Abstract
The aim of our study was to evaluate the association between polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and the risk for congenital heart disease (CHD). Electronic literature databases were searched to identify eligible studies published before Jun, 2014. The association was assessed by the odds ratio (OR) with a 95% confidence interval (CI). The publication bias was explored using Begg's test. Sensitivity analysis was performed to evaluate the stability of the crude results. A total of 35 studies were included in this meta-analysis. For the MTHFR C677T polymorphism, we detected significant association in all genetic models for Asian children and the maternal population. Significant association was also detected in T vs. C for a Caucasian paediatric population (OR = 1.163, 95% CI: 1.008–1.342) and in both T vs. C (OR = 1.125, 95% CI: 1.043–1.214) and the dominant model (OR = 1.216, 95% CI:b1.096–1.348) for a Caucasian maternal population. For the MTHFR A1298C polymorphism, the association was detected in CC vs. AC for the Caucasian paediatric population (OR = 1.484, 95% CI: 1.035–2.128). Our results support the MTHFR -677T allele as a susceptibility factor for CHD in the Asian maternal population and the -1298C allele as a risk factor in the Caucasian paediatric population.
Congenital heart disease (CHD) is the most frequently occurring congenital disorder in newborns and is the most frequent cause of infant death from birth defects. The aetiology of CHD is largely unknown. Epidemiological studies reveal a significant environmental contribution to the pathogenesis of CHD1,2. Familial aggregation and twin studies indicate the presence of genetic factors for susceptibility to this condition3,4,5. Except for a few types of CHD induced by a single gene mutation, the majority of CHDs are polygenic diseases affected by both genetic and environmental factors.
The importance of genetic factors in the development of CHD is also supported by recent data from genome-wide association studies (GWASs). Data from these studies have confirmed that a region on chromosome 4p16 adjacent to the MSX1 and STX18 genes was associated with the risk of ostium secundum atrial septal defect (ASD)6, and rs2228638 in NRP1 on 10p11 significantly increased the risk of Tetralogy of Fallot (TOF)7. In our studies, we identified HOMEZ and PLAGL1 as pathogenic genes in Chinese patients with isolated ventricular septal defects (VSDs)8,9. In addition, our proteomic study revealed plasma protein changes in CHD patients10.
The 5,10-methylenetetrahydrofolate reductase (MTHFR) gene is located on chromosome 1 at 1p36.3. MTHFR is the key metabolic enzyme of homocysteine (Hcy). It catalyses 5,10-methylenetetrahydrofolate reduction to 5-methyltetrahydrofolate, which as a methyl donor induces Hcy remethylation to methionine11. A common C677T mutation (rs1801133) in the MTHFR gene has been described, which results in the conversion of the amino acid alanine to valine at position 226 in the protein. This mutation was associated with a 50% reduction of MTHFR enzyme activity, an increase in plasma Hcy concentration and a decrease in plasma folic acid concentration. Another polymorphism (A1298C, rs1801131) is located in exon 7, within the presumptive regulatory domain, and results in a glutamate-to-alanine change with decreased enzyme activity in vitro12. It has been reported that MTHFR polymorphisms play important roles in diseases. For example, neural tube defects and pregnancy complications appear to be linked to impaired MTHFR function13,14.
Since Wenstrom first noted an association between MTHFR gene polymorphism and susceptibility to CHD15, other studies have been undertaken to replicate this work. However, previous case-control reports have yielded inconsistent results. Wang and co-workers carried out a meta-analysis involving 2,554 CHD patients and 3,838 controls by searching the electronic literature for articles published before July 22, 2012. They suggested that the infant and maternal MTHFR C667T polymorphism may be associated with an increased occurrence of CHD16. By contrast, Mamasoula and co-workers indicated that the MTHFR C677T polymorphism, which directly influences plasma folate levels, is not associated with the risk of CHD17. Therefore, we performed an up-dated meta-analysis of all published studies (until Jun, 2014) to investigate the association between MTHFR polymorphisms (C677T and A1298C) and the risk of CHD.
Methods
Search strategy
We conducted a comprehensive search of Embase, Ovid, Web of Science, the Cochrane database, Medline (PubMed), the Chinese Biomedical Literature Database (CBM-disc, 1979–2014), the database of National Knowledge Infrastructure (CNKI, 1979–2014) and the full paper database of Chinese Science and Technology of Chongqing (VIP, 1989–2014) to identify suitable studies published before Jun, 2014. The following keywords were used for searching: (“congenital heart” OR “congenital cardiac” OR “heart defect*” OR “congenital car*”) AND (“polymorphism*” OR “variant*”) AND (“methylenetetrahydrofolate reductase” OR “MTHFR”). The most complete and recent results were used when there were multiple publications from the same study group. The references of reviews and retrieved articles were also searched simultaneously to find additional eligible studies.
Inclusion criteria
Two investigators reviewed all identified studies independently to determine whether an individual study was eligible for inclusion. The selection criteria for studies to be considered for this meta-analysis were as follows: 1) MTHFR polymorphisms in CHD; 2) case-control or case-cohort study; 3) proper CHD diagnosis criteria; 4) original data; 5) human subjects, not animal studies. We expected the clinical assessment of the patients to include anthropometric measurement and physical examination for dysmorphism and malformation, and diagnostic studies to include chest X-ray examination, electrocardiogram, ultrasonic echocardiogram, etc. Studies would be excluded if the necessary information could not be obtained.
Data extraction
Two investigators extracted the data independently, and a third investigator reviewed the result. The following information was extracted from each study: first author, year of publication, study population (country, ethnicity), the number of patients and controls in the study, genotype information, genotype methods, and main types of CHD. If any data essential to the analysis were not available from a study, best efforts were made to contact the authors to fill in the missing data.
Statistical analysis
Allele frequencies for the MTHFR (C677T and A1298C) polymorphisms from each study were determined by the allele counting method18. The genotype distributions of controls were used to estimate the frequency of the putative risk allele (-677T and -1298C) using the inverse variance method19,20. The Hardy-Weinberg Equilibrium (HWE) is the most fundamental rule of population genetics. It prescribes the genotype frequencies at a locus in terms of its allele frequencies in a population. In the most general form, it states that selection, migration, and random genetic drift occur with random mating in a population in the absence of mutation21. The deviation from HWE for the distribution of the allele frequencies was analysed by Fisher's exact test in control groups. We examined the contrast of a vs. A, aa vs. AA, aa vs. Aa and also examined the recessive genetic model (aa vs. AA+Aa) and the dominant genetic model (Aa+aa vs. AA). The associations between MTHFR polymorphisms and CHD susceptibility were estimated by OR and its 95% CI. The significance of the pooled OR was determined by the Z-test; P < 0.05 was considered statistically significant. To evaluate the specific effects of ethnicity, stratified analyses were performed.
Heterogeneity across the eligible studies was tested using the Q-test, and the results were considered statistically significant when P < 0.122,23. Heterogeneity was also quantified with the I2 metric (I2 = (Q - df)/Q × 100%; I2 < 25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 > 75%, extreme heterogeneity). When the effects were assumed to be homogenous (P > 0.1, I2 < 50%), the fixed-effects model was used; otherwise, the random-effects model was more appropriate24,25,26. Sensitivity analysis was performed to evaluate the stability of the results. If more than seven studies were included, Begg's test was used to measure publication bias, which was shown as a funnel plot27,28. P < 0.05 was considered representative of statistically significant publication bias. All analyses were performed using STATA software, version 10.0 (Stata Corporation, College Station, TX, USA), Review Manager (RevMan version 5.1.1, The Nordic Cochrane Centre: http://ims.cochrane.org/revman/download) and R statistical software (version 2.15.2, http://www.r-project.org).
Results
Studies included in the meta-analysis
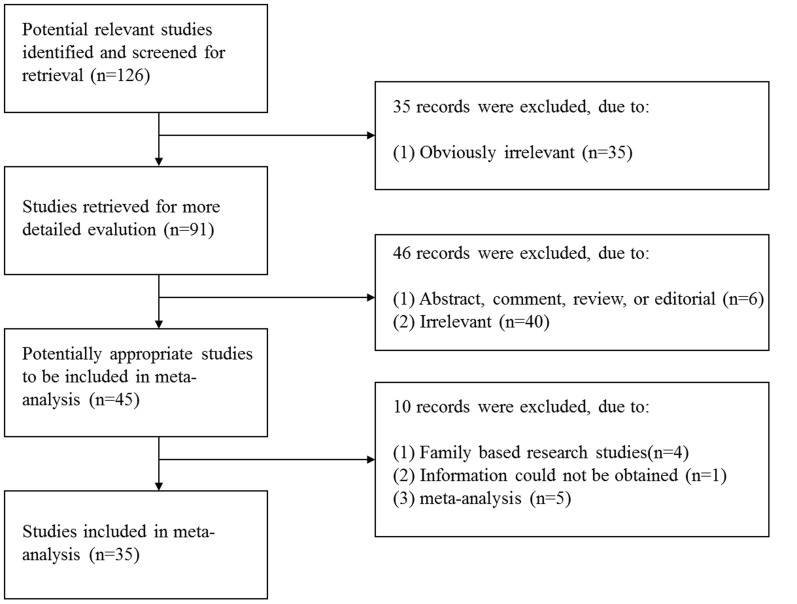
A total of 126 abstracts that met the inclusion criteria were retrieved through the databases. Two reviewers then selected the relevant studies independently. Forty-five relevant studies that described the association between the MTHFR polymorphism and CHD were identified. However, after reading the full articles and contacting the authors, we excluded five meta-analysis studies29,30,31,32,33, four family-based studies34,35,36,37, and one study in which information could not be obtained even after the authors were contacted38. Figure 1 shows the process of study selection and exclusion, with specification of reasons. Finally, 35 studies that met the inclusion criteria, corresponding to 9,329 CHD children and 15,076 normal controls, 3,232 mothers with CHD offspring and 27,174 normal controls for the C677T polymorphism and 1,761 CHD children and 1,868 normal controls/705 mothers with CHD offspring and 15,458 controls for the A1298C polymorphism, were considered in the meta-analysis15,17,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71. The main characteristics of the included studies are listed in Table 1–2.
Figure 1. Flow chart of the study selection process and specific reasons for exclusion from the meta-analysis.
Table 1. The detailed characteristics of all eligible studies for MTHFR C677T polymorphism.
| MTHFR C677T | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Mother | |||||||||||||||||
| Cases | Controls | Cases | Controls | |||||||||||||||
| Study | Year | Country (Ethnicity **) | CC | CT | TT | CC | CT | TT | HWE | CC | CT | TT | CC | CT | TT | HWE | Methods | Main types of CHD *</p> |
| Junker et al | 2001 | Germany (C) | 52 | 42 | 21 | 129 | 78 | 21 | 0.087 | — | — | — | — | — | — | — | PCR-RFLP | PS, HLHS, CoA, AVS, d-TGA, ASD, VSD, AVSD, TOF, PDA, DIV, PA, TA, Ebstein's Anomaly. |
| Wenstrom et al | 2001 | USA(90% C) | 17 | 8 | 1 | 104 | 9 | 3 | 0.006 | — | — | — | — | — | — | — | PCR-RFLP | HLV, HRV, CoA, PS, PA, TA, LVA, Atrioventricular Canal, Truncus Arteriosus, DORA, ASD, VSD. |
| Liu et al | 2002 | China (A) | — | — | — | — | — | — | — | 5 | 14 | 8 | 2 | 15 | 3 | 0.068 | PCR-RFLP | VSD ASD, TOF, PDA, Single Atrium/Ventricle |
| Storti et al | 2003 | Italy (C) | 28 | 55 | 20 | 52 | 108 | 40 | 0.259 | 27 | 53 | 23 | 52 | 108 | 40 | 0.259 | PCR-RFLP | VSD,TOF, DORV, PA, d-TGA, AC |
| Nurk et al | 2004 | Norway (C) | — | — | — | — | — | — | — | 12 | 12 | 1 | 7165 | 6037 | 1282 | 0.842 | RT-PCR | Congenital Anomalies Heart |
| Li et al | 2005 | China (A) | 32 | 94 | 61 | 20 | 57 | 25 | 0.320 | 32 | 90 | 61 | 20 | 57 | 25 | 0.320 | PCR-RFLP | VSD, ASD, PDA, TOF, |
| LEE et al | 2005 | China (A) | 110 | 89 | 14 | 114 | 68 | 13 | 0.556 | — | — | — | — | — | — | — | PCR-DHPLC | AP Window, ASD, CoA, PS, DILV, DORV, ECD, IAA, LAI, PA, PDA, RAI, TGA, TOF, VSD. |
| Shaw et al | 2005 | USA (C) | 69 | 68 | 16 | 180 | 202 | 52 | 0.753 | — | — | — | — | — | — | — | ARRAY | TOF, d-TGA, Truncus Arteriosus, DORV, PA, VSD, AP-Window |
| Hobbs et al | 2006 | USA (C) | — | — | — | — | — | — | — | 127 | 118 | 30 | 48 | 56 | 14 | 0.841 | SEQUENCE | Nonsyndromic Septal, Conotruncal, or right- or left-sided ObstructiveHeart Defect |
| Zhu et al | 2006 | China (A) | 7 | 22 | 27 | 22 | 57 | 24 | 0.328 | 6 | 27 | 23 | 20 | 57 | 25 | 0.320 | PCR-RFLP | ASD, PDA |
| Zhong et al | 2006 | China (A) | — | — | — | — | — | — | — | 67 | 33 | 15 | 76 | 34 | 5 | 0.558 | PCR-RFLP | Congenital Heart Disease |
| van Beynum et al | 2006 | Netherlands (C) | 79 | 66 | 20 | 98 | 104 | 18 | 0.216 | 72 | 68 | 18 | 131 | 107 | 23 | 0.881 | PCR-RFLP | TOF, VSD, Truncus Arteriosus, TGA, AP-Window, TVA, AVSD, PS, AS, HLHS, CoA, PDA, |
| Galdieri et al | 2007 | Brazil (M) | 30 | 21 | 7 | 18 | 14 | 6 | 0.286 | 27 | 15 | 5 | 10 | 15 | 1 | 0196 | PCR-RFLP | Congenital Heart Defects |
| Wintner et al | 2007 | Austria (C) | — | — | — | — | — | — | — | 16 | 12 | 3 | 10 | 17 | 4 | 0.708 | ARRAY | TOF,HLHS, TGA, DORV, VSD, AS, CoA, PS, Anomalies of the Aortic Arch |
| Liu et al | 2007 | China (A) | 30 | 68 | 34 | 46 | 48 | 13 | 0.829 | — | — | — | — | — | — | — | PCR-RFLP | Congenital Heart Disease |
| van Driel et al | 2008 | Netherlands(C) | 99 | 103 | 27 | 119 | 107 | 25 | 0.884 | 91 | 117 | 22 | 111 | 104 | 36 | 0.166 | PCR-RFLP | TOF, TGA, ASD, VSD, CoA, AS, PS, HLHS, |
| Marinho et al | 2009 | Portugal (M) | 12 | 20 | 6 | 113 | 124 | 14 | 0.073 | — | — | — | — | — | — | — | PCR-RFLP | TOF |
| Obermann-Borst et al | 2010 | Netherlands (C) | 64 | 66 | 9 | 92 | 76 | 15 | 1.000 | — | — | — | — | — | — | — | PCR-RFLP | TOF, TGA, ASD, VSD, CoA, AS, PS, HLHS |
| Hobbs et al | 2010 | USA (C) | — | — | — | — | — | — | — | 285 | 203 | 65 | 191 | 128 | 37 | 0.036 | SEQUENCE | Nonsyndromic Septal, Conotruncal, or Right- or Left-sided ObstructiveHeart Defect |
| Xu et al | 2010 | China (A) | 162 | 244 | 96 | 151 | 261 | 115 | 0.930 | — | — | — | — | — | — | — | PCR-RFLP | Cyanotic Cardiac Disease, ASD, VSD, PDA, Left-sided Obstruction Defects |
| García-Fragoso et al | 2010 | Puerto Rico (M) | 9 | 14 | 4 | 84 | 115 | 21 | 0.056 | 10 | 11 | 6 | 84 | 115 | 21 | 0.056 | PCR-RFLP | HLHS, TOF, DORV, TGA, VSD, PS, AS, CoA, ASD, Ebstein's Anomaly. |
| Kuehl et al | 2010 | USA (C) | 12 | 33 | 10 | 134 | 124 | 32 | 0.688 | — | — | — | — | — | — | — | ARRAY | CoA |
| Weiner et al | 2012 | Russia (C) | — | — | — | — | — | — | — | 18 | 21 | 6 | 173 | 149 | 26 | 0.514 | RT-PCR | Congenital Anomalies-cardiovascular System |
| Zhou et al | 2012 | China (A) | 23 | 60 | 53 | 88 | 126 | 63 | 0.183 | — | — | — | — | — | — | — | PCR-RFLP | TOF |
| Pishva et al | 2013 | Malaysia (SA) | 63 | 60 | 0 | 71 | 54 | 0 | 0.001 | — | — | — | — | — | — | — | PCR-RFLP | VSD |
| Mamasoula et al | 2013 | UK(M) | 2759 | 2430 | 625 | 4826 | 4114 | 1116 | 0.000 | 336 | 396 | 97 | 4826 | 4114 | 1116 | 0.000 | SEQUENCE | Congenital Heart Disease |
| Wang et al | 2013 | China(A) | 59 | 76 | 25 | 53 | 100 | 35 | 0.377 | — | — | — | — | — | — | — | SEQUENCE | Congenital Heart Disease |
| Jing et al | 2013 | China (A) | 46 | 42 | 16 | 39 | 114 | 55 | 0.164 | — | — | — | — | — | — | — | PCR-RFLP | Congenital Heart Disease |
| Sahiner et al | 2013 | Turkey(C) | 69 | 53 | 14 | 47 | 39 | 7 | 1.000 | — | — | — | — | — | — | — | PCR-RFLP | Obstruction in LV Output, Left-to-right Shunt, Conotruncal Anomalies, Complex Anomalies |
| Zidan et al | 2013 | Egypt (AR) | 18 | 21 | 41 | 32 | 21 | 27 | 0.000 | 21 | 30 | 29 | 31 | 25 | 24 | 0.001 | PCR-RFLP | ASD, VSD, PDA, PS, TOF, HLHS, Combined Lesion |
| Balderra' bano-Saucedo et al | 2013 | Mexico (M) | — | — | — | — | — | — | — | 7 | 12 | 12 | 24 | 31 | 7 | 0.595 | PCR-RFLP | Complex Congenital Heart Disease |
| Christensen et al *** | 2013 | USA (C) | 68 | 61 | 28 | 35 | 26 | 8 | 0.395 | 67 | 89 | 26 | 27 | 29 | 9 | 0.791 | PCR-RFLP | VSD, TOF, AS, TGA, AVSD, DORV, PS, CoA, Truncus Arteriosus |
| Wang et al | 2013 | China (A) | 33 | 92 | 111 | 88 | 126 | 63 | 0.183 | 39 | 100 | 96 | 82 | 129 | 66 | 0.279 | PCR-RFLP | VSD, ASD, PDA, TOF, DORV |
| Huang et al | 2014 | China (A) | 63 | 45 | 60 | 84 | 72 | 48 | 0.000 | — | — | — | — | — | — | — | MASS SPECTRUM | TOF |
| Chao et al | 2014 | China (A) | 10 | 5 | 2 | 19 | 12 | 3 | 0.660 | — | — | — | — | — | — | — | PCR-RFLP | PDA |
*: PS: Pulmonary Stenosis; HLHS: Hypoplastic Left Heart Syndrome; CoA: Coarctation of the Aorta; AVS: Aortic Valve Stenosis; TGA: Transposition of Great Arteries; ASD: Atrial Septal Defect; VSD: Ventricular Septal Defect; AVSD: Atrioventricular Septal Defect; TOF: Tetralogy of Fallot; PDA: Patent Ductus Arteriosus; DIV: Double Inlet Ventricle; PA: Pulmonary Atresia; TA: Tricuspid Atresia; HLV: Hypoplastic Left Ventricle; HRV: Hypoplastic Right Ventricle; LVA: Left Ventricular Aneurysm; DORV: Double-outlet Right Ventricle; AC: Aortic Coarctation; AP window: Atriopulmonary window; ECD: Endocardial Cushion Defect; IAA: Interrupted Aortic Arch; LAI: Left Atrial Isomerism; RAI: Right Atrial Isomerism; TVA: Tricuspid Valve Atresia; AS: Aortic Stenosis.
**: C: Caucasians; A: South Asians; M: Mixed; AR: Arabian.
***: The data was respectively provided by author of Dr. Karen E. Christensen (see Acknowledgements).
Table 2. The detailed characteristics of all eligible studies for MTHFR A1298C polymorphism.
| MTHFR A1298C | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Mother | |||||||||||||||||
| Cases | Controls | Cases | Controls | |||||||||||||||
| Study | Year | Country (Ethnicity **) | AA | AC | CC | AA | AC | CC | HWE | AA | AC | CC | AA | AC | CC | HWE | Methods | Main types of CHD * |
| Storti et al | 2003 | Italy (C) | 45 | 47 | 11 | 101 | 86 | 13 | 0.387 | 49 | 46 | 8 | 101 | 86 | 13 | 0.387 | PCR-RFLP | VSD,TOF, DORV, PA, d-TGA, AC |
| Nurk et al | 2004 | Norway (C) | — | — | — | — | — | — | — | 9 | 13 | 3 | 6607 | 6342 | 1525 | 0.955 | RT-PCR | Congenital Anomalies Heart |
| Galdieri et al | 2007 | Brazil (M) | 35 | 21 | 1 | 19 | 16 | 3 | 1.000 | 26 | 17 | 4 | 15 | 10 | 1 | 1.000 | PCR-RFLP | Congenital Heart Defects |
| van Driel et al | 2008 | Netherlands (C) | 112 | 90 | 27 | 97 | 129 | 25 | 0.073 | 104 | 102 | 24 | 116 | 104 | 31 | 0.319 | PCR-RFLP | TOF, TGA, ASD, VSD, CoA, AS, PS, HLHS, |
| Obermann-Borst et al | 2010 | Netherlands (C) | 69 | 57 | 13 | 75 | 90 | 18 | 0.256 | — | — | — | — | — | — | — | PCR-RFLP | TOF, TGA, ASD, VSD, CoA, AS, PS, HLHS |
| Xu et al | 2010 | China (A) | 316 | 168 | 18 | 326 | 185 | 16 | 0.110 | — | — | — | — | — | — | — | PCR-RFLP | Cyanotic Cardiac Disease, ASD, VSD, PDA, Left-sided Obstruction Defects. |
| Weiner et al | 2012 | Russia (C) | — | — | — | — | — | — | — | 33 | 13 | 2 | 168 | 152 | 42 | 0.403 | RT-PCR | Congenital Anomalies-cardiovascular System |
| Wang et al | 2013 | China (A) | 115 | 45 | 10 | 133 | 47 | 8 | 0.186 | — | — | — | — | — | — | — | SEQUENCE | Congenital Heart Disease |
| Sahiner et al | 2013 | Turkey (C) | 45 | 68 | 24 | 31 | 54 | 8 | 0.029 | — | — | — | — | — | — | — | PCR-RFLP | Obstruction in LV Output, Left-to-right Shunt, Conotruncal Anomalies, Complex Anomalies |
| Zidan et al | 2013 | Egypt (AR) | 16 | 27 | 37 | 26 | 24 | 27 | 0.001 | 13 | 32 | 25 | 33 | 25 | 22 | 0.001 | PCR-RFLP | ASD, VSD, PDA, PS, TOF, HLHS, Combined lesion |
| Christensen et al *** | 2013 | USA (C) | 78 | 67 | 12 | 38 | 26 | 5 | 0.764 | 98 | 71 | 13 | 36 | 22 | 7 | 0.220 | PCR-RFLP | VSD, TOF, AS, TGA, AVSD, DORV, PS, CoA, Truncus Arteriosus |
| Huang et al | 2014 | China (A) | 111 | 56 | 3 | 146 | 56 | 6 | 0.800 | — | — | — | — | — | — | — | MS | TOF |
*: PS: Pulmonary Stenosis; HLHS: Hypoplastic Left Heart Syndrome; CoA: Coarctation of the Aorta; TGA: Transposition of Great Arteries; ASD: Atrial Septal Defect; VSD: Ventricular Septal Defect; AVSD: Atrioventricular Septal Defect; TOF: Tetralogy of Fallot; PDA: Patent Ductus Arteriosus; PA: Pulmonary Atresia; DORV: Double-outlet Right Ventricle; AC: Aortic Coarctation; AS: Aortic Stenosis.
**: C: Caucasians; A: South Asians; M: Mixed; AR: Arabian.
***: The data was respectively provided by author of Dr. Karen E. Christensen (see Acknowledgements).
Pooled Prevalence of MTHFR -677T and -1298C in the Controls
The pooled MTHFR –677T allele frequency determined using the random-effects model was 28.99% (95 CI: 26.14%–32.02%) in the Caucasian paediatric population and was 42.28% (95% CI: 34.17%–50.83%) in the Asian paediatric population. There was no heterogeneity among the Caucasian and Asian maternal population studies. The MTHFR –677T allele frequency was 31.76% (95 CI: 30.14%–33.43%) in the Caucasian maternal population and was 41.51% (95% CI: 37.50%–45.64%) in the Asian maternal population.
The pooled –1298C allele frequency in the fixed-effects model was 33.12% (95 CI: 29.80%–36.61%) in the Caucasian paediatric population and was 31.09% (95% CI: 25.34%–37.46%) in the Caucasian maternal population using the random-effects model.
Association between MTHFR C677T polymorphism and risk of CHD
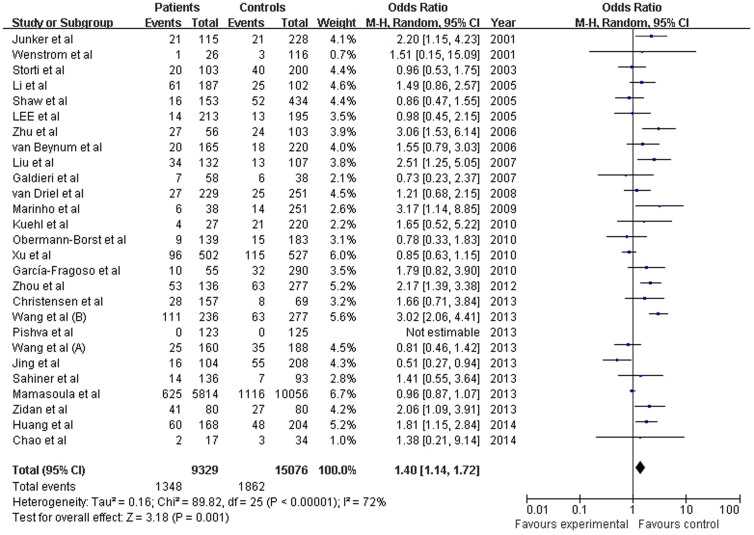
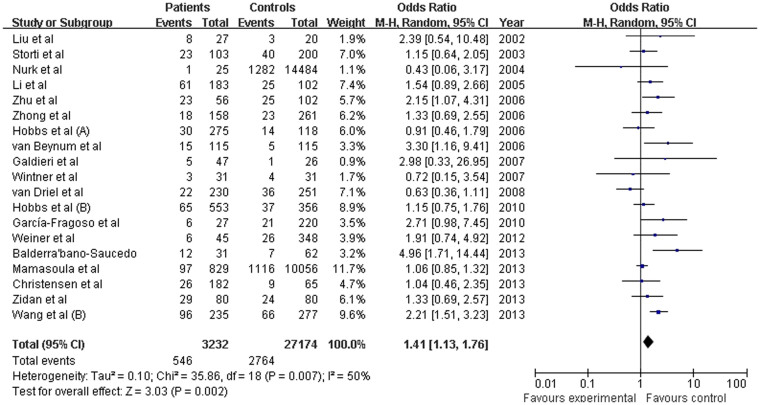
We investigated the association between the MTHFR C677T polymorphism and the risk of CHD for each study. When all the eligible studies were pooled in the overall population of children with random-effects models, significant associations were observed in all genetic models: T versus C (OR = 1.248, 95% CI: 1.093–1.426; P = 0.001), TT versus CC (OR = 1.485, 95% CI: 1.140–1.935; P = 0.003), and TT versus CT (OR = 1.312, 95% CI: 1.100–1.565; P = 0.003), the dominant model (OR = 1.240, 95% CI: 1.053–1.461; P = 0.010), and the recessive model (OR = 1.410, 95% CI: 1.139–1.724; P = 0.001;(Figure 2). In addition, significant associations were observed in the overall maternal population in all genetic models for T versus C (OR = 1.215, 95% CI: 1.085–1.361; P = 0.001), TT versus CC (OR = 1.488, 95% CI: 1.169–1.859; P = 0.001), TT versus CT (OR = 1.315, 95% CI: 1.042–1.659; P = 0.021), the dominant model (OR = 1.258, 95% CI: 1.144–1.383; P = 2.14e-6), and the recessive model (OR = 1.408, 95% CI: 1.128–1.757; P = 0.002; (Figure 3). The Z-test indicated that the pooled ORs were statistically significant.
Figure 2. Pooled OR (recessive model) and 95% CI for individual studies and pooled data for the association between the polymorphism C677TT and congenital heart disease (CHD) in the overall paediatric population.
Figure 3. Pooled OR (recessive model) and 95% CI for individual studies and pooled data for the association between the polymorphism C677TT and congenital heart disease (CHD) in the overall maternal population.
In the stratified analysis by ethnicity, significant associations were found when all studies were pooled with fixed or random-effects models for T versus C (OR = 1.163, 95% CI: 1.008–1.342; P = 0.039) in Caucasian children, and for T versus C (OR = 1.125, 95% CI: 1.043–1.214; P = 0.002), dominant model (OR = 1.216, 95% CI: 1.096–1.348; P = 2.24e-4) in the Caucasian maternal population. In addition, significant associations were found when all studies were pooled in fixed or random-effects models for all genetic models in Asian children and the maternal population. The main results of meta-analysis are shown in Table 3.
Table 3. Main results of association between MTHFR C677T polymorphism and CHD.
| Sample size | Test of heterogeneity | Test of association | Test of publication bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Genetic model | Patients | Controls | Q | P | I2 (%) | OR | 95% CI | Z | P | z | P |
| Children Overall | T vs. C | 9,329 | 15,076 | 146.67 | 0.000 | 82.3 | 1.248 | 1.093–1.426 | 3.27 | 0.001 | 1.13 | 0.260 |
| TT vs. CC | 118.35 | 0.000 | 78.9 | 1.485 | 1.140–1.935 | 2.93 | 0.003 | 0.48 | 0.628 | |||
| TT vs. CT | 53.62 | 0.001 | 53.4 | 1.312 | 1.100–1.565 | 3.02 | 0.003 | 0.66 | 0.508 | |||
| Dominant model | 102.79 | 0.000 | 74.4 | 1.240 | 1.053–1.461 | 2.58 | 0.010 | 1.54 | 0.123 | |||
| Recessive model | 89.82 | 0.000 | 72.2 | 1.401 | 1.139–1.724 | 3.19 | 0.001 | 0.18 | 0.860 | |||
| Maternal Overall | T vs. C | 3,232 | 2,7174 | 34.32 | 0.011 | 47.6 | 1.215 | 1.085–1.361 | 3.38 | 0.001 | 0.35 | 0.726 |
| TT vs. CC | 32.94 | 0.017 | 45.4 | 1.488 | 1.169–1.895 | 3.23 | 0.001 | 1.33 | 0.174 | |||
| TT vs. CT | 35.13 | 0.009 | 48.8 | 1.315 | 1.042–1.659 | 2.31 | 0.021 | 0.98 | 0.327 | |||
| Dominant model | 25.69 | 0.107 | 29.9 | 1.258 | 1.144–1.383 | 4.74 | 2.14e-6 | 0.70 | 0.484 | |||
| Recessive model | 35.86 | 0.007 | 49.8 | 1.408 | 1.128–1.757 | 3.03 | 0.002 | 0.91 | 0.363 | |||
| Caucasian Children | T vs. C | 7,092 | 12,150 | 26.94 | 0.003 | 62.9 | 1.163 | 1.008–1.342 | 2.06 | 0.039 | 2.18 | 0.029 |
| TT vs. CC | 18.09 | 0.073 | 44.7 | 1.273 | 0.978–1.658 | 1.79 | 0.073 | 0.93 | 0.350 | |||
| TT vs. CT | 9.29 | 0.505 | 0.0 | 0.986 | 0.892–1.090 | 0.28 | 0.781 | 0.62 | 0.533 | |||
| Dominant model | 24.13 | 0.007 | 58.6 | 1.182 | 0.982–1.422 | 1.77 | 0.077 | 2.34 | 0.020 | |||
| Recessive model | 13.12 | 0.217 | 23.8 | 1.012 | 0.921–1.113 | 0.26 | 0.798 | 0.47 | 0.640 | |||
| Caucasian Maternal | T vs. C | 2,431 | 26,170 | 9.22 | 0.417 | 2.4 | 1.125 | 1.043–1.214 | 3.04 | 0.002 | 0.89 | 0.371 |
| TT vs. CC | 7.25 | 0.611 | 0.0 | 1.157 | 0.977–1.370 | 1.69 | 0.690 | 0.72 | 0.474 | |||
| TT vs. CT | 6.95 | 0.643 | 0.0 | 0.945 | 0.800–1.116 | 0.67 | 0.504 | 0.00 | 1.00 | |||
| Dominant model | 11.03 | 0.274 | 18.4 | 1.216 | 1.096–1.348 | 3.69 | 2.24e-4 | 0.54 | 0.592 | |||
| Recessive model | 6.58 | 0.681 | 0.0 | 1.074 | 0.894–1.227 | 0.57 | 0.566 | 0.54 | 0.592 | |||
| Asian Children | T vs. C | 1,911 | 2,222 | 74.39 | 0.000 | 86.6 | 1.449 | 1.117–1.880 | 2.79 | 0.005 | 0.16 | 0.876 |
| TT vs. CC | 62.4 | 0.000 | 83.9 | 1.960 | 1.203–3.192 | 2.70 | 0.007 | 0.16 | 0.876 | |||
| TT vs. CT | 31.57 | 0.000 | 68.3 | 1.649 | 1.209–2.248 | 3.16 | 0.002 | 0.47 | 0.640 | |||
| Dominant model | 43.94 | 0.000 | 77.2 | 1.441 | 1.049–1.978 | 2.26 | 0.024 | 0.16 | 0.876 | |||
| Recessive model | 49.87 | 0.000 | 79.9 | 1.761 | 1.227–2.526 | 3.07 | 0.002 | 0.62 | 0.533 | |||
| Asian Maternal | T vs. C | 467 | 616 | 3.96 | 0.412 | 0.0 | 1.595 | 1.348–1.886 | 5.45 | 5.04e-8 | – | – |
| TT vs. CC | 3.49 | 0.479 | 0.0 | 2.548 | 1.788–3.631 | 5.18 | 2.22e-7 | – | – | |||
| TT vs. CT | 1.51 | 0.825 | 0.0 | 1.884 | 1.415–2.509 | 4.34 | 1.42e-5 | – | – | |||
| Dominant model | 4.93 | 0.295 | 18.9 | 1.605 | 1.215–2.121 | 3.33 | 0.001 | – | – | |||
| Recessive model | 2.05 | 0.727 | 0.0 | 2.073 | 1.583–2.716 | 5.29 | 1.22e-7 | – | – | |||
Association between MTHFR A1298C polymorphism and risk of CHD
We investigated the association between the MTHFR A1298C polymorphism and the risk of CHD for each study. Overall, when all the eligible studies were pooled in the fixed-effects model, significant associations were observed for CC vs. AC (OR = 1.354, 95% CI: 1.022–1.793; P = 0.034), and for the recessive model (OR = 1.322, 95% CI: 1.015–1.732; P = 0.038) in the overall paediatric population. The main results of the meta-analysis are shown in Table 4.
Table 4. Main results of association between MTHFR A1298C polymorphism and CHD.
| Sample size | Test of heterogeneity | Test of association | Test of publication bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Genetic model | Patients | Controls | Q | P | I2 (%) | OR | 95% CI | Z | P | z | P |
| Children Overall | C VS. A | 1,834 | 1,744 | 14.21 | 0.077 | 43.7 | 1.044 | 0.890–1.225 | 0.53 | 0.595 | 0.31 | 0.754 |
| CC vs. AA | 9.05 | 0.338 | 11.6 | 1.260 | 0.950–1.671 | 1.60 | 0.109 | 0.10 | 0.917 | |||
| CC vs. AC | 4.56 | 0.804 | 0.00 | 1.354 | 1.022–1.793 | 2.11 | 0.034 | 1.56 | 0.118 | |||
| Dominant model | 14.34 | 0.073 | 44.2 | 0.978 | 0.792–1.206 | 0.21 | 0.832 | 0.36 | 0.175 | |||
| Recessive model | 5.83 | 0.666 | 0.0 | 1.322 | 1.015–1.732 | 2.07 | 0.038 | 0.52 | 0.602 | |||
| Maternal Overall | C VS. A | 705 | 15,458 | 16.60 | 0.011 | 63.9 | 1.041 | 0.781–1.386 | 0.27 | 0.785 | 0.60 | 0.548 |
| CC vs. AA | 11.15 | 0.084 | 46.2 | 1.085 | 0.631–1.864 | 0.29 | 0.769 | 0.00 | 1.000 | |||
| CC vs.AC | 2.07 | 0.913 | 0.0 | 0.841 | 0.587–1.205 | 0.94 | 0.346 | 0.60 | 0.548 | |||
| Dominant model | 17.61 | 0.007 | 65.9 | 1.107 | 0.748–1.639 | 0.51 | 0.612 | 0.60 | 0.548 | |||
| Recessive model | 5.39 | 0.495 | 0.00 | 0.966 | 0.690–1.352 | 0.20 | 0.839 | 0.30 | 0.764 | |||
| Caucasian Children | C VS. A | 765 | 796 | 6.76 | 0.149 | 40.8 | 0.989 | 0.848–1.154 | 0.14 | 0.891 | – | – |
| CC vs. AA | 4.15 | 0.386 | 3.60 | 1.177 | 0.819–1.691 | 0.88 | 0.378 | – | – | |||
| CC vs. AC | 2.22 | 0.695 | 0.00 | 1.484 | 1.035–2.128 | 2.15 | 0.032 | – | – | |||
| Dominant model | 7.83 | 0.098 | 48.9 | 0.916 | 0.681–1.231 | 0.58 | 0.559 | – | – | |||
| Recessive model | 2.92 | 0.571 | 0.00 | 1.332 | 0.944–1.878 | 1.63 | 0.103 | – | – | |||
| Caucasian Maternal | C VS. A | 588 | 15,352 | 9.17 | 0.057 | 56.4 | 0.920 | 0.693–1.223 | 0.57 | 0.567 | – | – |
| CC vs. AA | 4.43 | 0.364 | 7.4 | 0.850 | 0.565–1.278 | 0.78 | 0.434 | – | – | |||
| CC vs. AC | 1.25 | 0.870 | 0.00 | 0.802 | 0.531–1.212 | 1.05 | 0.295 | – | – | |||
| Dominant model | 9.22 | 0.056 | 56.6 | 0.943 | 0.652–1.363 | 0.31 | 0.753 | – | – | |||
| Recessive model | 2.77 | 0.597 | 0.00 | 0.824 | 0.557–1.217 | 0.97 | 0.330 | – | – | |||
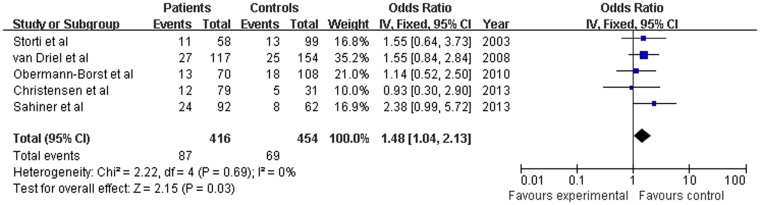
In the analysis stratified by ethnicity, significant associations were found in the Caucasian paediatric population when all studies were pooled in the fixed-effects model for CC versus AC (OR = 1.484, 95% CI: 1.035–2.128; P = 0.032; Figure 4). The main results of the meta-analysis are shown in Table 4.
Figure 4. Pooled OR (CC vs. AC) and 95% CI of individual studies and pooled data for the association between the polymorphism A1298C and congenital heart disease (CHD) in the Caucasian paediatric population.
Sensitivity analyses
We removed the studies due to the genotype distribution in the control groups deviating from HWE. We found that the corresponding ORs for the C677T polymorphism for the TT vs. CT and recessive models in the overall paediatric population and for all genetic types in the overall maternal population and the Asian maternal population were not substantially altered (Table 5). This finding supports the reliability of the results.
Table 5. Sensitivity analysis of association between MTHFR C677T polymorphism and CHD.
| Test of heterogeneity | Test of association | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | Genetic model | Q | P | I2 (%) | OR | 95% CI | Z | P |
| Children Overall | TT vs. CT | 32.42 | 0.020 | 44.5 | 1.303 | 1.064–1.596 | 2.56 | 0.010 |
| Recessive model | 61.61 | 0.000 | 70.8 | 1.335 | 1.028–1.735 | 2.16 | 0.030 | |
| Maternal Overall | T vs. C | 32.48 | 0.006 | 53.8 | 1.215 | 1.042–1.425 | 2.48 | 0.013 |
| TT vs. CC | 29.99 | 0.012 | 50.0 | 1.570 | 1.125–2.192 | 2.65 | 0.008 | |
| TT vs. CT | 26.09 | 0.037 | 42.5 | 1.462 | 1.104–1.937 | 2.65 | 0.008 | |
| Dominant model | 22.10 | 0.105 | 32.1 | 1.198 | 1.035–1.386 | 2.43 | 0.015 | |
| Recessive model | 29.49 | 0.014 | 49.1 | 1.527 | 1.149–2.030 | 2.92 | 0.004 | |
| Asian Maternal | T vs. C | 3.96 | 0.412 | 0.0 | 1.595 | 1.348–1.886 | 5.45 | 5.04e-8 |
| TT vs. CC | 3.49 | 0.479 | 0.0 | 2.548 | 1.788–3.631 | 5.18 | 2.22e-7 | |
| TT vs. CT | 1.51 | 0.825 | 0.0 | 1.884 | 1.415–2.509 | 4.34 | 1.42e-5 | |
| Dominant model | 4.93 | 0.295 | 18.9 | 1.605 | 1.215–2.121 | 3.33 | 0.001 | |
| Recessive model | 2.05 | 0.727 | 0.0 | 2.073 | 1.583–2.716 | 5.29 | 1.22e-7 | |
Publication bias
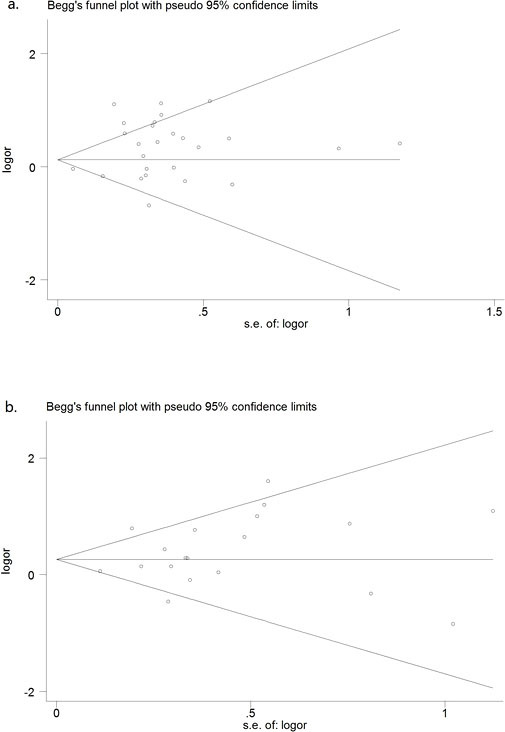
Begg's test and a funnel plot were performed to assess the publication bias of the literature. We detected publication biases for the C677T polymorphism for the T vs. C and dominant models in the Caucasian paediatric population (Table 3). This might represent a limitation of our analysis because the studies with null findings, especially those with small sample size, were less likely to be published. By using the trim and fill method, we showed that, if the publication bias was the only source of the funnel plot asymmetry, they needed two and one more studies, respectively, to balance the funnel plot. The adjusted risk estimate was attenuated. The adjusted OR for T vs. C was 1.142 (95% CI: 0.729–1.786) and for the dominant model was 1.253 (95%CI: 0.738–2.133). The results suggest no evidence of publication biases in other genetic models and populations (Figure 5).
Figure 5. Funnel plot of the C1858T polymorphism and susceptibility to CHD (recessive model) in (a) the overall paediatric population (z = 0.18, P = 0.860) and (b) the overall maternal population (z = 0.91, P = 0.363).
Discussion
It is estimated that 7.9 million children are born with a serious birth defect of genetic or partially genetic origin each year in the world. CHDs are the most commonly occurring conditions. However, the aetiology of CHDs is largely unknown, and there are no established strategies for reducing their public health impact.
Many studies have demonstrated that genetic factors play important roles in the pathogenesis of CHD. In our previous studies, we have detected several novel variations of the PLAGL1 and HOMEZ genes in Chinese patients with isolated VSD. We believe that these two genes are directly linked aetiologically with isolated VSD in the population8,9. In addition, the results of recent genome-wide association studies indicated that a region on chromosome 4p16 adjacent to the MSX1 and STX18 genes was associated (P = 9.5 × 10−7) with the risk of ostium secundum ASD6. These studies also showed that 1p12 (rs2474937 near TBX15; P = 8.44 × 10−10) and 4q31.1 (rs1531070 in MAML3; P = 4.99 × 10−12) were associated with congenital heart malformations in Han Chinese populations72.
In 1999, Kapusta and associates first reported that maternal hyperhomocysteinaemia is correlated with an increased risk of CHDs73. More recently, Hobbs and co-workers studied mothers whose pregnancies were affected by congenital heart defects (224 case subjects) or unaffected by any birth defect (90 control subjects) and identified Hcy, S-adenosylhomocysteine, and methionine as the most important biomarkers predictive of case or control status36. The MTHFR protein is a key enzyme in Hcy metabolism. The MTHFR gene is located on chromosome 1 at 1p36.3. The major product of the MTHFR gene is a catalytically active 77 kDa protein that catalyses the conversion of 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate, the major circulating form of folate. Two common genetic polymorphisms associated with reduced MTHFR activity have been identified. The C677T polymorphism is located in exon 4 at the folate-binding site and results in an alanine-to-valine substitution. In healthy homozygous subjects, the 677TT genotype is associated with higher total Hcy and lower folate plasma level. The other polymorphism (A1298C) is in exon 7 within the presumptive regulatory domain and results in a glutamate-to-alanine change. Heterozygosity and homozygosity are associated neither with higher total Hcy nor lower folate plasma concentration. The MTHFR gene polymorphisms are directly linked with many diseases20,74. Our recent meta-analysis demonstrated that the MTHFR C677T polymorphism is associated with the risk of myocardial infarction in young/middle-aged Caucasians and is associated with susceptibility to preeclampsia20,74.
A number of studies have investigated the association between MTHFR genotype and the risk of CHD. In fact, in the last few years, several case–control studies were performed on this topic. However, the results are inconclusive. The two most recent meta-analyses for associations between polymorphism and CHD also led to conflicting conclusions. By reviewing all studies published before April, 2011, Yin and co-workers suggested that the foetal and paternal MTHFR C667T gene may be associated with an increased occurrence of CHD32. By contrast, after analysis of 7,698 cases and 13,159 controls by reviewing studies published before 2010, Mamasoula and co-workers indicated that the same polymorphism, which directly influences plasma folate levels, is not associated with CHD risk17. Others also conducted meta-analysis to evaluate the association between MTHFR polymorphism and CHD29,30,31. It is possible that the relatively small sample size of these studies affected the accuracy of the results. Therefore, it is essential to re-perform a meta-analysis to evaluate the association. In our present study, we enlarged the sample size to 24,405 participants (9,329 CHD children and 15,076 normal controls), and performed sensitivity analysis to evaluate the stability of the results. In addition, we are the first to evaluate the association between the MTHFR A1298C polymorphism and CHD by meta-analysis. We are indebted to Dr. Christensen from McGill University for kindly allowing us access to his previously un-published data for this meta-analysis.
Our results indicate that the frequency of the putative risk allele -677T was 28.99% in Caucasian children and 31.76% in the Caucasian maternal population, whereas the frequency of -677T was 42.28% in Asian paediatric and 41.51% in the Asian maternal population. In addition, the pooled –1298C allele frequency was 33.12% in Caucasian children and 31.09% in the Caucasian maternal population. The meta-analysis results showed that associations exist between the MTHFR C677T polymorphism and susceptibility to CHD for all genetic models in all paediatric and maternal populations, especially in the Asian population. We also detected a significant association in the genetic model for T vs. C in the Caucasian paediatric population and in T vs. C and TT vs. CT for the Caucasian maternal population (Table 3). In our analysis of the A1298C polymorphism, we detected an association in the genetic model for TT vs. CT in the Caucasian paediatric population (Table 4). The results showing significant association for all genetic models in the overall maternal population and the Asian maternal population, and for the TT vs. CT and recessive models in the overall paediatric population were found to be stable and reliable by sensitivity analyses (Table 5).
Some limitations of this meta-analysis should be discussed. First, significant heterogeneity was observed in some genetic models when we pooled ORs. Under this condition, we used the random-effects model to pool the data. Sensitivity analysis was performed to evaluate the stability of the crude results. Second, publication biases appear to substantially contaminate the literature with regard to some genetic associations. The results of the trim and fill method demonstrated that the publication biases may affect the stability of positive results.
In conclusion, our results support the MTHFR –677T allele as a susceptibility factor for CHD in the Asian maternal population and the -1298C allele as a risk factor in the Caucasian paediatric population. Because of the heterogeneity and publication bias, we believe that other positive results may not be stable in our meta-analysis. A large number of homogeneous studies should be performed to evaluate these crude results in the future.
Author Contributions
Conception and design of the study: C.X. and L.M.L. Acquisition of data: H.L., J.X.Z. and H.W.W. Analysis and interpretation of the data: C.X., H.L., J.X.Z., Y.W., C.P.N., Z.L. and B.B.Z. Writing and revision of the manuscript: C.X., L.M.L. G.W.H. All authors reviewed the manuscript.
Acknowledgments
We thank Karen E. Christensen (Departments of Pediatrics and Human Genetics, McGill University-Montreal Children's Hospital Research Institute, Quebec, Canada) for providing data from her group's study. The work was fully supported by grants from the National Natural Science Foundation of China (No. 81301485 & 81170148) and the Shangdong Young Scientists Award Foundation (No. BS2013YY036)
References
- Kappagoda C. T. & Macartney F. J. Effect of environmental temperatures on oxygen consumption in infants with congenital disease of the heart. Br Heart J 38, 1–4 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage K. et al. Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl Acad Sci USA 109, 14035–14040 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi G. et al. The Italian Multicentric Study on Epidemiology of Congenital Heart Disease: first step of the analysis. Working Party of the Italian Society of Pediatric Cardiology. Cardiol Young 9, 291–299 (1999). [DOI] [PubMed] [Google Scholar]
- Caputo S. et al. Congenital heart disease in a population of dizygotic twins: an echocardiographic study. Int J Cardiol 102, 293–296 (2005). [DOI] [PubMed] [Google Scholar]
- Seliem M. A., Bou-Holaigah I. H. & Al-Sannaa N. Influence of consanguinity on the pattern of familial aggregation of congenital cardiovascular anomalies in an outpatient population: studies from the eastern province of Saudi Arabia. Community Genet 10, 27–31 (2007). [DOI] [PubMed] [Google Scholar]
- Cordell H. J. et al. Genome-wide association study of multiple congenital heart disease phenotypes identifies a susceptibility locus for atrial septal defect at chromosome 4p16. Nat Genet 45, 822–824 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. Genetic variants at 10p11 confer risk of tetralogy of fallot in chinese of nanjing. PLoS One 9, e89636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. et al. A novel variation of PLAGL1 in Chinese patients with isolated ventricular septal defect. Genet Test Mol Biomarkers 16, 984–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. et al. Identification of two novel mutations of the HOMEZ gene in Chinese patients with isolated ventricular septal defect. Genet Test Mol Biomarkers 17, 390–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. et al. Proteomic study reveals plasma protein changes in congenital heart diseases. Ann Thorac Surg 97, 1414–1419 (2014). [DOI] [PubMed] [Google Scholar]
- Biselli P. M. et al. Genetic polymorphisms involved in folate metabolism and concentrations of methylmalonic acid and folate on plasma homocysteine and risk of coronary artery disease. J Thromb Thrombolysis 29, 32–40 (2010). [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S. et al. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343, 1608–1614 (2000). [DOI] [PubMed] [Google Scholar]
- Christensen B. et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 84, 151–157 (1999). [DOI] [PubMed] [Google Scholar]
- Ueland P. M. et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 22, 195–201(2001). [DOI] [PubMed] [Google Scholar]
- Wenstrom K. D. et al. Association of the C677T methylenetetrahydrofolate reductase mutation and elevated homocysteine levels with congenital cardiac malformations. Am J Obstet Gynecol 184, 806–817 (2001). [DOI] [PubMed] [Google Scholar]
- Wang W. et al. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 8, e58041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamasoula C. et al. Association between C677T polymorphism of methylene tetrahydrofolate reductase and congenital heart disease: meta-analysis of 7697 cases and 13,125 controls. Circ Cardiovasc Genet 6, 347–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug W. S. Population and evolutionary genetics. Concepts of genetics. Wilbur, B.(ed.). 700–702 (Pearson Benjamin Cummings, CA, 2011). [Google Scholar]
- Xuan C. et al. No association between APOE epsilon 4 allele and multiple sclerosis susceptibility: a meta-analysis from 5472 cases and 4727 controls. J Neurol Sci 308, 110–116 (2011). [DOI] [PubMed] [Google Scholar]
- Xuan C. & Lun L. M. Association between the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to preeclampsia: the need for data clarification in a recent meta-analysis. Hypertens Res 36, 463–464 (2013). [DOI] [PubMed] [Google Scholar]
- Chakraborty R. Hardy–Weinberg Equilibrium. Encyclopedia of Biostatistics 4, 1–2 (2005). [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Bowden J. et al. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol 11, 41(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. et al. Association between OCTN1/2 gene polymorphisms (1672C-T, 207G-C) and susceptibility of Crohn's disease: a meta-analysis. Int J Colorectal Dis 27, 11–9 (2012). [DOI] [PubMed] [Google Scholar]
- Xuan C. et al. PTPN22 gene polymorphism (C1858T) is associated with susceptibility to type 1 diabetes: a meta-analysis of 19,495 cases and 25,341 controls. Ann Hum Genet 77, 191–203 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang B. B. et al. Genetic 135G/C polymorphism of RAD51 gene and risk of cancer: a meta-analysis of 28,956 cases and 28,372 controls. Fam Cancer 10.1007/s10689-014-9729-0 (2014). [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Tian Q. W. et al. Diagnostic accuracy of glycosylated hemoglobin in chinese patients with gestational diabetes mellitus: a meta-analysis based on 2,812 patients and 5,918 controls. Genet Test Mol Biomarkers 17, 687–695 (2013). [DOI] [PubMed] [Google Scholar]
- van Beynum I. M. et al. The MTHFR 677C->T polymorphism and the risk of congenital heart defects: a literature review and meta-analysis. QJM 100, 743–753 (2007). [DOI] [PubMed] [Google Scholar]
- Verkleij-Hagoort A. et al. Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: a meta-analysis. Am J Med Genet A 143A, 952–960 (2007). [DOI] [PubMed] [Google Scholar]
- Nie Y. et al. Methylenetetrahydrofolate reductase C677T polymorphism and congenital heart disease: a meta-analysis. Clin Chem Lab Med 49, 2101–2108 (2011). [DOI] [PubMed] [Google Scholar]
- Yin M. et al. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann Hum Genet 76, 9–16 (2012). [DOI] [PubMed] [Google Scholar]
- Chen K. H. et al. Maternal MTHFR C677T polymorphism and congenital heart defect risk in the Chinese Han population: a meta-analysis. Genet Mol Res 12, 6212–6219 (2013). [DOI] [PubMed] [Google Scholar]
- McBride K. L. et al. A family-based association study of congenital left-sided heart malformations and 5,10 methylenetetrahydrofolate reductase. Birth Defects Res A Clin Mol Teratol 70, 825–830 (2004). [DOI] [PubMed] [Google Scholar]
- Pereira A. C. et al. Lack of evidence of association between MTHFR C677T polymorphism and congenital heart disease in a TDT study design. Int J Cardiol 105, 15–8 (2005). [DOI] [PubMed] [Google Scholar]
- Hobbs C. A. et al. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr 81, 147–153 (2005). [DOI] [PubMed] [Google Scholar]
- Goldmuntz E. et al. Variants of folate metabolism genes and the risk of conotruncal cardiac defects. Circ Cardiovasc Genet 1, 126–132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M. et al. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 10, 49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker R. et al. Infant methylenetetrahydrofolate reductase 677TT genotype is a risk factor for congenital heart disease. Cardiovasc Res 51, 251–254 (2001). [DOI] [PubMed] [Google Scholar]
- Liu H. et al. Maternal homocysteine folic acid, MTHFR gene polymorphism and congenital heart defects in offspring. Chin J Perinatal Med 5, 102–105 (2002). [Google Scholar]
- Storti S. et al. Association between 5,10-methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and conotruncal heart defects. Clin Chem Lab Med 41, 276–280 (2003). [DOI] [PubMed] [Google Scholar]
- Nurk E. et al. Associations between maternal methylenetetrahydrofolate reductase polymorphisms and adverse outcomes of pregnancy: the Hordaland Homocysteine Study. Am J Med 117, 26–31 (2004). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Study of serum Hcy and polymorphisms of Hcy metabolic enzymes in 192families affected by congenital heart disease. J Peking Univ (Health Sci) 37, 75–80 (2005). [PubMed] [Google Scholar]
- Lee C. N. et al. Association of the C677T methylenetetrahydrofolate reductase mutation with congenital heart diseases. Acta Obstet Gynecol Scand 84, 1134–1140 (2005). [DOI] [PubMed] [Google Scholar]
- Shaw G. M. et al. Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Am J Med Genet A 138, 21–26 (2005). [DOI] [PubMed] [Google Scholar]
- Hobbs C. A. et al. Congenital heart defects and genetic variants in the methylenetetrahydroflate reductase gene. J Med Genet 43, 162–166 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W. L. et al. Maternal and offspring MTHFR gene C677T polymorphism as predictors of congenital atrial septal defect and patent ductus arteriosus. Mol Hum Reprod 12, 51–54 (2006). [DOI] [PubMed] [Google Scholar]
- Zhong Q. A. et al. Association of congenital heart diseases with MTHFR gene and CBS gene. Guangxi Med J 28, 1140–1142 (2006). [Google Scholar]
- van Beynum I. M. et al. Maternal MTHFR 677C>T is a risk factor for congenital heart defects: effect modification by periconceptional folate supplementation. Eur Heart J 27, 981–987 (2006). [DOI] [PubMed] [Google Scholar]
- Galdieri L. C. et al. Homocysteine concentrations and molecular analysis in patients with congenital heart defects. Arch Med Res 38, 212–218 (2007). [DOI] [PubMed] [Google Scholar]
- Wintner S. et al. Association of congenital cardiac defects and the C677T methylenetetrahydrofolate reductase polymorphism. Prenat Diagn 27, 704–708 (2007). [DOI] [PubMed] [Google Scholar]
- Liu Y. S. et al. Relation ship between genetic polymorphism of homocysteine metabolism enzyme and congenital heart disease. Chin J Cardio Rev 15, 210–213 (2007). [Google Scholar]
- van Driel L. M. et al. Two MTHFR polymorphisms, maternal B-vitamin intake, and CHDs. Birth Defects Res A Clin Mol Teratol 82, 474–481 (2008). [DOI] [PubMed] [Google Scholar]
- Marinho C. et al. The methylenetetrahydrofolate reductase gene variant (C677T) as a susceptibility gene for tetralogy of Fallot. Rev Port Cardiol 28, 809–812 (2009). [PubMed] [Google Scholar]
- Kuehl K. et al. Association of congenital cardiovascular malformations with 33 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Birth Defects Res A Clin Mol Teratol 88, 101–110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs C. A., Cleves M. A., Karim M. A., Zhao W. & MacLeod S. L. Maternal folate-related gene environment interactions and congenital heart defects. Obstet Gynecol 116, 316–232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. MTHFR c.1793G>A polymorphism is associated with congenital cardiac disease in a Chinese population. Cardiol Young 20, 318–326 (2010). [DOI] [PubMed] [Google Scholar]
- Garcia-Fragoso L. et al. MTHFR polymorphisms in Puerto Rican children with isolated congenital heart disease and their mothers. Int J Genet Mol Biol 2, 43–47 (2010). [PMC free article] [PubMed] [Google Scholar]
- Obermann-Borst S. A. et al. Congenital heart defects and biomarkers of methylation in children: a case-control study. Eur J Clin Invest 41, 143–150 (2011). [DOI] [PubMed] [Google Scholar]
- Weiner A. S. et al. Polymorphisms in folate-metabolizing genes and risk of having an offspring with congenital anomalies in the West Siberian region of Russia: a case-control study. Prenat Diagn 32, 1041–1048 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou S. Y. et al. Study on the association between MTHFR gene polymorphism and tetralogy of fallot. Shandong Med J 52, 1–3 (2012). [Google Scholar]
- Pishva S. R. et al. Analysis of MTHFR and MTRR Gene Polymorphisms in Iranian Ventricular Septal Defect Subjects. Int J Mol Sci 14, 2739–2752 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. et al. Association of SNPs in genes involved in folate metabolism with the risk of congenital heart disease. J Matern Fetal Neonatal Med 26, 1768–1777 (2013). [DOI] [PubMed] [Google Scholar]
- Jing X. A. et al. Associations of MTHFR gene polymorphism and environmental factors with congenital heart disease. Chin J Public Health 3,347–349 (2013). [Google Scholar]
- Balderrabano-Saucedo N. A. et al. Polymorphism 677C > T MTHFR gene in Mexican mothers of children with complex congenital heart disease. Pediatr Cardiol 34, 46–51 (2013). [DOI] [PubMed] [Google Scholar]
- Christensen K. E. et al. Risk of congenital heart defects is influenced by genetic variation in folate metabolism. Cardiol Young 23, 89–98 (2013). [DOI] [PubMed] [Google Scholar]
- Wang L. N. et al. Relationship between 5,10-methylenetetrahydrofolate gene polymorphism and congenital heart disease in nuclear family. Chin J Appl Clin Pediatr 1, 32–35 (2013). [Google Scholar]
- Zidan H. E., Rezk N. A. & Mohammed D. MTHFR C677T and A1298C gene polymorphisms and their relation to homocysteine level in Egyptian children with congenital heart diseases. Gene 529, 119–124 (2013). [DOI] [PubMed] [Google Scholar]
- Huang J. et al. rs1801133 C>T polymorphism is associated with an increased risk of tetralogy of Fallot. Biomed Rep 2, 172–176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C. S. et al. Correlation Between Methyltetrahydrofolate Reductase (MTHFR) Polymorphisms and Isolated Patent Ductus Arteriosus in Taiwan. Heart Lung Circ 23, 655–660 (2014). [DOI] [PubMed] [Google Scholar]
- Sahiner U. M. et al. Methylene tetrahydrofolate reductase polymorphisms and homocysteine level in heart defects. Pediatr Int 56, 167–172 (2014). [DOI] [PubMed] [Google Scholar]
- Hu Z. et al. A genome-wide association study identifies two risk loci for congenital heart malformations in Han Chinese populations. Nat Genet 45, 818–821 (2013). [DOI] [PubMed] [Google Scholar]
- Kapusta L. et al. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr 135, 773–774 (1999). [DOI] [PubMed] [Google Scholar]
- Xuan C. et al. Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls. Arch Med Res 42, 677–685 (2011). [DOI] [PubMed] [Google Scholar]