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Short SM, Boyer JL, Juliano RL. Integrins regulate the linkage between upstream and downstream events in G protein-coupled receptor signaling to mitogen-activated protein kinase. J Biol Chem 275: 12970–12977, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Sima AA, Li ZG. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes 54: 1497–1505, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Sima AA, Zhang W, Kreipke CW, Rafols JA, Hoffman WH. Inflammation in diabetic encephalopathy is prevented by C-peptide. Rev Diabet Stud 6: 37–42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sima AA, Zhang W, Sugimoto K, Henry D, Li Z, Wahren J, Grunberger G. C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia 44: 889–897, 2001. [DOI] [PubMed] [Google Scholar]
- 98.Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30: 208–213, 1987. [DOI] [PubMed] [Google Scholar]
- 99.Sjoquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int 54: 758–764, 1998. [DOI] [PubMed] [Google Scholar]
- 100.Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science 157: 697–700, 1967. [DOI] [PubMed] [Google Scholar]
- 101.Stevens MJ, Zhang W, Li F, Sima AA. C-peptide corrects endoneurial blood flow but not oxidative stress in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab 287: E497–E505, 2004. [DOI] [PubMed] [Google Scholar]
- 102.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic b cells. Trends Endocr Metabol 23: 477–487, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Sutherland DE, Kendall DM, Moudry KC, et al. Pancreas transplantation in nonuremic, type I diabetic recipients. Surgery 104: 453–464, 1988. [PubMed] [Google Scholar]
- 104.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96: 10857–10862, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46: 1733–1742, 1997. [DOI] [PubMed] [Google Scholar]
- 106.Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes 47: 1578–1585, 1998. [DOI] [PubMed] [Google Scholar]
- 107.Vejandla H, Hollander JM, Kothur A, Brock RW. C-peptide reduces mitochondrial superoxide generation by restoring complex I activity in high glucose-exposed renal microvascular endothelial cells. ISRN Endocrinol 2012: 162802, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vish MG, Mangeshkar P, Piraino G, Denenberg A, Hake PW, O'Connor M, Zingarelli B. Proinsulin C-peptide exerts beneficial effects in endotoxic shock in mice. Crit Care Med 35: 1348–1355, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Wahren J, Kallas Å. Loss of pulsatile insulin secretion: a factor in the pathogenesis of type 2 diabetes? Diabetes 61: 2228–2229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes 61: 761–772, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walcher D, Babiak C, Poletek P, Rosenkranz S, Bach H, Betz S, Durst R, Grub M, Hombach V, Strong J, Marx N. C-Peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res 99: 1181–1187, 2006. [DOI] [PubMed] [Google Scholar]
- 112.Wallerath T, Kunt T, Forst T, Closs EI, Lehmann R, Flohr T, Gabriel M, Schafer D, Gopfert A, Pfutzner A, Beyer J, Forstermann U. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 9: 95–102, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Yosten GL, Kolar GR, Redlinger LJ, Samson WK. Evidence for an interaction between proinsulin C- peptide and GPR146. J Endocrinol 218: B1–B8, 2013. [DOI] [PubMed] [Google Scholar]
- 114.Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol 303: R941–R949, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young LH, Ikeda Y, Scalia R, Lefer AM. C-peptide exerts cardioprotective effects in myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol 279: H1453–H1459, 2000. [DOI] [PubMed] [Google Scholar]
- 116.Zerbini G, Mangili R, Luzi L. Higher post-absorptive C-peptide levels in Type 1 diabetic patients without renal complications. Diabet Med 16: 1048, 1999. [DOI] [PubMed] [Google Scholar]
- 117.Zhang W, Kamiya H, Ekberg K, Wahren J, Sima AA. C-peptide improves neuropathy in type 1 diabetic BB/Wor-rats. Diabetes Metab Res Rev 23: 63–70, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Zhong Z, Davidescu A, Ehren I, Ekberg K, Jornvall H, Wahren J, Chibalin AV. C-peptide stimulates ERK1/2 and JNK MAP kinases via actvation of protein kinase C in human renal tubular cells. Diabetologia 48: 187–197, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Zhong Z, Kotova O, Davidescu A, Ehren I, Ekberg K, Jornvall H, Wahren J, Chibalin AV. C-peptide stimulates Na+,K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci 61: 2782–2790, 2004. [DOI] [PubMed] [Google Scholar]