Abstract
Fat-specific protein 27 (FSP27) plays a pivotal role in controlling the formation of large lipid droplet and energy metabolism. The cellular levels of FSP27 are tightly regulated through the proteasomal ubiquitin-mediated degradation. However, the upstream signals that trigger FSP27 degradation and the underlying mechanism(s) have yet to be identified. Here we show that AMP-activated protein kinase (AMPK) activation by AICAR (5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide) or phenformin induced the ubiquitination of FSP27 and promoted its degradation in 3T3-L1 adipocytes. The levels of FSP27 protein could be maintained by either knocking down AMPKα1 or blocking proteasomal pathway. Moreover, AICAR treatment induced multilocularization of LDs in 3T3-L1 adipocytes, reminiscent of the morphological changes in cells depleted of FSP27. Furthermore, mass spectrometry-based proteomic analysis identified heat shock cognate 70 (HSC70) as a novel binding protein of FSP27. The specific interaction was confirmed by co-immunoprecipitation of both ectopically expressed and endogenous proteins. Importantly, knockdown of HSC70 by small interference RNA resulted in increased half-life of FSP27 in cells treated with a protein synthesis inhibitor cycloheximide (CHX) or AICAR. However, silencing of the E3 ubiquitin ligase CHIP (COOH terminus of HSC70-interacting protein) failed to alter the stability of FSP27 protein under both conditions. Taken together, our data indicate that AMPK is a negative regulator of FSP27 stability through the proteasomal ubiquitin-dependent protein catabolic process. Promotion of FSP27 degradation may be an important factor responsible for the beneficial effect of AMPK activators on energy metabolism.
Keywords: fat-specific protein 27, AMP-activated protein kinase, heat shock cognate 70
adipose tissue plays a central role in the development of obesity, which occurs when energy intake exceeds its expenditure. There are two types of adipose tissue, white adipose tissue (WAT) for energy storage and brown adipose tissue (BAT) for energy consumption to support heat production (9). White adipocytes, characterized by a large unilocular lipid droplet (LD), are responsible for the intracellular storage of triglycerides (TGs) in the fed state and provision of fatty acids as an energy substrate via lipolysis for other tissues in response to fasting. Brown adipocytes, on the other hand, contain numerous smaller LDs along with a large number of mitochondria and high oxidative activity. Enlargement of LDs or adipocytes in WAT contributes to the development of obesity and related insulin resistance (10, 13, 32). Thus, the induction of fat-burning brown adipocyte-like capacity in white adipocytes may represent a potential therapeutic strategy to combat obesity and its related disorders.
Recent studies have implicated AMPK as a potential target for inducing “BAT-like phenotype” in WAT (6). For example, it was demonstrated recently that WAT metabolism was remodeled toward energy dissipation through 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR)-induced AMPK activation (7, 38). Specifically, prolonged AMPK activation by AICAR caused increases in the expression of peroxisome proliferator-activated receceptor (PPAR)γ, PPARα, and PPARδ in isolated rat epididymal adipocytes. Higher expression of PPARγ coactivator-1α and carnitine palmitoyl transferase-1b was accompanied by an increase in fatty acid oxidation in these cells. Moreover, a separate study indicated that mice injected with AICAR showed increased uncoupling protein 1 expression and induced accumulation of brown adipocytes within the WAT (40). However, the molecular mechanisms underlying the effect of AMPK activation on the WAT plasticity have remained incompletely defined.
Fat-specific protein 27 (FSP27), a member of the cell death-inducing DFF45-like effector family, is a LD-associated protein expressed abundantly in the WAT (16, 17, 19). FSP27 possesses a profound role in maintaining the integrity of large unilocular LDs and the function of WAT as a TG storage organ. Whereas overexpression of FSP27 in nonadipocyte cells often results in enlarged LDs with increased capacity to store TGs, knockdown of FSP27 in mature white adipocytes leads to the appearance of smaller multilocular LDs as well as enhanced lipolysis (14–16, 30, 37, 42). In the liver, increased FSP27 expression is also causally linked to the enhanced hepatic TG accumulation and formation of LDs in ob/ob mice and fasted wild-type mice (24, 41). Mice with FSP27 deficiency exhibit multilocularization of LDs, upregulation of mitochondrial activity, and eventual acquirement of brown adipose-like properties in the WAT. These animals are also resistant to diet-induced obesity, hepatic steatosis, and insulin resistance (29, 39). In humans, treatment of obese patients with a very low-calorie diet causes a reduction of FSP27 expression in WAT (23). FSP27 protein is known to be rapidly degraded and has a short half-life of less than 2 h (28, 31), making the modulation of its stability a potential therapeutic approach for the treatment of metabolic diseases.
In the present study, we have obtained evidence that AMPK activation plays a critical role in the control of protein stability of FSP27. We show that prolonged AMPK activation promotes FSP27 degradation through the proteasomal pathway and induces multilocularization of LDs in adipocytes. We also provide evidence that heat shock cognate 70 (HSC70) is a new FSP27-interacting protein and is required for mediating the degradation of FSP27 downstream of AMPK activation.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies against phosphorylated acetyl-CoA carboxylase (ACC), phosphorylated AMP-activated protein kinase (AMPK)α, AMPKα, COOH terminus of HSC70-interacting protein (CHIP), and ubiquitin were purchased from Cell Signaling Technology. Anti-perilipin 1 antibody was purchased from Abcam. Anti-ABHD5 (α/β-hydrolase domain-containing protein 5) antibody was purchased from Proteintech Group, and anti-β-actin and anti-FLAG antibodies were obtained from Sigma-Aldrich. Anti-HSC70 antibody was obtained from BD Bioscience. The rabbit antibody against FSP27 was a gift from Dr. Cynthia Smas (University of Toledo). Horseradish peroxidase-linked secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. The protease inhibitor minitablets were obtained from Roche Diagnostics. The RNeasy Plus Mini Kit was purchased from Qiagen. Reagents for tissue culture and Bodipy 493/503 were obtained from Invitrogen. AICAR, phenformin, cycloheximide (CHX), insulin, dexamethasone and 3-isobutyl-1-methylxanthine, chloroquine, cycloheximide, MG132, oligomycin, and epoxomicin were purchased from Sigma-Aldrich.
Cell culture.
Mouse 3T3-L1 preadipocytes were maintained in high glucose DMEM supplemented with 10% newborn calf serum, 100 U/ml penicillin G sodium, and 100 U/ml streptomycin sulfate. Differentiation to adipocytes was induced by treatment of postconfluent cells with 10% FBS, 1 μg/ml insulin, 0.25 μM dexamethasone, and 0.5 mM isobutyl-1-methylzanthine. The differentiation medium was withdrawn 3 days later and replaced with DMEM supplemented with 10% FBS and 1 μg/ml insulin. After 2 days in insulin-containing medium, the cells were then cultured for 2 more days in DMEM containing 10% FBS.
Adenoviral infection of adipocytes.
Recombinant adenoviruses coding murine FSP27 containing COOH-terminal FLAG tag under the control of a cytomegalovirus (CMV) promoter were custom generated by Vector Biolabs. A CMV-null virus (Ad-null) was also obtained for use in control experiments. Infection of differentiated adipocytes was performed as described previously (43). Briefly, Ad-FSP27 or Ad-null was diluted to 5 × 107 pfu/ml in serum-free DMEM containing 5.0 μg/ml polybrene and preincubated for 5 min at room temperature. Differentiated adipocytes plated in six-well dishes were washed once with PBS, and 1.5 ml of preincubated virus mix was added to each well. Cells were centrifuged at 800 g for 1 h at room temperature, followed by incubation at 37°C. Four hours later, fresh DMEM containing 10% FBS (1.5 ml/well) was added, and cells were incubated for another 48 h before use.
siRNA-mediated knockdown.
The following double-stranded stealth siRNA oligonucleotides (Invitrogen) were used: for mouse HSC70, 5′-GCGUAGGUUUGAUGAUGCUGUUGUU-3′ (sense) and 5′-AACAACAGCAUCAUCAAACCUACGC-3′ (antisense); for mouse CHIP, 5′-AGAAGUGCGCCUUCACAGACUGCCC-3′ (sense) and 5′-GGGCAGUCUGUGAAGGCGCACUUCU-3′ (antisense); for mouse AMPK, 5′-UCUCUUUCCUGAGGACCCAUCUUAU-3′ (sense) and 5′-AUAAGAUGGGUCCUCAGGAAAGAGA-3′ (antisense); for mouse FSP27, 5′-GCACAAUCGUGGAGACAGAAG-3′ (sense) and 5′-UCUUCUGUCUCCACGAUU-3′ (antisense). Control oligonucleotides with comparable guanine-cytosine (GC) content were also from Invitrogen. Four nanomoles of mixed oligonucleotides were delivered into cells via electroporation using a Bio-Rad Gene Pulser II at 950 μFaraday and 160 V, as described previously (43). Three days later, cells were processed for designated assays.
Immunoblotting and immunoprecipitation.
Tissues or 3T3-L1 cells were lysed in a buffer containing 50 mM Tris·HCl (pH 7.4), 135 mM NaCl, 10 mM NaF, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate, 1.0 mM EDTA, 10% glycerol, and 1× Complete protease inhibitor mixture. The lysates were clarified by centrifugation at 20,000 g for 10 min and then mixed with an equal volume of 2× SDS sample buffer. The solubilized proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Individual proteins were blotted with primary antibodies at appropriate dilutions. Peroxide-conjugated secondary antibodies were incubated with the membrane at a dilution of 1:5,000. The signals were then visualized using ECL substrate (Pierce).
For immunoprecipitation, cells were lysed in a buffer containing 50 mM Tris·HCl (pH 7.4), 135 mM NaCl, 10 mM NaF, 1% NP-40, 1 mM EDTA, and protease inhibitors. The clarified lysate was allowed to mix with anti-FLAG M2 gel (Sigma-Aldrich) or protein A/G plus agarose (Santa Cruz Biotechnology) containing HSC70 antibody at 4°C for 4 h. Following extensive washes with the same lysis buffer, the agarose was mixed with 1× SDS sample buffer, and proteins were detected by immunoblotting analysis.
RNA extraction and real-time PCR.
Total RNA was isolated from 3T3-L1 cells using the RNeasy Plus Mini Kit according to the manufacturer's instructions. cDNA was synthesized from total RNA by Long Range reverse transcriptase (Qiagen) with oligo(dT). For quantification of FSP27 expression, real-time PCR was performed using SYBR Green PCR Master Mix (Invitrogen) on an Applied Biosystems 7900 HT Real-Time PCR System. The following PCR primer were used: for FSP27, sense 5′-GTGTTAGCACCGCAGATCG-3′ and anti-sense 5′-CACGATTGTGCCATCTTC-3′; for β-actin, sense 5-′CTAAGGCCAACCGTGAAAAG-3′ and anti-sense 5-′ACCAGAGGCATACAGGGACA-′3. Data were analyzed using the comparative cycle threshold (ΔΔCT) method. The mRNA levels of genes normalized to β-actin were presented as relative to the control. PCR product specificity was verified by postamplification melting curve analysis and by running products on an agarose gel.
Immunofluorescence staining and confocal microscopy.
Adipocytes were maintained at proper densities on glass coverslips placed in six-well dishes. After the fixation with 3% paraformaldehyde in PBS for 15 min followed by permeabilization with 0.5% Triton X-100 in PBS for 5 min, cells were quenched with 100 mM glycine in PBS for 20 min and blocked with 1% BSA in PBS for 1 h. The cells were then exposed to anti-FLAG or HSC70 antibodies for 2 h at room temperature. Following three washes with PBS, the cells were incubated for 1 h with 1% BSA-PBS containing Alexa Fluor secondary antibodies. Samples were mounted on glass slides with Vecta shield mounting medium and examined under the Zeiss LSM 510 inverted confocal microscope.
In-gel digestion and mass spectrometry.
The immunoprecipitated samples were resolved by 4–15% 1D-SDS-PAGE. The proteins were then visualized by Coomassie blue staining (Sigma-Aldrich). Then, the gel portions were excised, destained, dehydrated, dried, and subjected to trypsin digestion overnight. The resulting peptides were desalted and analyzed by on-line HPLC on a linear trap Quadru pole Fourier transform ion cyclotron resonance.
Animal experiments.
Ten-week-old male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility at Mayo Clinic Arizona. All mice had free access to water and were fed a chow diet (no. 5001; Test Diet). Mice were injected intraperitoneally with vehicle (PBS) or AICAR (1 g/kg). Six hours later, epididymal fat was collected for immunoblotting analysis. The protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Statistical analysis.
Values are expressed as means ± SD. Statistical significance was evaluated by two-tailed unpaired Student's t-test. Differences were considered significant at P < 0.05.
RESULTS
AMPK activation decreases protein level of FSP27.
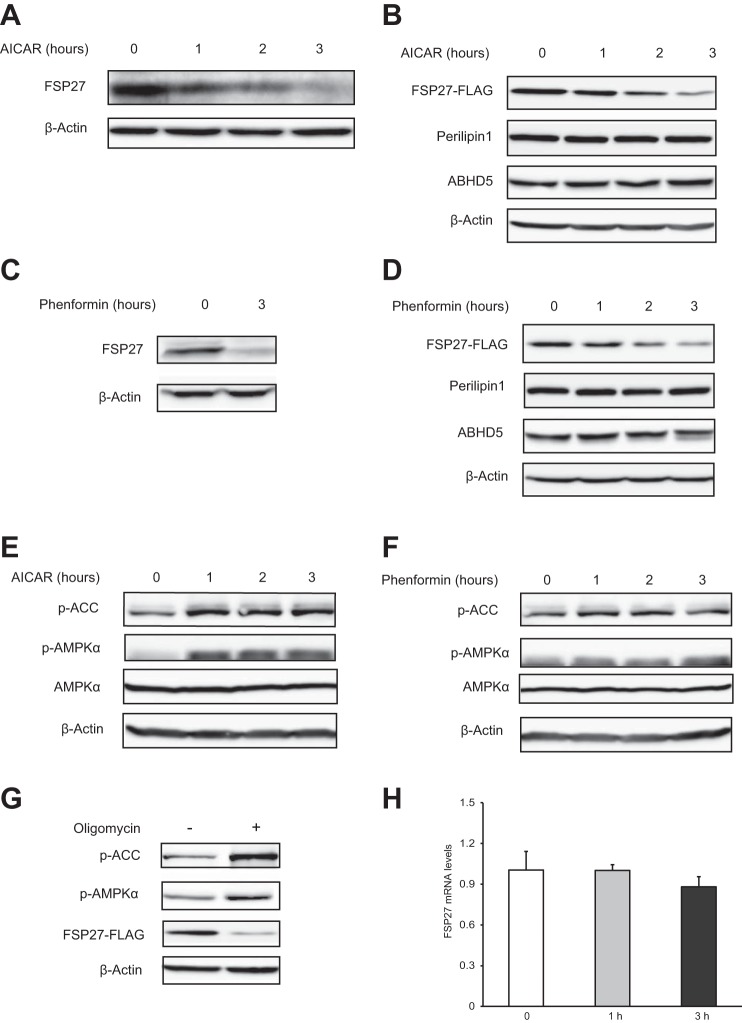
Since both AMPK activation and FSP27 ablation led to brown-like phenotypes in white adipocytes, we asked whether AMPK would be involved in the control of FSP27 expression. In differentiated 3T3-L1 white adipocytes, treatment with AICAR caused a time-dependent decrease in the levels of endogenous (Fig. 1A) as well as ectopically expressed FSP27 (Fig. 1B). The ectopic expression was achieved by infection with a recombinant adenovirus (Ad-FSP27-FLAG) that encodes mouse FSP27 with a COOH-terminal FLAG epitope tag under the control of a CMV promoter. Similar effects were observed when cells were treated with phenformin (Fig. 1, C and D), another pharmaceutical activator of AMPK. By contrast, neither AICAR nor phenformin affected the protein levels of perilipin 1 and ABHD5, two well-known LD-associated proteins (Fig. 1, B and D). Both AICAR and phenformin increased the phosphorylation of AMPKα and its target ACC (acetyl-CoA carboxylase) (Fig. 1, E and F), confirming the successful activation of AMPK. In addition, energy depletion by treatment of cells with an ATP synthase inhibitor, oligomycin, also activated AMPK and decreased the protein levels of FSP27 (Fig. 1G). Importantly, AICAR exhibited no significant influence on the mRNA levels of FSP27 (Fig. 1H). Thus, AMPK activation induced by different pharmaceutical compounds leads to decreased protein level of FSP27 in 3T3-L1 adipocytes.
Fig. 1.
AMP-activated protein kinase (AMPK) activation decreases the protein levels of fat-specific protein 27 (FSP27) in 3T3-L1 adipocytes. A: cells were treated with 2 mM 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR) for indicated times, and the levels of endogenous FSP27 protein were detected by immunoblotting using an anti-FSP27 antibody. B: cells infected with Ad-FSP27-FLAG were treated with 2 mM AICAR for indicated times. Immunoblotting was performed using antibodies against FLAG, perilipin 1, α/β-hydrolase domain-containing protein 5 (ABHD5), and β-actin. C: cells were treated with 2 mM phenformin, and the endogenous protein level of FSP27 was detected by immunoblotting. D: cells overexpressing FSP27-FLAG were treated with 2 mM phenformin and then lysed for immunoblotting analysis using indicated antibodies. E and F: cells were treated with 2 mM AICAR (E) or phenformin (F) for the indicated times, and then the phosphorylation (p) of acetyl-CoA carboxylase (ACC) or AMPKα was determined by immunoblotting using phosphorylated specific antibodies. G: cells overexpressing FSP27 were treated with 1 μM oligomycin for 3 h and then lysed for immunoblotting analysis. H: real-time PCR was used to determine the mRNA levels of FSP27 in cells treated with 2 mM AICAR. Data shown are representative of 3 independent experiments.
AMPKα1 is involved in the regulation of FSP27 protein level.
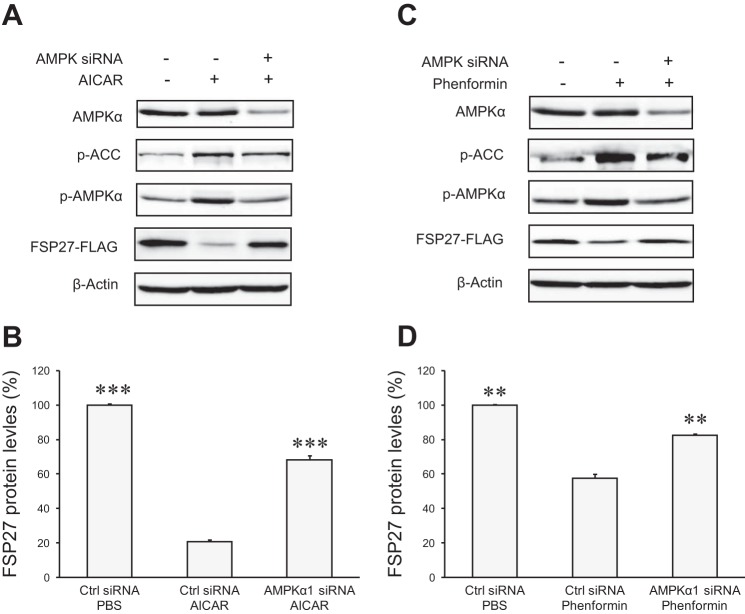
AMPKα1 was reported to be the major isoform of the AMPK catalytic α-subunit in adipocytes, accounting for ∼90% of the total AMPK activity (4, 21). To investigate whether AMPK mediates AICAR/phenformin-induced downregulation of FSP27 expression, we performed siRNA-mediated gene silencing to deplete AMPKα1 in 3T3-L1 adipocytes. Compared with the transfection with control siRNA, only about 50% of AMPKα1 remained in cells transfected with an AMPKα1-specific siRNA (Fig. 2, A and C). Importantly, AMPKα1 knockdown markedly inhibited AICAR/phenformin-induced phosphorylation of AMPKα subunit and a typical AMPK subunit ACC, demonstrating an effective blockade of AMPK activation by AICAR/phenformin. In cells treated with control siRNA, treatment with AICAR and phenformin reduced the protein levels of FSP27-FLAG by ∼80 and 40%, respectively (Fig. 2, B and D). However, in cells treated with AMPKα1 siRNA, FSP27-FLAG experienced reductions by only 32 and 17%, respectively. These results suggest that AICAR and phenformin induce the downregulation of FSP27 protein expression specifically via the activation of AMPK.
Fig. 2.
AMPKα1 mediates AICAR/phenformin-induced downregulation of FSP27 protein levels. 3T3-L1 adipocytes were transfected with control (Ctrl) or AMPKα1-specific siRNA followed by infection with Ad-FSP27-FLAG. Cells were treated with 2 mM AICAR (A and B) or phenformin (C and D) for 3 h. Immunoblotting was performed to detect different protein expression. The protein levels of FSP27-FLAG were quantified by densitometric measurement and normalized by the levels of β-actin (B and D). All data are shown as means ± SD and represent 3 independent experiments. ***P < 0.001 vs. Ctrl siRNA + AICAR; **P < 0.01 vs. Ctrl siRNA + phenformin.
AMPK activation induces FSP27 degradation via ubiquitin-dependent proteasomal pathway.
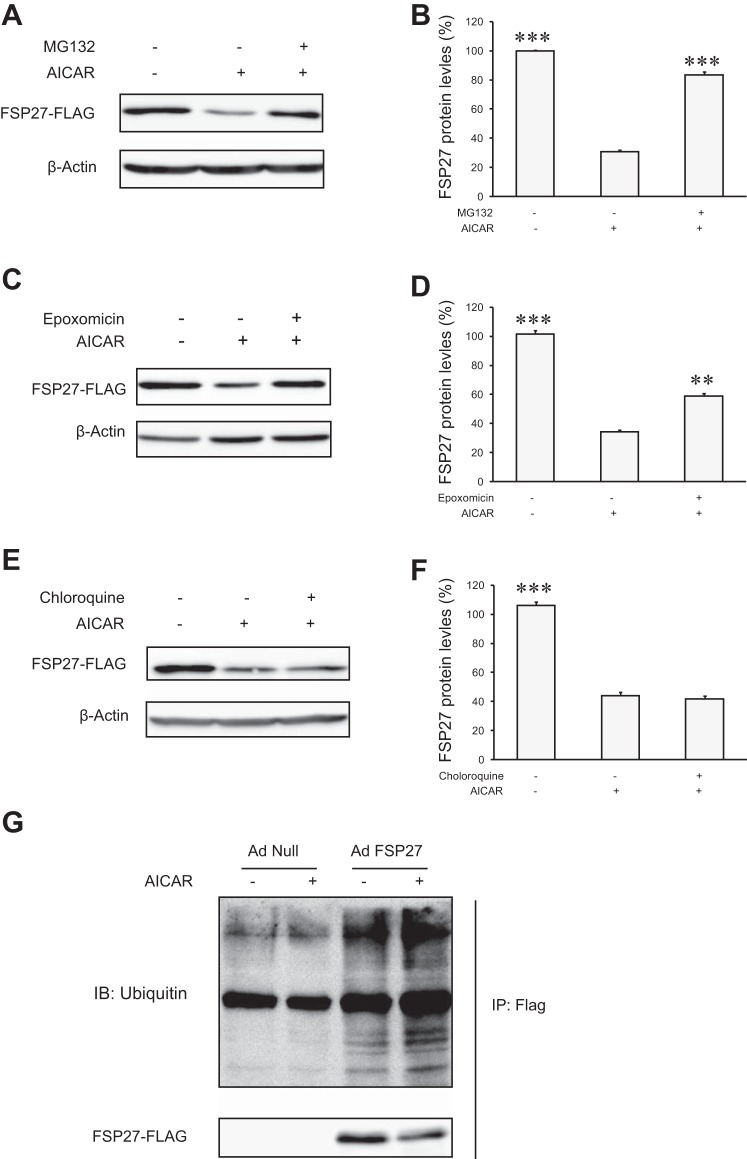
Next, we determined whether the protein level of FSP27 could be maintained by prevention of protein degradation. As shown in Fig. 3, A–D, addition of the proteasomal inhibitor, MG-132 or epoxomicin, blocked the degradation of FSP27 in 3T3-L1 adipocytes treated with AICAR. By contrast, a lysosomal protease inhibitor chloroquine was incapable of preventing such turnover (Fig. 3, E and F). To examine potential ubiquitination of FSP27, we infected cells with either Ad-FSP27-FLAG or a control null virus (Ad-null). Following a 2-h treatment with AICAR, FSP27-FLAG was immunoprecipitated and then subjected to anti-ubiquitin immunoblotting analysis. As expected, AICAR caused a decrease in the amount of FSP27 protein isolated from cells infected with Ad-FSP27 (Fig. 3G). However, FSP27 ubiquitination was increased in AICAR-treated cells compared with control cells. Thus, our data suggest that AMPK activation promotes FSP27 ubiquitination and degradation through the proteasomal pathway.
Fig. 3.
AICAR treatment increases FSP27 ubiquitination in 3T3-L1 adipocytes. FSP27-FLAG-overexpressed cells were pretreated with 10 μM MG-132 (A and B), 1 μM epoxomycin (C and D), or 100 μM chloroquine (E and F) for 30 min prior to the addition of 2 mM AICAR. After 3 h of AICAR treatment, cells were lysed for immunoblotting (IB) analysis. G: cells were infected with Ad-null or Ad-FSP27-FLAG for 2 days. After 1.5 h of treatment with 2 mM of AICAR, immunoprecipitation (IP) was performed by anti-FLAG affinity gel. Immunoprecipitates were subject to immunoblotting analysis using anti-ubiquitin and -FLAG antibodies. The experiments were repeated 3 times, and data are shown as means ± SD. **P < 0.01 and ***P < 0.001 vs. single AICAR treatment.
HSC70 specifically interacts with FSP27.
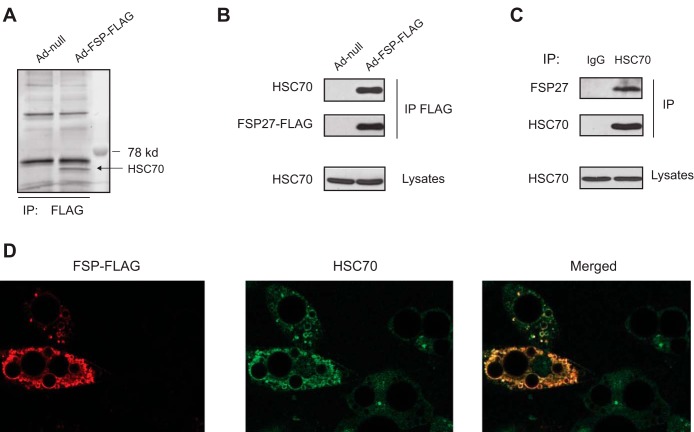
To uncover the mechanism by which the protein stability of FSP27 is regulated, we searched for the potential interacting protein(s) of FSP27 by combining the usage of coimmunoprecipitation and mass spectrometry-based protein identification methods. We expressed FSP27-FLAG protein in 3T3-L1 adipocytes via infection with Ad-FSP27-FLAG. As control, cells were infected with Ad-null. Following anti-FLAG immunoprecipitation and resolution on SDS-PAGE, SYPRO Ruby staining revealed the presence of a single, specific band at ∼73 kDa in FSP27-FLAG precipitates (Fig. 4A). Mass spectrometry analysis identified the corresponding protein as HSC70, which was confirmed by anti-HSC70 immunoblotting (Fig. 4B). Anti-HSC70 immunoprecipitation followed by anti-FSP27 immunoblotting confirmed that a specific interaction also existed between the endogenous proteins (Fig. 4C). It is well known that FSP27 is a LD-associated protein. Figure 4D shows that FSP27-FLAG localized at the surface of LDs in 3T3-L1 adipocytes. Interestingly, although it was mainly distributed scatterly or diffusely in the cytosol of control cells, HSC70 was recruited to the surface of LDs in the presence of overexpressed FSP27-FLAG. Taking together, these data indicate that HSC70 is a specific FSP27-binding protein.
Fig. 4.
Heat shock cognate (HSC70) interacts with FSP27 in 3T3-L1 adipocytes. A and B: cells were infected with null virus or Ad-FSP27-FLAG for 2 days. Anti-FLAG immunoprecipitates were resolved by SDS-PAGE and then subject to SyproRuby staining (A). Endogenous HSC70 was detected in anti-FLAG immunoprecipitates and cell lysates by anti-HSC70 IB (B). C: anti-HSC70 IP was performed and analyzed by anti-HSC70 immunoblotting. Coprecipitation of endogenous FSP27 was detected by immunoblotting for FSP27. D: immunofluorescence staining with anti-FLAG and anti-HSC70 antibodies was performed to reveal colocalization between FSP27-FLAG (red) and HSC70 (green) in 3T3-L1 adipocytes. The images shown are representative of 3 independent experiments.
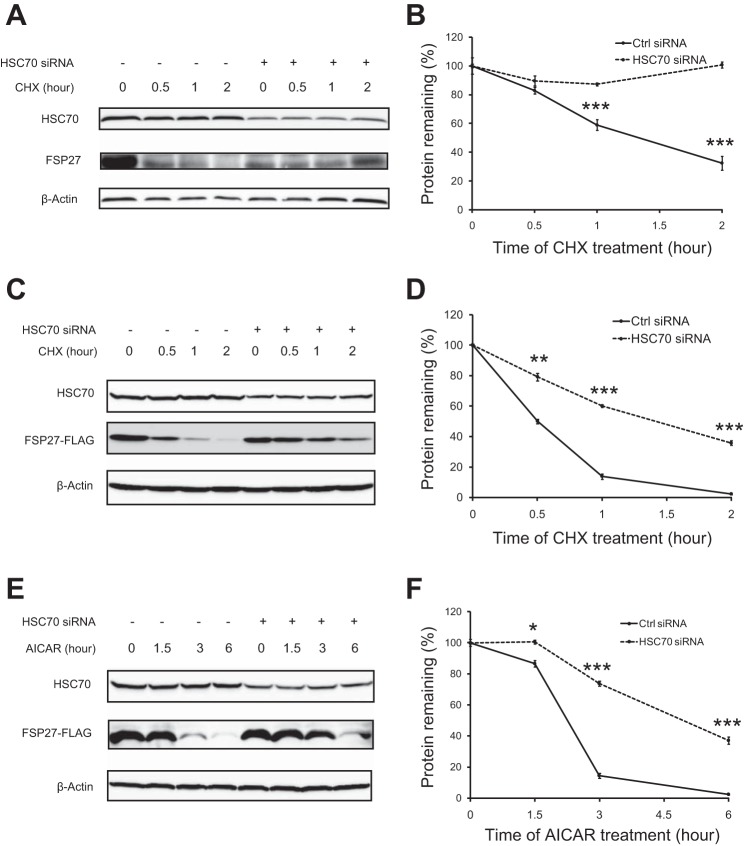
HSC70 mediates AICAR-induced FSP27 degradation.
HSC70 is a member of the heat shock protein 70 (HSP70) family and serves as a molecular chaperone to promote correct folding of nascent polypeptides or target proteins for degradation (3, 26). To test whether HSC70 would mediate FSP27 degradation, we silenced HSC70 expression via the HSC70-specific siRNA in 3T3-L1 adipocytes. Compared with the endogenous protein levels in cells transfected with a GC-matched control siRNA, ∼50% of HSC70 was present in cells transfected with HSC70-specific siRNA (Fig. 5A). Upon treatment of cells with cycloheximide to block protein synthesis, endogenous FSP27 experienced a rapid turnover in the control cells with a 1-h half-life, whereas in HSC70 knockdown cells the protein was relatively stable without significant degradation within 2 h (Fig. 5, A and B). Similarly, the half-life of ectopic FSP27 was determined to be 0.5 and 1.5 h in control and HSC70 knockdown cells, respectively(Fig. 5, C and D). In addition, FSP27 was highly unstable in the presence of AICAR and experienced a >85 and 97% reduction after 3 and 6 h, respectively (Fig. 5, E and F). However, in HSC70 knockdown cells, FSP27 stability was dramatically improved with a reduction of 25 and 63% after the AICAR treatment for 3 and 6 h, respectively (Fig. 5, E and F). Thus, HSC70 plays a critical role in the regulation of FSP27 protein stability and its degradation in response to the AMPK activation.
Fig. 5.
HSC70 participates in AICAR-induced FSP27 degradation. A and B: 3T3-L1 adipocytes were transfected with control or HSC70-specific siRNA. After 3 days, cells were treated with 10 μg/ml cycloheximide (CHX) for the indicated times. Endogenous FSP27 was detected by IB with anti-FSP27 antibody. C–F: 3T3-L1 adipocytes were transfected with control or HSC70-specific siRNA for 1 day and then infected with Ad-FSP27-FLAG. Two more days later, cells were treated with 10 μg/ml CHX (C and D) or 2 mM AICAR (E and F). FSP27-FLAG was detected by anti-FLAG immunoblotting. The levels of endogenous or ectopic FSP27 were quantified by the densitometric measurement, using β-actin as a loading control (B, D, and F). Data are presented as the mean ± SD of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl knockdown.
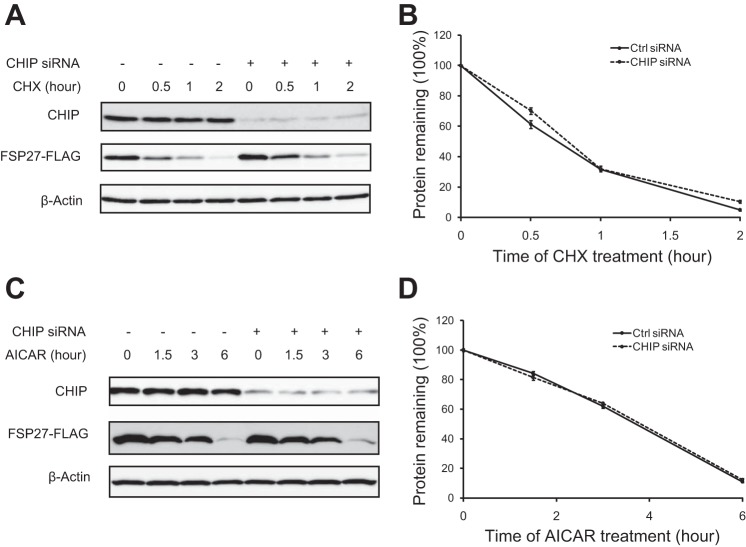
The effect of CHIP on FSP27 degradation.
CHIP (COOH terminus of HSC70-interacting protein) is an E3 ubiquitin ligase that commonly forms a preubiquitination complex with HSC70 and its client proteins targeting for degradation (25, 27). To determine whether CHIP mediates the degradation of FSP27, we overexpressed FSP27-FLAG in adipocytes where expression of endogenous CHIP was reduced via siRNA-mediated knockdown. Interestingly, CHIP did not affect FSP27 degradation in the presence of either CHX (Fig. 6A) or AICAR (Fig. 6C). Thus, we conclude that CHIP is not involved in FSP27 degradation.
Fig. 6.
COOH terminus of HSC70-interacting protein (CHIP) has no effect on FSP27 stability. 3T3-L1 adipocytes were transfected with control or CHIP-specific siRNA for 1 day and then infected with Ad-FSP27-FLAG. Two more days later, cells were treated with 10 μg/ml CHX (A and B) or 2 mM AICAR (C and D) for the indicated times. FSP27-FLAG was detected by IB, using β-actin as a loading control (A and C), and subjected to densitometric measurement (B and D). Data are presented as the mean ± SD of 3 independent experiments.
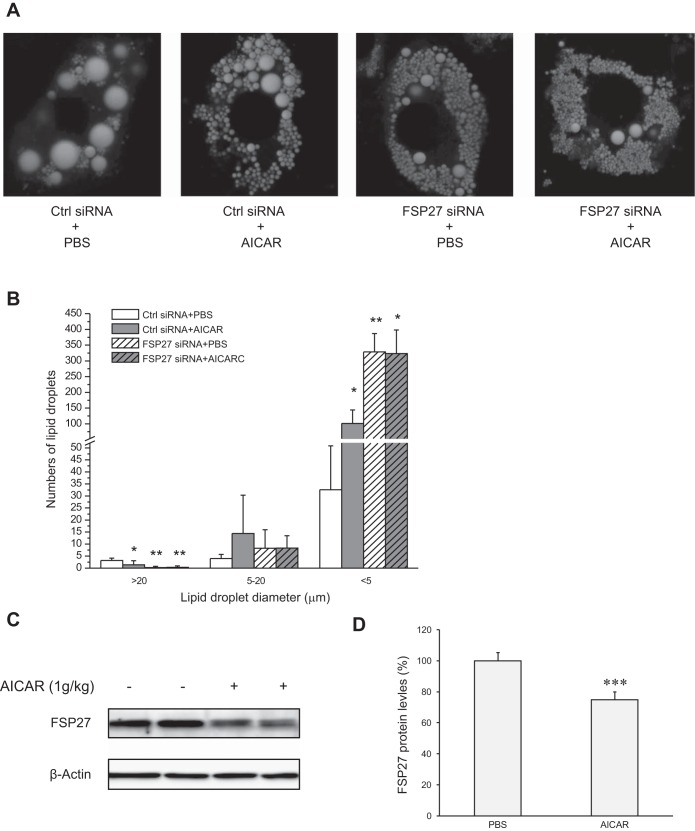
AICAR induces multilocularization of LDs.
FSP27 plays an important role in maintaining the integrity of large unilocular LDs. Next, we determined whether the degradation of FSP27 by AICAR would similarly affect the LD morphology as the FSP27 depletion. Consistent with our previous report (42), FSP27 knockdown produced a plenty of small LDs and fewer large droplets compared with the untreated control cells (Fig. 7, A and B). Interestingly, control cells treated with AICAR exhibited a reduction in the size of large LDs (>20 μM) along with a dramatic increase in the number of small LDs (<5 μM), indicating that AICAR induces multilocularization of LDs reminiscent of that produced by FSP27 knockdown. In addition, AICAR treatment was unable to further increase the number of small LDs in the FSP27-depleted cells. Furthermore, it was reported previously that AICAR treatment in mice increases the number of smaller LDs in WAT along with production of brown-like adipocytes (40). Figure 7, C and D, showed that a single intraperitoneal injection of AICAR in C57BL/6J mice decreased FSP27 protein levels in WAT. Taken together, these results suggest that AICAR may promote FSP27 degradation and induce multilocularization of LDs both in vitro and in vivo.
Fig. 7.
AICAR induces multilocularization of lipid droplets (LDs). A: 3T3-L1 adipocytes were transfected with Ctrl or FSP27-specific siRNA for 3 days and then treated with PBS or 2 mM AICAR for 16 h. A: fluorescence microscopy was performed to reveal intracellular LDs labeled with Bodipy 493/503. B: size distribution of LDs per cell was calculated based on results in A; n = 4/group. *P < 0.05 and **P < 0.01 vs. Ctrl siRNA + PBS; no significant difference between FSP27 siRNA + PBS and FSP27 siRNA + AICAR treatment (P > 0.05). C and D: male C57BL/6J mice were injected intraperitoneally with AICAR. Six hours later, the protein levels of FSP27 in epididymal adipose tissue were detected by IB with a FSP27 antibody (C) and subjected to densitometric measurement (D). ***P < 0.001 vs. PBS injection. The experiments were repeated 3 times.
DISCUSSION
FSP27 promotes lipid exchange between the smaller and larger LDs and is essential for the formation of the unilocular LDs in white adipocytes (11, 37). In this study, we demonstrate that AMPK, a crucial sensor of energy status, promotes ubiquitination and proteasomal degradation of FSP27 protein in differentiated adipocytes. We show that HSC70, a chaperon protein, associates with FSP27 and plays an important role in the degradation of FSP27. Our results thus provide a novel mechanism for the regulation of FSP27 protein stability.
AMPK plays a pivotal role in cellar lipid/energy metabolism. Activation of AMPK induced by its agonists (AICAR, phenformin, etc.) has been demonstrated to ameliorate obesity, promote lipid oxidation. and improve insulin sensitivity (1, 5, 8, 34, 44). A previous report has also shown that the chronic treatment of mice with AICAR causes the multilocularization of LDs in white adipocytes and the browning of WAT (40). Considering that these phenotypes are resonant with those caused by the ablation of FSP27 in mice, we speculated that a functional link might exist between these two key regulators of energy metabolism. Indeed, we found that both AICAR and phenformin activated AMPK and decreased both the endogenous and ectopic protein levels of FSP27 in 3T3-L1 adipocytes. That knockdown of AMPKα1 significantly prevented such an effect of AICAR and phenformin proves the specific involvement of AMPK in the regulation of FSP27 stability. Moreover, reminiscent of the LD morphological changes incurred by FSP27 depletion (11, 14, 15, 29, 39, 42), AMPK activation induced by AICAR led to multilocularization of LDs in 3T3-L1 adipocytes, implying a causal role for AMPK-induced FSP27 degradation. Multilocularization of LDs is a hallmark of brown and brown-like adipose tissue and is associated with higher mitochondrial oxidative capacity. In this regard, both FSP27 deletion and AMPK activation have been demonstrated to induce the browning of WAT along with enhanced energy metabolism. Injection of AICAR in mice reduced FSP27 protein content in the WAT (Fig. 7, C and D) as well as induced the browning of WAT (40). Therefore, we speculate that induction of FSP27 degradation and subsequent multilocularization of LDs may be one of the key mechanisms responsible for AMPK-induced browning of WAT.
Further studies demonstrated that AICAR reduced FSP27 stability through promoting its degradation via the ubiquitin-proteasomal pathway. The mass spectrometry analysis identified HSC70 in the FSP27-FLAG immunoprecipitates, offering the first piece of evidence that HSC70 is likely to be a FSP27-interacting protein. Coimmunoprecipitation later confirmed the specific interactions between both ectopically expressed and endogenous proteins. Consistent with the well-recognized notion that FSP27 is a LD-associated protein, our immunofluorescence staining experiment showed that a majority of ectopic FSP27 was located at the surface of LDs in 3T3-L1 adipocytes. The finding that the ectopic FSP27 was able to drive the endogenous HSC70 onto lipid droplets further strengthens our conclusion about the specific interaction between FSP27 and HSC70.
HSC70 is a member of the HSP70 family. Unlike canonical heat shock proteins, HSC70 is constitutively expressed and serves as a molecular chaperone to promote correct folding of nascent polypeptides/proteins or target its clients for degradation under nonstress conditions (20, 22, 36). Our results indicate that HSC70 is responsible for the short half-life of FSP27 protein as well as for mediating the degradation of FSP27 downstream of the AMPK activation. That FSP27 protein was more stable in AICAR-treated cells where HSC70 was knocked down strongly suggests that HSC70 associates with FSP27 and thereby targets it for degradation in response to the AMPK activation. Moreover, the E3 ubiquitin ligase responsible for FSP27 degradation remains to be identified. CHIP is a dual-function cochaperone/ubiquitin ligase that targets a number of Hsp70/90-associated proteins, including the glucocorticoid receptor, androgen receptor, Smad1/4, and ErbB2 for ubiquitination and degradation (2, 18, 35, 45). However, CHIP is unlikely the E3 ubiquitin ligase for FSP27, since knockdown of CHIP elicited no effect on FSP27 degradation in response to either blockade of protein synthesis or activation of AMPK. A separate study has shown that spartin, a protein truncated in the neurological disease Troyer syndrome, recruits and activates the E3 ubiquitin ligase atrophin-1-interacting protein 4 (AIP4) onto LDs, leading to the ubiquitination and degradation of droplet-associated proteins (46). It remains to be determined whether spartin/AIP4 is involved in the turnover of FSP27 protein mediated by HSC70.
In conclusion, we have found that AMPK activation can promote FSP27 degradation in adipocytes in a HSC70-dependent manner. The degradation of FSP27 by AMPK activation is associated with multilocularization of LDs as well as the browning of WAT in mice. The current study thus uncovers a novel mechanism underlying the beneficial effects of AMPK activators in the treatment of metabolic diseases.
GRANTS
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-089178) and the American Diabetes Association (1-10-JF-30) to J. Liu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Z. and J.L. conception and design of research; X.Z., B.L.H., X.X., and A.M.S. performed experiments; X.Z., B.L.H., X.X., and J.L. analyzed data; X.Z. and J.L. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; J.L. edited and revised manuscript; J.L. approved final version of manuscript.
REFERENCES
- 1.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 51: 2199–2206, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3: 93–96, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett 581: 3702–3710, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280: 25250–25257, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Drake JC, Alway SE, Hollander JM, Williamson DL. AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299: R1546–R1554, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaidhu MP, Ceddia RB. The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exerc Sport Sci Rev 39: 102–108, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, Ceddia RB. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 50: 704–715, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaidhu MP, Frontini A, Hung S, Pistor K, Cinti S, Ceddia RB. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res 52: 1702–1711, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 131: 242–256, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154: 2992–3000, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol 195: 953–963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr 93: 891S–896S, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Jambunathan S, Yin J, Khan W, Tamori Y, Puri V. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6: e28614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK, MacDougald OA. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem 283: 14355–14365, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Liu K, Zhou S, Tillison K, Wu Y, Smas CM. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am J Physiol Endocrinol Metab 294: E654–E667, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Zhang Y, Xu L, Zhou L, Wang Y, Xue B, Wen Z, Li P, Sang J. Regulation of gene expression by FSP27 in white and brown adipose tissue. BMC Genomics 11: 446, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Xin H, Xu X, Huang M, Zhang X, Chen Y, Zhang S, Fu XY, Chang Z. CHIP mediates degradation of Smad proteins and potentially regulates Smad-induced transcription. Mol Cell Biol 24: 856–864, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang L, Zhao M, Xu Z, Yokoyama KK, Li T. Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem J 370: 195–203, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Tang L. The critical roles of HSC70 in physiological and pathological processes. Curr Pharm Des 20: 101–107, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun 316: 853–858, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther 136: 354–374, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson B, Gummesson A, Glad CA, Goedecke JH, Jernas M, Lystig TC, Carlsson B, Fagerberg B, Carlsson LM, Svensson PA. Cell death-inducing DFF45-like effector C is reduced by caloric restriction and regulates adipocyte lipid metabolism. Metabolism 57: 1307–1313, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab 7: 302–311, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8: 303–308, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol 42: 1–9, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Murata S, Chiba T, Tanaka K. CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int J Biochem Cell Biol 35: 572–578, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Nian Z, Sun Z, Yu L, Toh SY, Sang J, Li P. Fat-specific protein 27 undergoes ubiquitin-dependent degradation regulated by triacylglycerol synthesis and lipid droplet formation. J Biol Chem 285: 9604–9615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118: 2808–2821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 282: 34213–34218, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Ranjit S, Boutet E, Gandhi P, Prot M, Tamori Y, Chawla A, Greenberg AS, Puri V, Czech MP. Regulation of fat specific protein 27 by isoproterenol and TNF-alpha to control lipolysis in murine adipocytes. J Lipid Res 52: 221–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magre J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S, Patch AM, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O'Rahilly S, Savage DB. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 1: 280–287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar S, Brautigan DL, Parsons SJ, Larner JM. Androgen receptor degradation by the E3 ligase CHIP modulates mitotic arrest in prostate cancer cells. Oncogene 33: 26–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stricher F, Macri C, Ruff M, Muller S. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy 9: 1937–1954, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu JW, Yang H, Yang M, Li P. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun 4: 1594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol 587: 1607–1617, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3: e2890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vila-Bedmar R, Lorenzo M, Fernández-Veledo S. Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 151: 980–992, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Vila-Brau A, De Sousa-Coelho AL, Goncalves JF, Haro D, Marrero PF. Fsp27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by FA oxidation rate. J Lipid Res 54: 592–601, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Heckmann BL, Zhang X, Smas CM, Liu J. Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol Endocrinol 27: 116–126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Wang X, He Y, Qi L, Yu L, Xue B, Shi H. The full capacity of AICAR to reduce obesity-induced inflammation and insulin resistance requires myeloid SIRT1. PLoS One 7: e49935, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P, Fernandes N, Dodge IL, Reddi AL, Rao N, Safran H, DiPetrillo TA, Wazer DE, Band V, Band H. ErbB2 degradation mediated by the co-chaperone protein CHIP. J Biol Chem 278: 13829–13837, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Hooper C1, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC. Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol 8: 72, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]