Abstract
Lungs are indispensable organs for the respiratory process, and maintaining their homeostasis is essential for human health and survival. However, during the lifetime of an individual, the lungs suffer countless insults that put at risk their delicate organization and function. Many cells of the immune system participate to maintain this equilibrium and to keep functional lungs. Among these cells, mast cells have recently attracted attention because of their ability to rapidly secrete many chemical and biological mediators that modulate different processes like inflammation, angiogenesis, cell proliferation, etc. In this review, we focus on recent advances in the understanding of the role that mast cells play in lung protection during infections, and of the relation of mast cell responses to type I hypersensitivity-associated pathologies. Furthermore, we discuss the potential role of mast cells during wound healing in the lung and its association with lung cancer, and how mast cells could be exploited as therapeutic targets in some diseases
Keywords: Allergic inflammation, cancer, lungs, mast cells, pathogens, wound healing.
INTRODUCTION
Paul Ehrlich described mast cells for the first time more than 100 years ago, through their characteristic metachromatic staining with aniline dyes. He noticed that these cells had abundant and dense granules; he assumed that the granules were caused by excessive feeding. These cells were abundant in areas near tumors, so Ehrlich postulated that they played a role in the protection against cancer [1]. Since then, many functions were proposed for mast cells in the regulation of organism homeostasis, but experimental evidence to support these assumptions was lacking [2]. The discovery of mice with a diminished number of mast cells, and their reconstitution with bone marrow-derived mast cells, provided a tool to test these hypotheses and to extend our knowledge about the functions of mast cells in the body [3].
Mast cells derive from hematopoietic precursors that are released from the bone marrow into the bloodstream and are recruited to connective tissues, where they complete their differentiation. Mast cells can live for months in the tissues, where they proliferate in response to different signals. The tissues that usually contain abundant mast cells are those that interact with the external environment (the skin and the gastrointestinal, urogenital and respiratory tracts), where mast cells are located near lymphatics and blood vessels, or close to nerve terminals or smooth muscle cells [4]. The morphology of mast cells depends on their anatomical location, ranging from rounded-shaped to elongated forms, when they are associated with blood vessels. Their size ranges from 5 to 20 µm and their cytoplasm contains abundant granules, with approximately 1,000 granules per cell [5].
Mast cell granules contain several chemical mediators, including histamine, proteoglycans, cytokines like TNF-α and IL-4, and neutral proteases like tryptase, chymase, carboxypeptidase and cathepsin G. Granule composition is not identical in all mast cells: human mast cells that reside in the skin have all the aforementioned neutral proteases, while epithelial lung mast cells have significant amounts of tryptase, but lack chymase, carboxypeptidase and cathepsin G. Accordingly, human mast cells are classified as either MCT (skin) or MT (lung) [6]. In addition to these pre-formed mediators that are stored in granules, mast cells are efficient producers of lipid-derived mediators, such as platelet-activating factor (PAF) and the arachidonic acid-derived prostaglandins, thromboxanes and leukotrienes. Mast cells are also efficient producers of many soluble protein mediators, such as cytokines, chemokines and antimicrobial peptides [6, 7]. Activation of mast cells induces the release of one group of these mediators or of all of them, depending on the triggering signal. However, not all the mediators are released at the same time: chemical mediators from the granules are secreted in seconds after stimulation, while lipid-derived mediators are produced minutes after activation and protein soluble mediators are detected hours after the initial trigger [4].
Mast cells produce abundant histamine, and since the discovery that histamine is a crucial element for the development of type I hypersensitivity, the relevance of mast cells for the initiation of allergic reactions was underscored [8]. By far, the most studied mast cell-activating signal is the cross-linking of FcεRI by IgE and allergen, an event that leads to mast cell degranulation and to the release of several chemical mediators that cause the immediate inflammatory response characteristic of type I hypersensitivity [9]. As a consequence, much of our knowledge of mast cell biology is biased towards their participation in this pathological process.
Type I hypersensitivity, or allergic inflammation, is an acute inflammation produced in sensitized individuals when they are exposed to the corresponding allergen. Sensitization occurs when dendritic cells capture an allergen in the airways, migrate to the regional lymph nodes and activate naïve T cells. In the presence of IL-4 (derived from mast cells, eosinophils or innate lymphoid cells), the T cells acquire a Th2 phenotype and produce IL-4 and IL-13. These cytokines promote class-switch recombination in B cells, which then produce allergen-specific IgE. IgE binds to FcεRI on mast cells, which are then primed for activation upon re-exposure to the allergen. During this subsequent exposure, the allergen cross-links FcεRI-bound IgE, which leads to mast cell activation and degranulation. The mediators derived from activated mast cells cause bronchoconstriction, vasodilation, increased vascular permeability, mucus production and recruitment of eosinophils and neutrophils. Repetitive or persistent exposure to the allergen leads to a chronic allergic inflammation, which causes tissue injury and remodeling; this last process includes the severe narrowing of the airway lumen observed in asthmatic patients [10].
However, mast cells are conserved in different groups of vertebrates [11], and from an evolutionary point of view, it makes no sense that humans should have conserved a cell population that causes a harmful and even deadly response if it conferred no evolutionary advantage. So, in the last years, the study of mast cells has been redirected to investigate their participation in processes such as innate immune response to pathogens, regulation of inflammation, wound healing and regulation of tumoral growth. In this review, we focus on the mechanisms used by mast cells in the lung to keep the proper function of this vital organ.
THE ROLE OF MAST CELLS DURING LUNG INFECTION
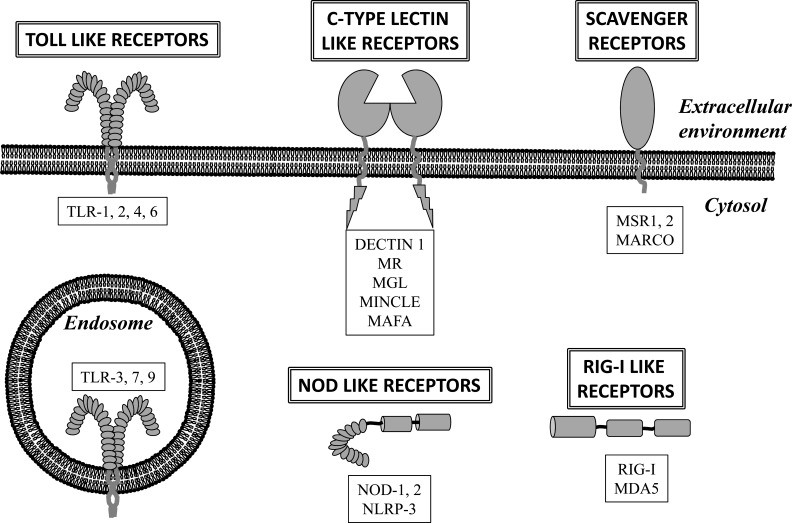
Mast cells have a vast collection of cell membrane receptors that allow them to sense the external environment. A set of these receptors are Pattern Recognition Receptors (PRR) that recognize Pathogen Associated Molecular Patterns (PAMP). These receptors are crucial for the initiation of the innate immune response, and they are classified in five groups: Toll-like receptors (TLR), Nod-like receptors (NLR), C-type Lectin Receptor (CLR), RIG-I-like Receptor (RIR) and scavenger receptors [12]. Mast cells express at least one receptor of each group, allowing them to recognize a multitude of microorganisms, including viruses, bacteria, protozoa, fungi and multicellular parasites [13-23] (Fig. 1).
Fig. (1).
Pattern Recognition Receptors (PRR) expressed by mast cells. Mast cells express receptors that belong to the five main families of PRR, which allows them to directly recognize pathogens. PRR are strategically distributed within the cellular environment to detect both extracellular and intracellular pathogens. TLR: Toll-like receptor, MR: Mannose receptor, MGL: Macrophage galactose-type lectin, MINCLE: Macrophage-Inducible C-type Lectin, MAFA: Mast cell function-associated antigen, NOD: Nucleotide-binding oligomerization domain, NLRP3: NLR family, pyrin-domain containing 3, MSR: Macrophage scavenger receptor, MARCO: Macrophage receptor with collagenous structure, RIG-I: Retinoic acid inducible gene 1, MDA5: Melanoma differentiation-associated gene 5.
The first evidence that mast cells are crucial elements of the immune response to pathogens came from a study of the infection of the rat gastrointestinal tract with multicellular parasites [24]. There are few studies that investigate if mast cells play a role in the containment of parasitic diseases in the lungs. Fungi are a group of pathogens that can establish infections in the lungs [25]. One of the firs studies that established a role for mast cells in fungal infections in vivo reported that, in a mouse model of infection with Sporothrix schenckii, mast cell depletion decreased the severity of cutaneous lesions [26]. Whether mast cells in the lungs plays a role in fungal infections needs to be evaluated, but in vitro experiments suggest that they do have a role, because they are activated by Aspergillus fumigates [27].
In contrast, bacterial infections of the lungs have been more studied. Malaviya et al. in 1996 identified for the first time that mast cells are essential for the early containment of bacterial infections in the lungs [28]; Klebsiella pneumonie, a gram-negative bacteria that causes community-acquired pneumonia [29], was among the bacteria tested in this study. The authors observed that the lung bacterial load was higher in mice deficient in mast cells, compared with wild-type mice, and this correlated with a defective recruitment of neutrophils to the lung. These results indicated that mast cells did not have a direct antimicrobial activity, but they induced inflammation through TNF-α, which allowed the recruitment of neutrophils [28].
Mast cells also participate in the immune response to Mycoplasma pneumoniae, a bacteria that is among the smallest free-living organisms and lacks a classical bacterial cell wall [30]. Airway infection of mice deficient in mast cells induced faster weight loss, more severe pneumonia, higher mortality and increased bacterial burden in the lungs, compared with wild-type mice. This work showed that mast cells play an important role in innate immune response against bacteria that infect lungs [31].
Tularemia is a life-threatening disease caused by Francisella tularensis, a gram-negative coccobacillus that can be spread through aerosols [32]. Intranasal infection of wild-type mice with F. tularensis induces an increased accumulation of mast cells in the lungs. Accordingly, mast cell-deficient mice showed impaired bacterial control and a significant decrease in IL-4 production. In fact, mast cells confer protection by inhibiting bacterial entry and replication in macrophages, thus showing a different mechanism by which mast cells confer protection against bacterial infection in the lungs [33].
Tuberculosis is one of the leading causes of death in the world. It is caused by Mycobacterium tuberculosis (Mtb), a bacteria that has a cell wall unique among bacteria, and that infects and usually persists in the lungs [34]. Studies with humans have shown an increase of mast cells in the lymph nodes of patients with tuberculous lymphadenitis [35]. In vitro studies demonstrated that Mtb activates mast cells, inducing degranulation, production of IL-6 and TNF-α and even bacterial internalization through lipid rafts [36, 37]. Moreover, in vivo experiments have suggested a role of mast cells in the containment of Mtb infection. In one of the first studies, mice received C48/80, a compound that induces mast cell degranulation, before infection with Mtb. These mice showed an altered production of cytokines and chemokines, which induced a decrease in lung inflammation and an increase in Mtb load, compared with untreated mice [38]. A second study used TLR-2 knockout mice, which are highly susceptible to Mtb infection. When these mice received mast cells that express TLR-2 before infection with Mtb, they had an improved inflammatory response, with myeloid cell recruitment and granuloma formation that correlated with increased protection against the bacilli [39]. These studies reinforce the notion of mast cells as crucial players of the lung innate immune response against bacterial pathogens.
Cytomegalovirus is a beta-herpes virus that causes interstitial pneumonia in immunocompromised patients and is a serious problem in patients with an organ transplant [40]. Infection of mast cell-deficient mice with cytomegalovirus is followed by high viral replication and delayed virus clearance from the lungs, compared with wild-type mice. Interestingly, this phenomenon was associated with a defective production of the chemokine CCL5 (also known as RANTES) and a decreased recruitment of CD8+ T cells into the infected lungs, providing evidence that mast cells can recognize viral infection and induce the recruitment of essential effector cells to the site of infection in the lung [41].
Influenza is a frequent infectious disease of the human respiratory tract, which is caused by a group of viruses from the Orthomyxoviridae family; the most common of these viruses is influenza A. These viruses have attracted attention because they are associated with pandemics that have severely affected human health, like the ‘Spanish flu’ of 1918 [42]. Interestingly, studies with this strain have reported alterations in lung architecture, which are caused by an excessive inflammatory response promoted by an uncontrolled production of inflammatory cytokines and chemokines, also known as ‘cytokine storm’ [43]. In a mouse model of infection with a pathogenic strain of H5N1 virus, lung lesions were reduced if mice were treated with ketotifen, a well-known mast cell degranulation inhibitor. Moreover, mice survival was dramatically increased if mice received a combined treatment of ketotifen and oseltamivir, an antiviral drug. These results suggest that mast cell activation correlated with pathology development during H5N1 infection, and that inhibition of mast cell activation improved host health [44]. Furthermore, Graham A et al. recently showed that the pathology associated with influenza A virus infection is significantly decreased in mast cell-deficient mice, compared with wild-type mice. This event is associated with a lower production of the inflammatory cytokine TNF-α and the chemokines CCL2, CCL3, CCL4, CXCL2 and CXCL10 in the lungs, suggesting a critical role of mast cells during the induction of the ‘cytokine storm’ [45].
The studies in mice described above show that mast cells play a pro-inflammatory role against bacterial and viral infections, with both beneficial and detrimental effects. On the one hand, mast cells are critical for the innate immune response because they recruit effector cells that are fundamental for the containment of the pathogens during the early stages of infection; this event has been observed in other organs besides the lungs. On the other hand, excessive mast cell activation by pathogens can lead to damage and loss of lung function, as evinced by influenza studies. This excessive mast cell activation has also been observed in calves with paroxystic respiratory distress syndrome caused by bovine respiratory syncitial virus [46]. Recent evidence indicates that mast cells also play a predominant role in the development of pathology during dengue virus infection. St John A, et al. observed a direct correlation between the serum levels of chymase and the severity of dengue disease in humans; interestingly, treatment of mice with drugs that block mast cell degranulation or with an antibody that blocks leukotriene receptors improved the vasculopathy associated with dengue virus infection [47]. These studies point to mast cells as a new therapeutic target to limit the damage provoked by excessive inflammation during infectious diseases, such as influenza and dengue.
THE REGULATORY FUNCTIONS OF MAST CELLS IN OTHER ORGANS
In contrast with their classical pro-inflammatory function, it has been reported that mast cells can produce significant amounts of anti-inflammatory cytokines, like IL-10. Mast cell-produced IL-10 plays a crucial role in limiting the sterile inflammation provoked by allergic contact dermatitis or UV radiation [48]. Moreover, mast cell-produced IL-10 is essential for the induction of immune suppression by UV exposure [49, 50]. Bacteria can also induce IL-10 production by mast cells; Chan C et al. recently demonstrated that mast cells produce IL-10 during in vivo bladder infection with uropathogenic Escherichia coli, and this event was necessary to maintain the tolerance that allowed bacterial persistence [51]. This study demonstrated that microorganisms can tune mast cells into a pro-inflammatory or an anti-inflammatory program in order to subvert the immune response.
Several microorganisms directly target mast cells in order to avoid an effective immune response. Choi et al, recently showed that Salmonella typhimurium injects a potent phosphatase (SptP) into the cytosol of mast cells; SptP interferes with mast cell activation, even with a very strong signal (FcεRI cross-linking). This strategy is also exploited by Yersinia pestis, which uses a phosphatase (YopH) with high homology to SptP to dampen mast cell activation [52]. Pathogenic bacteria are not the only ones that effectively suppress mast cell activation, symbiotic bacteria have established a delicate balance with the immune response in order to colonize and survive in different anatomical locations of the host [53]. Breakdown of such balance usually results in the development of different pathologies [54]. So, it is not surprising that bacteria that usually colonize the gastrointestinal tract can regulate mast cell activation. One of the first evidences of this strategy came from a study in which murine mast cells were stimulated with non-pathogenic E. coli from human gut. Unexpectedly, mast cells did not degranulate, even after incubation with a high bacterial load, which contrast with the swift degranulation in response to pathogenic E. coli. Furthermore, mast cells incubated with non-pathogenic E. coli lost the ability to degranulate in response to strong activators, such as A23187 and FcεRI cross-linking [55]. A similar phenomenon is also observed with Lactobacillus and Bifidobacterium, other probiotic and symbiotic bacteria that reside in the human gut [56, 57]. These results are of great interest in view of the association that exists between the use of probiotics and the prevention and treatment of several atopic diseases [58]. However, how this prevention and treatment are achieved is still a matter of discussion, and several mechanisms have been proposed to explain this phenomenon. Recent evidence derived from a mouse model of allergic inflammation points out that probiotics have the ability to decrease the effector phase in allergic diseases, even in the presence of specific IgE antibodies. This effect is related to the inhibition of mast cell activation through the alteration of the signaling pathways triggered by FcεRI [59]. The probiotics Bifidobacterium breve and Lactobacillus rhamnosus have been proposed as treatments for atopic diseases because of their potent effect in suppressing mucosal mast cell degranulation during chronic asthma [60].
IS THERE A CORRELATION BETWEEN MAST CELLS RESPONSE TO INFECTION AND TYPE I HYPERSENSITIVITY IN THE LUNGS?
As described in the previous section, mast cells play an essential role in the initiation of the innate immune response to pathogens. In fact, all the cells that participate in type I hypersensitivity also participate in type 2 immune responses, which protect against infections with bacteria, viruses and multicellular parasites, and participate in the clearance of venoms, xenobiotics and irritants [61].Type 2 innate lymphoid cells and Th2 lymphocytes produce IL-5, which induces eosinophil activation and recruitment; they also produce IL-9, which causes basophil and mast cell proliferation, and IL-13, which increases mucus production and causes fibrosis [61]. These cell populations constantly interact with each other: for example, type 2 innate lymphoid cells produce IL-13 in response to mast cell-derived prostaglandin D2, for which they have a specific receptor, CRTH2 [62].
Since mast cells play a fundamental role during the early stages of type I hypersensitivity and also during the initiation of the innate immune response to pathogens, two important questions arise: 1. Is the response of mast cells to pathogens affected in an allergic environment? 2. Can pathogens exacerbate type I hypersensitivity through mast cell activation?
In relation to the first question, there is considerable epidemiological evidence that asthma is a risk factor for viral and bacterial infections in the lungs, but what promotes this increased susceptibility to infections is poorly understood [63-67]. A deficient immune response could explain this increased susceptibility; for example, Jung J et al. observed that asthmatic patients have decreased titers of specific pneumococcal antibodies when compared to not-asthmatic individuals [68]. Other authors suggest a defective innate immune response as the cause of this increase in the susceptibility to infections. Several mechanisms of the innate immune response are altered in allergic patients, including a defective epithelial barrier, an excessive mucus production and a decreased production of cytokines [69]. However, this defective innate immune response apparently does not affect the potential of mast cell to combat infections, as is demonstrated in a mouse model of infection with Mycoplasma pneumonie in an allergic setting [70].
In relation to the second question, infectious diseases, especially those caused by bacteria and viruses, are considered important environmental elements that exacerbate atopic diseases [71]. Several reports indicate that infections with bacteria and viruses that affect the respiratory tract induce the production of IgE antibodies specific for bacterial or viral components. Infection of newborn mice with respiratory syncital virus (RSV) induces the production of virus-specific IgE, and reinfection of these mice caused the airway symptoms classically associated with an allergic response [72]. Moreover, in a mouse model of acute infection with influenza A virus, an enhanced susceptibility of the host to develop a severe allergic response to house dust-mite was described [73]. These studies suggest that the induction and the exacerbation of allergic responses by pathogens are associated with a modification of the environment that favors IgE production; however, another mechanism could be the exacerbation of mast cell responses. As we discussed earlier, several bacterial and viral pathogens can induce mast cell activation, inducing a pro-inflammatory environment that recruits effector immune cells. But how does a mast cell respond if it is simultaneously activated through FcεRI and through receptors that sense infection? Early studies suggested that this double stimuli results in an increased activation of mast cells. For example, mast cell lines activated through FcεRI and infected with rhinovirus release more histamine and produce more cytokines than mast cell lines activated with a single stimuli [74]. Patients with allergic rhinitis that were experimentally infected with rhinovirus showed increased levels of histamine and eosinphil recruitment in bronchoalveolar lavages, compared with allergic individuals that were not infected with rhinovirus, suggesting an altered mast cell response [75].
Several reports also associate infection with pathogenic bacteria with the development of an environment that facilitates or exacerbates the development of type I hypersensitivity. For example, the pathogenic bacteria Mycoplasma pneumoniae, Streptococcus pneumoniae, Haemophilus influenza and Chlamydia pneumoniae induce the production of IgE specific for bacterial components, suggesting that infection promotes an environment favorable for the development of allergies [76-78]. However, little information exists about how these pathogenic bacteria could affect mast cell activation through FcεRI. As mentioned above, the only bacteria that have been analyzed in this respect are symbiotic bacteria from the gastrointestinal tract (E. coli, Lactobacillus spp and Bifidobacterium spp), and these bacteria ameliorated FcεRI-mediated activation of mast cells, showing a therapeutic potential of these bacteria for allergic reactions in the gut. However, other symbiotic bacteria that colonize a different environment, the vagina, apparently do not mimic this effect [79]. Interestingly, new evidence has demonstrated that the human respiratory tract is home to several symbiotic bacteria, and environments that were thought to be sterile, like the bronchial tree, in fact contain about 2,000 bacterial genomes per cm2. Moreover, asthmatic patients showed altered bacterial communities in their airways, when compared with healthy individuals, with an increase in Haemophillus spp and a decrease in Prevotella spp [80]. How these changes in microbiota airway affect the progress of type I hypersensitivity and how they alter mast cell function are fields that need to be clarified in the future.
THE ROLE OF MAST CELLS IN WOUND HEALING OF THE LUNG
Wound healing is a biological process critical to maintain body homeostasis. This process develops in an orderly fashion, involving hemostasis, inflammation, cell proliferation and remodeling of the affected tissue. Alterations in the sequence, duration or intensity of any of these stages result in an impaired tissue repair that is usually reflected in the development of different pathologies [81]. Several cell populations are involved in this process, and cells of the immune system have been implied in different steps of the process. Mast cells are considered as important players in this process for several reasons. First, they tend to accumulate and degranulate in the wounded tissue, favoring the recruitment of effector cells, mainly neutrophils and macrophages [82]. The neutral proteases chymase and tryptase favor the degradation of the extracellular matrix [83, 84]; mast cell production of arachidonic acid-derivatives induces the production of type I collagen by fibroblasts [85], and mast cell production of Fibroblast Growth Factor (FGF), Vascular Endothelial Growth Factor (VEGF) and Transforming-Growth Factor β1 (TGF-β1) is considered important for fibroblast proliferation and for the formation of new blood vessels [86]. However, much of the knowledge about the role of mast cells during wound healing comes from studies in the skin, and little work has been performed in the lung, perhaps because of the lack of an appropriate model of lung injury, making this an interesting field that needs more research to clarify the role of mast cells in this environment.
THE ROLE OF MAST CELLS IN LUNG CANCER
Paul Ehrlich described that mast cells were abundant in areas near tumors, and assumed that these cells played an important role in the regulation of tumor growth more than 100 years ago [1]. Since then, several reports have confirmed that mast cells usually accumulate in areas near tumors, and that this accumulation is associated with a poor prognosis in breast cancer [87], melanoma [88], Hodgkin’s lymphoma [89], oral squamous cell carcinoma [90], renal carcinoma [91], pancreatic cancer [91], endometrial cancer [92], cervical cancer [93], etc. In 2000, Imada et al. observed that patients with stage I non-small cell lung adenocarcinoma had an increased number of mast cells in tumor areas around blood vessels, and those patients with higher mast cells counts had a worse prognosis [94]. The mechanisms that lead to mast cell accumulation in different tumors are still unclear. It has been suggested that tumor cells produce chemotactic mediators that attract mast cells to the tumor, but few of these chemotactic mediators have been identified. One example is Stem Cell Factor (SCF), which is produced by several tumor-derived cell lines, including a human alveolar adenocarcinoma cell line. SCF interacts with the receptor c-kit, which is expressed on mast cells, and directs their migration to the tumor [95].
How mast cells contribute to tumor growth is still matter of research, but three main mechanisms have been proposed: 1) production of tumor growth factors, 2) facilitation of tumor angiogenesis and 3) modification of the interaction between the epithelial cells and the stroma [96]. A mast cell product that leads to the proliferation of tumor cells is histamine, which promotes the proliferation of alveolar basal carcinoma cells [97]. Another product is adrenomedullin, which promotes the proliferation of lung cancer cells. Adrenomedullin-stimulated mast cells produce cytokines, such as Fibroblast Growth Factor (FGF) and Vascular Endothelial Growth Factor (VEGF), which are associated with increased angiogenesis in vivo [98]. Imada et al. observed an increase in the number of mast cells that produce VEGF in patients with lung adenocarcinoma [94]. In contrast with previous reports by several authors, Welsh et al. reported that, in non-small cell lung adenocarcinoma, the number of mast cells in the tumor stroma had no correlation with tumor progression, while an increased number of mast cells in the tumor cell islets correlated with an improved prognosis [99]. These results suggested an anti-tumor role for the infiltrating mast cells, but the mechanism was not investigated, and further studies are needed to clarify this issue.
CONCLUSION
Mast cells can trigger different mechanisms that contribute to the homeostasis and adequate function of the lungs. Disequilibrium in the function of mast cells can lead to pathological states, of which asthma is the most recognized. Mast cells play an essential role in type I hypersensitivity (allergic inflammation) and also during the early stages of the innate immune response to pathogens. Several causes, from infections to tumor growth, alter the function of mast cells (Fig. 2). The regulation of mast cell activation by conventional drugs or microorganisms, either pathogenic or symbiotic, opens a new window of opportunities to develop new treatments that could preserve and restore lung health.
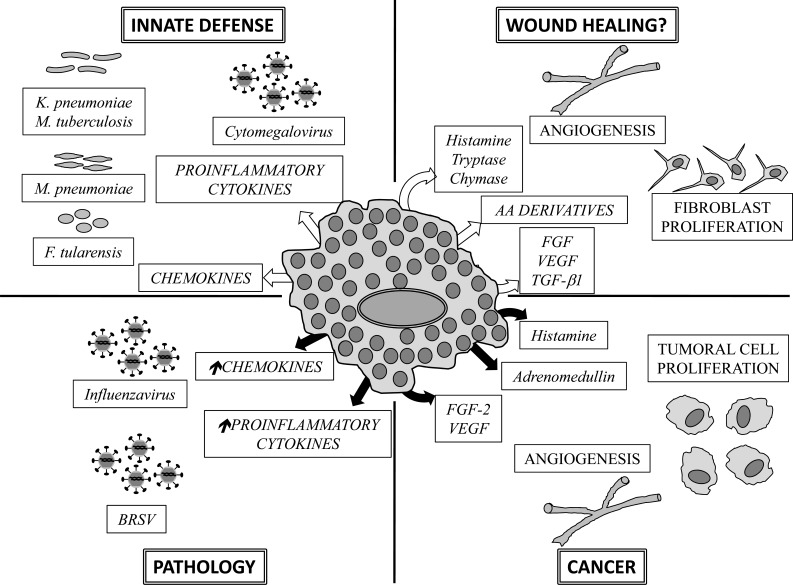
Fig. (2).
Functions of mast cells in the lungs. The dual role of mast cells in lung functions is depicted. White arrows indicate release of mediators that contribute to lung homeostasis. Black arrows designate liberation of mediators that contribute to pathological states in the lungs. Some mediators participate in lung homeostasis or contribute to pathology, depending on their concentration or on the timing of their production. AA: Arachidonic acid, FGF: Fibroblast Growth Factor, VEGF: Vascular Endothelial Growth Factor, TGF-β1: Transforming Growth Factor β1, BRSV: Bovine Respiratory Syncitial Virus. Arrows pointing up indicate exacerbated production.
ACKNOWLEDGEMENTS
This work was supported by SIP, IPN and Conacyt (157100 CB-2010-01).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Crivellato E, Beltrami C, Mallardi F, Ribatti D. Paul Ehrlich's doctoral thesis a milestone in the study of mast cells. Br J Haematol . 2003;123(1):19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 2.Maurer M, Theoharides T, Granstein RD , et al. What is the physiological function of mast cellsκ. Exp Dermatol. 2003;12(6):886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakano T, Sonoda T, Hayashi C , et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice.Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162(3):1025–43. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong LC. The mast cell origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997;49(6):409–24. doi: 10.1016/S0940-2993(97)80129-7. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 7.Di Nardo A, Vitiello A, Gallo RL. Cutting edge mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170(5):2274–8. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 8.Riley JF, West GB. The presence of histamine in tissue mast cells. J Physiol. 1953;120(4):528–37. doi: 10.1113/jphysiol.1953.sp004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2(10):773–86. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crivellato E, Ribatti D. The mast cell an evolutionary perspective. Biol Rev Camb Philos Soc . 2010;85(2):347–60. doi: 10.1111/j.1469-185X.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20(5):530–7. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Guthmann MD, Tal M, Pecht I. A new member of the C-type lectin family is a modulator of the mast cell secretory response. Int Arch Allergy Immunol. 1995;107(1-3):82–6. doi: 10.1159/000236938. [DOI] [PubMed] [Google Scholar]
- 14.Ribbing C, Engblom C, Lappalainen J , et al. Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy. 2011;66(1):110–9. doi: 10.1111/j.1398-9995.2010.02437.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Marshall JS. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology. 2009;214(4):321–30. doi: 10.1016/j.imbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Vukman KV, Ravida A, Aldridge AM, O'Neill SM. Mannose receptor and macrophage galactose-type lectin are involved in Bordetella pertussis mast cell interaction. J Leukoc Biol. 2013;94(3):439–48. doi: 10.1189/jlb.0313130. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Ushio H, Kawasaki J , et al. Expression and functional characterization of retinoic acid-inducible gene-I-like receptors of mast cells in response to viral infection. J Innate Immun . 2013;5(2):163–73. doi: 10.1159/000343895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JM, Swindle EJ, Kushnir-Sukhov NM, Holian A, Metcalfe DD. Silica-directed mast cell activation is enhanced by scavenger receptors. Am J Respir Cell Mol Biol. 2007;36(1):43–52. doi: 10.1165/rcmb.2006-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Feng BS, He SH , et al. Bacterial peptidoglycan breaks down intestinal tolerance via mast cell activation the role of TLR2 and NOD2. Immunol Cell Biol. 2007;85(7):538–45. doi: 10.1038/sj.icb.7100079. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Kambe N, Saito M , et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med. 2009;206(5):1037–46. doi: 10.1084/jem.20082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enoksson M, Ejendal KF, McAlpine S, Nilsson G, Lunderius-Andersson C. Human cord blood-derived mast cells are activated by the Nod1 agonist M-TriDAP to release pro-inflammatory cytokines and chemokines. J Innate Immun. 2011;3(2):142–9. doi: 10.1159/000321933. [DOI] [PubMed] [Google Scholar]
- 22.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol . 2002;14(9):1065–74. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173(1):531–41. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 24.Woodbury RG, Miller HR, Huntley JF , et al. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312(5993):450–2. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 25.Romani L, Puccetti P. Immune regulation and tolerance to fungi in the lungs and skin. Chem Immunol Allergy. 2008;94:124–37. doi: 10.1159/000154957. [DOI] [PubMed] [Google Scholar]
- 26.Romo-Lozano Y, Hernandez-Hernandez F, Salinas E. Mast cell activation by conidia of Sporothrix schenckii role in the severity of infection. Scand J Immunol . 2012;76(1):11–20. doi: 10.1111/j.1365-3083.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 27.Urb M, Pouliot P, Gravelat FN, Olivier M, Sheppard DC. Aspergillus fumigatus induces immunoglobulin E-independent mast cell degranulation. J Infect Dis. 2009;200(3):464–72. doi: 10.1086/600070. [DOI] [PubMed] [Google Scholar]
- 28.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381(6577):77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 29.Podschun R, Ullmann U. Klebsiella spp.as nosocomial pathogens epidemilogy taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Vargas FM, Gomez-Duarte OG. Mycoplasma pneumoniae-an emerging extra-pulmonary pathogen. Clin Microbiol Infect. 2008;14(2):105–17. doi: 10.1111/j.1469-0691.2007.01834.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Zhang D, Lyubynska N , et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173(2):219–25. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15(4):631–46. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ketavarapu JM, Rodriguez AR, Yu JJ , et al. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc Natl Acad Sci U S A. 2008;105(27):9313–8. doi: 10.1073/pnas.0707636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dannenberg AM ., Jr Perspectives on clinical and preclinical testing of new tuberculosis vaccines. Clin Microbiol Rev. 2010;23(4):781–94. doi: 10.1128/CMR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taweevisit M, Poumsuk U. High mast cell density associated with granulomatous formation in tuberculous lymphadenitis. Southeast Asian J Trop Med Public Health. 2007;38(1):115–9. [PubMed] [Google Scholar]
- 36.Munoz S, Hernandez-Pando R, Abraham SN, Enciso JA. Mast cell activation by Mycobacterium tuberculosis mediator release and role of CD48. J Immunol. 2003;170(11):5590–6. doi: 10.4049/jimmunol.170.11.5590. [DOI] [PubMed] [Google Scholar]
- 37.Munoz S, Rivas-Santiago B, Enciso JA. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand J Immunol. 2009;70(3):256–63. doi: 10.1111/j.1365-3083.2009.02295.x. [DOI] [PubMed] [Google Scholar]
- 38.Carlos D, de Souza Junior DA, de Paula L , et al. Mast cells modulate pulmonary acute inflammation and host defense in a murine model of tuberculosis. J Infect Dis . 2007;196(9):1361–8. doi: 10.1086/521830. [DOI] [PubMed] [Google Scholar]
- 39.Carlos D, Frantz FG, Souza-Junior DA , et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect . 2009;11(8-9):770–8. doi: 10.1016/j.micinf.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 40.José AG, Jesús CF, Dina VG , et al. Overview of clinical and molecular aspects involved in the development of symptomatic infection and end-organ disease caused by cytomegalovirus in pediatric patients.ytomegalovirus Infections Risk Fators. Causes and Management. 2012: 293–324. [Google Scholar]
- 41.Ebert S, Becker M, Lemmermann NA , et al. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog. 2014;10(4):e1004100. doi: 10.1371/journal.ppat.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe . 2010;7(6):440–51. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobasa D, Jones SM, Shinya K , et al. Aberrant innate immune response in lethal infection of macaques with the 1918, influenza virus. Nature. 2007;445(7125):319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Jin Y, Han D , et al. Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J Virol. 2012;86(6):3347–56. doi: 10.1128/JVI.06053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham AC, Hilmer KM, Zickovich JM, Obar JJ. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J Immunol . 2013;190(9):4676–84. doi: 10.4049/jimmunol.1202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jolly S, Detilleux J, Desmecht D. Extensive mast cell degranulation in bovine respiratory syncytial virus-associated paroxystic respiratory distress syndrome. Vet Immunol Immunopathol. 2004;97(3-4):125–36. doi: 10.1016/j.vetimm.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 47.St John AL, Rathore AP, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife. 2013;2:e00481. doi: 10.7554/eLife.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8(10):1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 49.Chacon-Salinas R, Limon-Flores AY, Chavez-Blanco AD, Gonzalez-Estrada A, Ullrich SE. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186(1):25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chacon-Salinas R, Chen L, Chavez-Blanco AD , et al. An essential role for platelet-activating factor in activating mast cell migration following ultraviolet irradiation. J Leukoc Biol. 2014;95(1):139–48. doi: 10.1189/jlb.0811409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38(2):349–59. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi HW, Brooking-Dixon R, Neupane S , et al. Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity. 2013;39(6):1108–20. doi: 10.1016/j.immuni.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 54.Zeissig S, Blumberg RS. Life at the beginning perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15(4):307–10. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 55.Magerl M, Lammel V, Siebenhaar F , et al. Non-pathogenic commensal Escherichia coli bacteria can inhibit degranulation of mast cells. Exp Dermatol. 2008;17(5):427–35. doi: 10.1111/j.1600-0625.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 56.Harata G, He F, Takahashi K , et al. Bifidobacterium suppresses IgE-mediated degranulation of rat basophilic leukemia (RBL-2H3):cells. Microbiol Immunol. 2010;54(1):54–7. doi: 10.1111/j.1348-0421.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 57.Oksaharju A, Kankainen M, Kekkonen RA , et al. Probiotic Lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J Gastroenterol. 2011;17(6):750–9. doi: 10.3748/wjg.v17.i6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalliomaki M, Salminen S, Arvilommi H , et al. Probiotics in primary prevention of atopic disease a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 59.Schiffer C, Lalanne AI, Cassard L , et al. A strain of Lactobacillus casei inhibits the effector phase of immune inflammation. J Immunol. 2011;187(5):2646–55. doi: 10.4049/jimmunol.1002415. [DOI] [PubMed] [Google Scholar]
- 60.Sagar S, Morgan ME, Chen S , et al. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir Res. 2014;15:46. doi: 10.1186/1465-9921-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484(7395):465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnig C, Cernadas M, Dutile S , et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jung JA, Kita H, Yawn BP , et al. Increased risk of serious pneumococcal disease in patients with atopic conditions other than asthma. J Allergy Clin Immunol. 2010;125(1):217–21. doi: 10.1016/j.jaci.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juhn YJ, Kita H, Yawn BP , et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122(4):719–23. doi: 10.1016/j.jaci.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baraldo S, Contoli M, Bazzan E , et al. Deficient antiviral immune responses in childhood distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130(6):1307–14. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Kim YJ, Ryu SL, Jung SH , et al. Increased Prevalence of H1N1-Induced Severe Lower Respiratory Tract Diseases in Children With Atopic Sensitization. Allergy Asthma Immunol Res. 2012;4(5):277–83. doi: 10.4168/aair.2012.4.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections in adults with atopic disease and IgE antibodies to common aeroallergens. PLoS One. 2013;8(7):e68582. doi: 10.1371/journal.pone.0068582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung JA, Kita H, Dhillon R , et al. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad Med. 2010;122(5):116–24. doi: 10.3810/pgm.2010.09.2208. [DOI] [PubMed] [Google Scholar]
- 69.James KM, Peebles RS , Jr, Hartert TV. Response to infections in patients with asthma and atopic disease an epiphenomenon or reflection of host susceptibilityκ. J Allergy Clin Immunol. 2012;130(2):343–51. doi: 10.1016/j.jaci.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michels NM, Chu HW, LaFasto SC , et al. Mast cells protect against airway Mycoplasma pneumoniae under allergic conditions. Clin Exp Allergy. 2010;40(9):1406–13. doi: 10.1111/j.1365-2222.2010.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sykes A. Asthma and infections An update on asthma exacerbations. Clinical Pulmonary Medicine. 2013;20(2):56–60. [Google Scholar]
- 72.Dakhama A, Lee YM, Ohnishi H , et al. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol. 2009;123(1):138–45 e5. doi: 10.1016/j.jaci.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Al-Garawi AA, Fattouh R, Walker TD , et al. Acute, but not resolved, influenza A infection enhances susceptibility to house dust mite-induced allergic disease. J Immunol. 2009;182(5):3095–104. doi: 10.4049/jimmunol.0802837. [DOI] [PubMed] [Google Scholar]
- 74.Hosoda M, Yamaya M, Suzuki T , et al. Effects of rhinovirus infection on histamine and cytokine production by cell lines from human mast cells and basophils. J Immunol. 2002;169(3):1482–91. doi: 10.4049/jimmunol.169.3.1482. [DOI] [PubMed] [Google Scholar]
- 75.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94(6):2200–8. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seggev JS, Sedmak GV, Kurup VP. Isotype-specific antibody responses to acute Mycoplasma pneumoniae infection. Ann Allergy Asthma Immunol. 1996;77(1):67–73. doi: 10.1016/S1081-1206(10)63482-5. [DOI] [PubMed] [Google Scholar]
- 77.Kjaergard LL, Larsen FO, Norn S , et al. Basophil-bound IgE and serum IgE directed against Haemophilus influenzae and Streptococcus pneumoniae in patients with chronic bronchitis during acute exacerbations. APMIS. 1996;104(1):61–7. [PubMed] [Google Scholar]
- 78.Emre U, Sokolovskaya N, Roblin PM, Schachter J, Hammerschlag MR. Detection of anti-Chlamydia pneumoniae IgE in children with reactive airway disease. J Infect Dis. 1995;172(1):265–7. doi: 10.1093/infdis/172.1.265. [DOI] [PubMed] [Google Scholar]
- 79.Brzeziκska-Blaszczyk E, Wasiela M. Vaginal bacterial flora activates rat peritoneal mast cells. International Journal of Immunopathology and Pharmacology. 2002;15(3):233–8. doi: 10.1177/039463200201500310. [DOI] [PubMed] [Google Scholar]
- 80.Hilty M, Burke C, Pedro H , et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azevedo LH, De Sousa SCOM, Correa L , et al. Mast cell concentration in the wound healing process of incisions made by different instruments. Lasers in Medical Science. 2009;24(4):585–90. doi: 10.1007/s10103-008-0616-5. [DOI] [PubMed] [Google Scholar]
- 83.Lazaar AL, Plotnick MI, Kucich U , et al. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J Immunol. 2002;169(2):1014–20. doi: 10.4049/jimmunol.169.2.1014. [DOI] [PubMed] [Google Scholar]
- 84.Fajardo I, Pejler G. Human mast cell beta-tryptase is a gelatinase. J Immunol. 2003;171(3):1493–9. doi: 10.4049/jimmunol.171.3.1493. [DOI] [PubMed] [Google Scholar]
- 85.Abe M, Kurosawa M, Ishikawa O, Miyachi Y. Effect of mast cell-derived mediators and mast cell-related neutral proteases on human dermal fibroblast proliferation and type I collagen production. J Allergy Clin Immunol. 2000;106(1 Pt 2):S78–84. doi: 10.1067/mai.2000.106058. [DOI] [PubMed] [Google Scholar]
- 86.Shiota N, Nishikori Y, Kakizoe E , et al. Pathophysiological role of skin mast cells in wound healing after scald injury study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol. 2010;151(1):80–8. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- 87.Kankkunen JP, Harvima IT, Naukkarinen A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer. 1997;72(3):385–8. doi: 10.1002/(sici)1097-0215(19970729)72:3<385::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 88.Duncan LM, Richards LA, Mihm MC ., Jr Increased mast cell density in invasive melanoma. J Cutan Pathol. 1998;25(1):11–5. doi: 10.1111/j.1600-0560.1998.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 89.Molin D, Edstrom A, Glimelius I , et al. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol. 2002;119(1):122–4. doi: 10.1046/j.1365-2141.2002.03768.x. [DOI] [PubMed] [Google Scholar]
- 90.Iamaroon A, Pongsiriwet S, Jittidecharaks S , et al. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32(4):195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 91.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur Urol. 2006;50(3):530–4. doi: 10.1016/j.eururo.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 92.Ribatti D, Finato N, Crivellato E , et al. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193(6):1961–5. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 93.Benitez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49(8):1061–2. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- 94.Imada A, Shijubo N, Kojima H, Abe S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir. J. 2000;15(6):1087–93. doi: 10.1034/j.1399-3003.2000.01517.x. [DOI] [PubMed] [Google Scholar]
- 95.Huang B, Lei Z, Zhang GM , et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269–79. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galinsky DS, Nechushtan H. Mast cells and cancer--no longer just basic science. Crit Rev Oncol Hematol. 2008;68(2):115–30. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Stoyanov E, Uddin M, Mankuta D, Dubinett SM, Levi-Schaffer F. Mast cells and histamine enhance the proliferation of non-small cell lung cancer cells. Lung Cancer. 2012;75(1):38–44. doi: 10.1016/j.lungcan.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 98.Zudaire E, Martinez A, Garayoa M , et al. Adrenomedullin is a cross-talk molecule that regulates tumor and mast cell function during human carcinogenesis. Am J Pathol. 2006;168(1):280–91. doi: 10.2353/ajpath.2006.050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Welsh TJ, Green RH, Richardson D , et al. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23(35):8959–67. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]