Abstract
The benefits of regular exercise, physical fitness and sports participation on cardiovascular and brain health are undeniable. Physical activity reduces the risk for cardiovascular disease, type 2 diabetes, hypertension, obesity, and stroke, and produces beneficial effects on cholesterol levels, antioxidant systems, inflammation, and vascular function. Exercise also enhances psychological health, reduces age-related loss of brain volume, improves cognition, reduces the risk of developing dementia, and impedes neurodegeneration. Nonetheless, the play of sports is associated with risks, including a risk for mild TBI (mTBI) and, rarely, catastrophic traumatic injury and death. There is also growing awareness that repetitive mTBIs, such as concussion and subconcussion, can occasionally produce persistent cognitive, behavioral, and psychiatric problems as well as lead to the development of a neurodegeneration, chronic traumatic encephalopathy (CTE). In this review, we summarize the beneficial aspects of sports participation on psychological, emotional, physical and cognitive health, and specifically analyze some of the less common adverse neuropathological outcomes, including concussion, second-impact syndrome, juvenile head trauma syndrome, catastrophic sudden death, and CTE. CTE is a latent neurodegeneration clinically associated with behavioral changes, executive dysfunction and cognitive impairments, and pathologically characterized by frontal and temporal lobe atrophy, neuronal and axonal loss, and abnormal deposits of paired helical filament (PHF)-tau and 43 kDa TAR deoxyribonucleic acid (DNA)-binding protein (TDP-43). CTE often occurs as a sole diagnosis, but may be associated with other neurodegenerative disorders, including motor neuron disease (CTE-MND). Although the incidence and prevalence of CTE are not known, CTE has been reported most frequently in American football players and boxers. Other sports associated with CTE include ice hockey, professional wrestling, soccer, rugby, and baseball.
Introduction
Each year, an estimated 38 million children and adolescents and 170 million adults participate in organized sports in the United States (US) [47]. Participation in sports has many clear benefits, including increased physical fitness, reduced body fat, improved cardiovascular and metabolic disease risk profiles, improved psychological health, enriched interpersonal relationships, and reduced symptoms of depression and anxiety [55, 154]. At the same time, the play of sports is also associated with a small, but unpredictable risk for sudden death and catastrophic traumatic injury [48], and there is growing awareness that mTBI, such as concussion and subconcussion, can sometimes produce persistent cognitive, behavioral, and psychiatric problems [48]. In addition, repetitive mTBIs experienced during the play of certain sports are associated with the development of a progressive neurodegeneration, chronic traumatic encephalopathy (CTE) [38, 65, 88, 92, 126–128, 147, 148]. The purpose of this review is to broadly summarize the beneficial aspects of sports participation on psychological, physical and cognitive health, and to specifically analyze some of the less common adverse neuropathological outcomes.
The benefits of exercise and sports participation
The psychological benefits of sports participation
Sports participation is associated with improved psycho-social health as well as increased physical health, and participation in team sports is associated with improved health outcomes compared to individual sports [55]. In a recent systematic review of the psychological and social health benefits of participation in sport for children and adolescents, the most commonly reported benefits of sports participation were higher self-esteem, better social skills, fewer depressive symptoms, higher confidence, and a sense of higher competence among sport participants compared to non-sport participants [55]. Cross-sectional studies using surveys of US high school students have found that sports participation is associated with increased life satisfaction, increased emotional self-efficacy, as well as reduced hopelessness and suicidality [185]. In another cross-sectional survey, Boone and Leadbeater found that involvement in team sports is positively associated with social acceptance and negatively associated with depressive symptoms possibly related to enhanced perceived social acceptance and reduced body dissatisfaction [13]. In a longitudinal study of adolescents, team sport participation was found to be protective against depressed mood associated with school performance levels 1 year later [73]. Other studies have shown increased self-esteem 3 years later in females [151] and lower social isolation 12 years later, compared with other school-based activities [7]. In studies of adolescents classified as ‘athletes’ or ‘non-athletes’ based on school and club participation, athletes reported superior well-being, including feeling better adjusted, less nervous or anxious, happier, more energetic, less depressed, and having improved body image with fewer suicide attempts than non-athletes [60, 172]. In a large population-based sample of adults, exercisers were found to be less anxious, less depressed, less neurotic, more extroverted, and higher in self-rated health than non-exercisers [49, 50].
The physical benefits of sports participation
The beneficial effects of exercise, physical fitness, and sports participation on cardiovascular and brain health are undeniable. Regular physical activity is essential to the maintenance of a healthy lifestyle and exercise prescription is a cornerstone in the management of many disease conditions. Higher levels of physical activity, exercise training, and overall cardiorespiratory fitness reduce the risk for hypertension, obesity, type 2 diabetes mellitus, stroke, and metabolic syndrome, as well as lower cardiovascular mortality [104, 110, 178, 200]. Regular exercise produces beneficial effects in high-density lipoprotein-cholesterol levels, glucose control, weight control, and resting heart rate and blood pressure [178]. Physical activity increases antioxidant enzymes, including superoxide dismutase and heat shock proteins, and reduces reactive oxygen species [178]. Exercise training is also associated with improved endothelial function, decreased arterial stiffness, and greater vascular reactivity associated with coronary artery disease, heart failure, and peripheral vascular disease [110]. In patients with pre-existing coronary artery or peripheral vascular disease, exercise therapy promotes enhanced vasoresponsiveness (angiogenesis, arteriogenesis, mitochondrial synthesis), enhances oxygen delivery and metabolic responses, delays disease progression, and extends longevity [143]. Exercise training also suppresses chronic low-grade inflammation and reduces levels of C-reactive protein and TNF-alpha [152].
Exercise as a neuroprotectant against age-related cognitive decline
Although regular exercise improves cognitive status indirectly by enhancing sleep, reducing stress and depression, and bettering cardiovascular health [9], physical exercise also directly promotes neurogenesis, cell survival [187], synaptogenesis, synaptic plasticity [54, 96] and angiogenesis [99], improves cognitive function, learning and memory, and significantly moderates age-related cognitive decline [2, 97, 104, 110, 187, 188, 200], reviewed in [10]. The molecular mechanisms by which exercise directly affects the brain most likely involve growth factors, such as brain derived neurotrophic factor (BDNF). BDNF acts on neurons to promote growth, increase synaptic plasticity, and enhance resistance to injury and neurodegeneration. In the hippocampus, BDNF promotes the production and survival of new neurons from stem cells and the integration of new neurons into existing neuronal circuits [141], reviewed in [160].
Cross-sectional studies show that improved physical fitness is associated with more efficient cognitive functions and longitudinal studies show less cognitive decline in working memory, processing speed, attention, and executive function in older adults without dementia [170]. Middle-aged and older adults who complete an aerobic training program show a significant improvement in cognitive performance and executive control. Furthermore, resistance training, motor learning, and coordinative exercise also enhance neurocognitive function in older adults (reviewed in [10, 190]).
Imaging studies indicate that physical exercise and higher fitness levels are associated with a reduced loss of hippocampal, gray and white matter volume with aging [34, 56, 57, 90, 189]. Aerobic exercise increases hip-pocampal and cortical volume and significantly improves spatial memory after 1 year [57]. Furthermore, functional MRI has documented that aerobic fitness is associated with improved cortical connectivity and activation [35, 191].
Exercise has also been shown to be neuroprotective, to delay the onset and progression, and reduce the risk for many neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [17, 39, 58, 89, 108, 163, 193, 199]. Epidemiological research shows that persons with higher levels of physical activity have a reduced risk of dementia [17, 107, 163, 199]. Prospective epidemiological studies show that increased baseline physical activity reduces the risk of developing dementia by 28 % and of developing AD by 45 % [58, 83]. Although most epidemiological studies assess physical activity later in life and the incidence of dementia, a multicenter, prospective study of women 65 and older showed that physical activity at three time points—age 30, age 50 and late-life—was associated with reduced odds of developing cognitive impairment [132]. Geda and colleagues also observed that moderate activity during midlife was associated with a 39 % lower risk of mild cognitive impairment in later life [64]. In studies using objective measures of activity such as actigraphy instead of self-report, older women with the highest quartile of activity performed better than those with the lowest quartile of activity on cognitive and executive functioning assessment [8]. In addition, those with the lowest baseline activity were two times more likely to develop AD 3.5 years later than those with the highest baseline activity [199]. There is also evidence that physical activity may slow cognitive decline in individuals already experiencing cognitive impairment. Fitness levels correlate with higher whole-brain and white matter volume in patients with early AD [18, 108]. In older adults with MCI and AD, higher muscle strength was associated with a slower rate of global cognitive decline [14]. Furthermore, moderate-intensity exercise for 6 months in older subjects with memory loss produced significantly better cognition at 6 months compared to sedentary subjects, and the effect persisted at least for 18 months [108].
In addition to enhanced cognition, exercise also improves motor performance in individuals with Parkinson’s disease [153], lowers disability in multiple sclerosis [161], improves functionality in individuals with motor neuron disease [46], and improves cognitive, mood and motor recovery from TBI, although the precise timing and intensity of the beneficial exercise after TBI remain to be determined [62, 197].
The mechanisms underlying improved cognition, enhanced motor function, increased brain volumes, lowered dementia risk, and resilience to neurodegeneration in physically active individuals are most likely exercise-induced enhanced neuroplasticity and upregulation of proteins involved in signal transduction, synaptic trafficking and transcriptional regulation. Numerous animal studies have demonstrated the beneficial effects of exercise, particularly aerobic endurance exercise, on the structure and function of the brain. Rodents that run voluntarily on running wheels exhibit increased numbers of dendritic spines and synapses in hippocampal neurons, increased neurogenesis, and improved performance in some behavioral tests of cognitive function [59, 185]. Treadmill exercise also improves hippocampus-dependent spatial learning and memory as well as aversive memory [2, 3, 25, 97, 112, 174, 186, 187]. Furthermore, in rodent models, BDNF, insulin– like growth factor 1 (IGF-1), mitogen-activated protein kinase phosphatase-1 (MKP-1), and glial-derived neurotrophic factor (GDNP) are elevated by exercise [1, 53, 179] and early post-injury exercise has been shown to reverse memory deficits and inhibit the progression of neurodegeneration following TBI [27].
Adverse neuropathological outcomes associated with sports participation
Sudden death in young athletes
Overall, athletes are at a low risk of sudden death, but physical exertion in the presence of clinically unsuspected cardiovascular disease increases this risk. Hypertrophic cardiomyopathy, congenital coronary artery anomalies, valvular heart disease, and myocarditis are the most common cardiovascular disorders encountered in young athletes that result in unforeseen fatalities [117]. Sudden death may also occur in athletes without antecedent heart disease as a result of blunt trauma to the chest that produces ventricular fibrillation (commotio cordis). Children and adolescents with compliant chest walls and undeveloped musculature are most susceptible, as commotion cordis is typically the result of a projectile, such as a hockey puck, lacrosse ball or baseball, traveling at high velocity and striking the precordium [117]. Other less common causes of sudden death in young athletes include heat stroke, uncontrolled bronchial asthma, ruptured cerebral artery aneurysm, and drug use [117].
Catastrophic traumatic head and neck injuries also occur. Analysis of the 30-year US National Registry of Sudden Death in Young Athletes from 1980 to 2009 found 1,827 deaths in athletes aged 21 years or younger (mean 16 – 2 years; 90 % male) including 261 (14 %) due to trauma, most often to the head and neck. The mortality rate was 0.11 in 100,000 participants (95 % confidence interval 0.08–0.15) with the largest number of deaths reported in American football (148 [57 %]), including 17 high school athletes who sustained concussions shortly before a fatal head trauma (“second-impact syndrome”) [181]. Catastrophic head and neck injuries caused by direct contact during sports participation result in fatal, nonfatal permanent, or serious nonpermanent injury, and include skull fracture, subdural and epidural hematoma, ruptured vertebral artery with subarachnoid hemorrhage, second-impact syndrome, and juvenile head trauma syndrome. Of these, the most common cause of death or disability in sports-related head injury is subdural hematoma [113, 133]. Since 1982, there have been 133 non-professional American football players who died or experienced incomplete neurological recovery following catastrophic head and neck injury. Over 90 % of these injuries occurred in high school athletes, 8 % occurred in college participants, and 1 % involved sandlot players [139].
Second-impact syndrome
Second-impact syndrome (SIS) occurs when an athlete sustains an initial mild head injury or concussion, then suffers a second head injury before the symptoms associated with the first impact have cleared, with resultant diffuse cerebral swelling and grave deterioration [19, 20, 113, 133, 137, 164]. Typically, the second head injury is only a minor blow to the head and is not followed by immediate loss of consciousness. However, within minutes of the injury, the athlete precipitously collapses into coma from severe cerebrovascular engorgement, cerebral edema, and brain herniation. All reported cases of SIS have involved young athletes, predominantly males (90 %) ranging in age from 10 to 24 years, mean age 17.9 years [137]. 71 % of the affected athletes have been American football players, usually at the high school level, but younger players and collegiate athletes have occasionally been reported. 14 % of SIS cases occurred during boxing competition, and isolated cases have been reported in association with karate, skiing, and ice hockey. Although the definition of SIS is controversial and some investigators have challenged its existence [123], SIS is thought to result from abrupt post-traumatic loss of cerebral blood flow autoregulation and catecholamine release that create a rapid increase in intracranial blood volume and catastrophic cerebral edema [32, 105]. In two-thirds of cases, a thin, acute subdural hematoma has been reported on neuroimaging or at autopsy [19, 137], which may be a result of the hyperemic state, in the absence of other major hematomas or space-occupying lesions. The relationship of SIS to juvenile head trauma syndrome, or malignant cerebral edema after mTBI, is uncertain, and both may be manifestations of the same underlying pathophysiology.
Juvenile head trauma syndrome
Minor craniocerebral trauma complicated by severe, often fatal, cerebral edema and coma, known as juvenile head trauma syndrome, has been reported primarily in children. The neurological deterioration may be immediate or delayed, occurring after a “lucid interval”. The cause of this rapid vasodilation and redistribution of blood into the brain parenchyma is not clear, but the process may involve a functional channelopathy or a disturbance of ion channel subunits. Several individuals with this juvenile head trauma syndrome have been reported to have a mutation in the calcium channel subunit gene (CACNA1A) associated with familial hemiplegic migraine [101]. In some cases of juvenile head trauma syndrome, the rapidly developing cerebral edema occurs in a young athlete who experiences two head injuries, with the second injury occurring before complete recovery from the first impact, similar to SIS [129].
Acute concussive injury
The Centers for Disease Control estimates that 1.6 to 3.8 million concussions occur in sports and recreational activities annually in the US [47]. A concussion is an mTBI induced by an impulsive force transmitted to the head resulting from a direct or indirect impact to the head, face, neck, or elsewhere [37, 125]. Symptoms from concussion and other forms of mTBI are usually self-limited and resolve spontaneously over a period of several weeks, although 10–30 % of individuals develop prolonged symptoms. If symptoms persist for more than 3 months, the condition is referred to as post-concussive syndrome (PCS) [51]. Signs and symptoms of concussion and PCS include loss of consciousness, amnesia, as well as sleep disturbances, behavioral changes (e.g., irritability), cognitive impairment (e.g., slowed reaction times), somatic symptoms (e.g., headaches), cognitive symptoms (e.g., feeling “in a fog”), and/or emotional symptoms (e.g., emotional lability) [125]. Neuropsychological testing in PCS may reveal persistent, yet subtle, cognitive deficits, most often in the executive domain [192]. Impact injuries less severe than concussion that do not produce overt neurological symptoms, yet are associated with subtle neuropsychiatric deficits or changes in functional MRI, are referred to as “subconcussion” [81, 180]. Sub-concussive injuries can be substantial in some sports: for instance, it has been reported that an offensive lineman in American football can experience over 1,000 subconcussive hits over the level of 10g in the course of a single collegiate season [41]. Closed head injury with concussion and subconcussion occurs in a wide variety of sports, including American football, boxing, wrestling, rugby, ice and field hockey, lacrosse, and soccer [21, 22, 36, 40, 47, 68, 80, 86, 93, 95, 116, 125, 131, 140, 182]. A recent study comparing the concussion rate in high school football compared to collegiate football found that high school football was associated with concussion rate of 0.21 per 1,000 athlete exposures (A–E) in practice and 1.55 concussions per 1,000 A–E in competitions, compared to 0.39 per 1,000 A-E in practice and a rate of 3.02 concussions per 1,000 A-E in competitions in collegiate football players [68]. Multiple concussions were noted in 81 of 233 (34.9 %) high school football athletes [106]. Concussions are also frequent in soccer, the most popular sport worldwide with an estimated 265 million male and female players [102]. Causes of concussion in soccer include heading, or using the head to advance or redirect the ball—a unique feature of the sport—or collisions with another player, the goalpost, or the ground. Estimates of the concussion rate in high school soccer range from 1.38 concussions per 1,000 game time A-E for boys to 1.80 for girls [68]. Concussions in girls high school soccer rank second only to boys high school football [116]. Studies using accelerometers to measure the peak accelerations of the head during soccer heading found linear accelerations as high as 54.7g in high school players heading a soccer ball kicked from a distance of 30 yards, more than the average peak accelerations of 29.2 or 35g that occur in football or ice hockey [142]. Recently, head accelerations associated with heading in girls’ youth soccer were found to range from 4.5 to 62.9g and included substantial rotational acceleration at times [85]. Concussions also occur in rugby and Australian Rules football, although the data are not as widely available. In a cohort of 3,207 male nonprofessional rugby players followed for one or more seasons, the incidence of mTBI was 7.97 per 1,000 player game hours, with 313 players (9.8 %) sustaining 1 or more mTBIs during the study. Players who wore protective headgear during games were at a reduced risk (incident rate ratio 0.57; 95 % confidence interval [CI], 0.40–0.82), while the risk nearly doubled for players who had sustained one or more mTBIs within the previous 12 months [94].
Pathophysiology of concussion
Concussion and subconcussion are produced by acceleration and deceleration forces on the brain, either linear or rotational [130]. When the brain is subjected to rapid acceleration, deceleration and rotational forces, the brain elongates and deforms, stretching individual components such as neurons, glial cells, and blood vessels and altering membrane permeability. These traumatic stretch injuries affect neuronal cell bodies, axons, dendrites, blood vessels, and glial cells; axons are especially vulnerable as they often extend long distances from the neuronal cell bodies and may be injured even without the death of the neuron of origin [155, 156]. Traumatic axonal injury (TAI) does not uniformly affect all axonal populations; smaller, unmyelinated axons may be more susceptible to damage from concussive forces than larger myelinated axons [158]. In addition, immediately after biomechanical injury to the brain there is a “neurometabolic cascade of concussion”, characterized by a rapid release of neurotransmitters, efflux of potassium, influx of calcium, and acceleration of the cellular sodium– potassium (NaC–KC) pump to maintain membrane homeostasis, requiring large increases in glucose metabolism [71]. This post-concussive hypermetabolism occurs in the setting of diminished cerebral blood flow, with a widening disparity between glucose supply and demand producing a cellular energy crisis [71].
Pathology of concussion
Pathological studies of acute concussion and PCS are rare and often include subjects with more severe traumatic injuries, but multifocal traumatic axonal injury TAI, microhemorrhage, and microglial activation have been reported [12, 150]. Oppenheimer described microscopic petechial hemorrhages, axonal injury, and microglial clusters that were often perivascular [150]. Blumbergs and colleagues examined five cases of human concussive head injury using amyloid precursor protein (APP) immunohistochemistry and reported multifocal axonal injury in the fornices, a major hippocampal projection pathway involved in memory [12]. The authors suggested that damage to the fornix might underlie some of the persisting memory deficits that occur in patients after concussion. In general, the amount and distribution of TAI is dependent on the severity of the TBI, with mild injury producing only microscopic axonal damage and moderate and severe TBI producing more severe axonal injury.
Over the last 5 years, 117 brains of athletes, defined as a player over the age of 12 years who participated in organized sports for at least 3 consecutive years, have been analyzed at the mTBI brain bank at VA Boston/Boston University School of Medicine. Of these 117 athletes, there were 115M:2F, ranging in age at death from 14 to 98 years, mean 54.1 – 22.3 years, including 6 athletes who died within 6 months of a reported concussion (6 M:0F, 14–28 years old at death, mean 18.6 – 3.75 years) (Table 1).
Table 1.
Neuropathological findings in acute concussive injury compared to age-matched controls
| ID | Sport | Age | Race | Sex | Cause of death | Conc | Recent conc | TAI | Micro | Astro | PV Mac |
PVCI | PHF-tau | Micro Bleed | TDP43 | Ab | CAA | aSYN | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HS FB, BS KB | 17 | C | M | SIS | 3 | 27D, 6D, 1D | 4C | 4C | 4C | 3C | 0 | I | 0 | 1C | 0 | 0 | 0 | Subdural hematoma, subarachnoid hem- orrhage |
| 2 | HS FB | 17 | C | M | Suicide | 2 | 2D | 3C | 3C | 4C | 2C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Small Cavum |
| 3 | HS RG, FB | 18 | C | M | Cerebral edema | 4 | 19D, 6D | 4C | 3C | 4C | 3C | 3C | I | 0 | 1C | 0 | 0 | 0 | |
| 4 | FB,WR | 14 | C | M | Suicide | 6 | 21D, 15D , 10D | 1C | 2C | 2C | 0 | 1C | 0 | 0 | 0 | 0 | 0 | 0 | Large cavum |
| 5 | Snow, HS FB | 17 | C | M | Suicide | 10 | 126D | 1C | 3C | 3C | 2C | 0 | F | 1C | 0 | 0 | 0 | 0 | |
| 6 | HCK | 28 | C | M | Overdose | >20 | 153D | 3C | 2C | 2C | 3C | 0 | II | 0 | 2C | 0 | 1C | 0 | Mammillary body gliotic |
| 7 | HS FB | 17 | C | M | Overdose | 1or 2 | >3 YR | 0 | 0 | 0 | 1C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | HS FB | 19 | C | M | Cardiac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | None | 21 | C | M | Suicide | 0 | 0 | 0 | 1C | 0 | 1C | 1C | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | HS FB | 22 | C | M | Suicide | 5 | >7 YR | 0 | 0 | 0 | 1C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | HS FB | 22 | C | M | Seizure | 6 | >1 YR | 0 | 0 | 0 | 3C | 2C | 0 | 0 | 0 | 0 | 0 | 0 |
I Stage I CTE, II Stage II CTE, aSYN alpha synuclein, A-beta amyloid plaques, Micro activated microglia, Astro astrocytosis, BSBK basketball, C Caucasian, CAA cerebral amyloid angiopathy, Conc concussion, D day, F focal, FB football, HCK ice hockey, HS high school, M male, PHF-TAU hyperphosphorylated tau, PV CI perivascular chronic inflammation, PV Mac perivascular macrophages, RG rugby, SIS second-impact syndrome, Snow snowboarding, TAI traumatic axonal injury, WR wrestling, YR year
In the players who died within 6 months of a reported concussion, neuropathological examination showed multifocal and perivascular axonal injury in the corpus callosum, fornix, subcortical white matter, and cerebellum detectable using APP and phosphorylated neurofilament immuno-histochemistry (Fig. 1). The finding of multifocal axonal injury in the cerebrum, cerebellum, and brainstem after concussion is consistent with the earlier literature. There was also striking perivascular microgliosis and astrocytosis. In addition, three of the six cases showed TDP-43 immunopositive neurites in the white matter and one case displayed mild beta amyloid (Ab) deposition in leptomeningeal vessels. Focal accumulations of paired helical filament (PHF)-tau as neurofibrillary tangles (NFTs) and dot and spindle-shaped neurites around small blood vessels were found at the depth of the sulci in four cases; in two cases the PHF-tau immunoreactivity was consistent with Stage I CTE and in one case with Stage II CTE. One subject, an 18-year-old who suffered a concussion with temporary loss of consciousness after a fall off a 28-foot jump while snowboarding, had a single focal microbleed characterized by perivascular and parenchymal hemosiderin and axonal spheroids in the uncus, with focal PHF-tau immunopositive neurons and neurites at the periphery of the microbleed (Table 1; Fig. 1). The finding of focal PHF-tau abnormalities in the brains of recently concussed young individuals in close proximity to focal axonal injury and foci of micro-hemorrhage suggests that axonal injury, loss of microvascular integrity, and breach of the blood brain barrier may be mechanistically linked to the development of PHF-tau pathology.
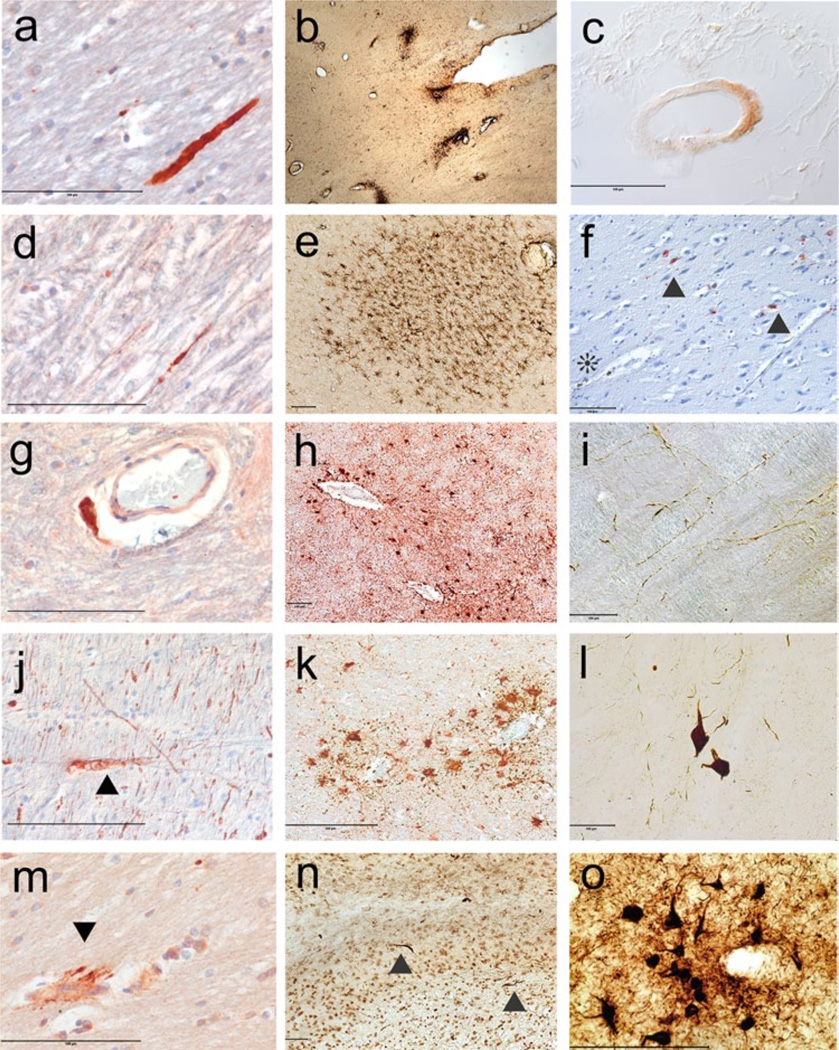
Fig. 1.
The neuropathology of acute concussive injury. a Axonal swelling in the corpus callosum (Table 1, case 2), APP immunostaining, 10 m-paraffin section. b Perivascular clusters of activated microglia in the corpus callosum and subcortical white matter (Table 1, case 1), LN3 immunostain, 50 mm free-floating section. c Vascular amyloid in leptomeningeal vessel (Table 1, case 6), Ab 42 immunostain, 10 m paraffin section. d Persistent axonal swelling in the cerebellar white matter (Table 1, case 6), APP immunostain, 10 m paraffin section. e Perivascular microglia around deep white matter vessel (Table 1, case 3), 50 mm free-floating section. f Hemosiderin-laden macrophages around a small blood vessel indicating a remote microbleed (asterisk) in close proximity to focal PHF-tau NFTs and neurites in the uncus (arrowhead) (Table 1, case 5). AT8 immunostain with hematoxylin counterstain, 10 m paraffin section. g Perivascular axonal spheroid in the cerebellar white matter (Table 1, case 6). APP immunostain, 10 m paraffin section. h Perivascular reactive astrocytes in white matter (Table 1, case 3), GFAP immunostain, 10 m paraffin section. i PHF-tau immunoreactive neurites in the medulla (Table 1, case 3), AT8 immunostain, 50 mm free-floating section. j Axonal swelling with digestion chamber in the midbrain tegmentum (Table 1, case 5), SMI 34 immunostain, 10 m-paraffin section. k Perivascular reactive astrocytes in white matter (Table 1, case 2), GFAP immunostaining, 10 m-paraffin section. l PHF-tau immunoreactive NFTs in the reticular formation of the medulla (Table 1, case 3), AT8 immunostain, 50 mm free-floating section. m Perivascular axonal swellings (arrowhead) (Table 1, case 2), APP immunostaining, 10 m-paraffin section. n TDP-43 immunoreactive neurites in the fornix (arrowheads) (Table 1, case 1), TDP-43 immunostain, 50 mm free-floating section. o PHF-tau immunoreactive perivascular NFTs and neurites in the frontal cortex (Table 1, case 3), AT8 immunostain, 50 mm free-floating section. All magnification bars 100 mm
Imaging studies after concussion
The structural changes in the brain after concussive injury, including traumatic axonal injury (TAI), are not detectable with conventional structural imaging studies, including computed tomography (CT) scan and magnetic resonance imaging (MRI). Diffusion tensor imaging (DTI), however, provides information about the white matter microstructure and fiber tract integrity and is emerging as a valuable tool in refining the diagnosis, prognosis, and management of mTBI [5, 149, 196]. In addition, the severity of symptoms after mTBI correlates with reduction of white matter integrity on DTI, suggesting that persistent microstructural brain injury underlies the symptoms of PCS [45]. There have also been reports of alterations in brain activation through blood oxygen level-dependent (BOLD) signals, resting state functional connectivity [120, 168], magnetic resonance spectroscopy [184], and SPECT imaging after concussion, although the neuroanatomical sites involved vary across different studies. Functional MRI (fMRI) studies have detected significant alterations in brain activation patterns in individuals with persistent symptoms after mTBI [26, 74]. These abnormal brain activation patterns can remain for months after injury, despite normal neurocognitive task performance [115, 122]. The discrepancy between fMRI and neurocognitive testing may be the result of functional re-allocation of neurocognitive resources as a compensatory mechanism, followed by a more prolonged period of microstructural recovery [45]. In a study of mTBI patients using fMRI to assess the neural correlate of working memory, patients with more severe post-concussive symptoms showed increased brain activity in the normal working memory network, as well as the recruitment of brain regions outside this network [171].
Chronic neurodegeneration including CTE
Clinical studies
Several studies have examined the relationship between exposure to repetitive brain trauma and long-term cognitive deficits and depression. Specifically, surveys of retired professional football players found that players who reported sustaining three or more concussions were significantly more likely to report cognitive symptoms, including a threefold increase in self-reported significant memory impairment and a fivefold increase in diagnosed mild cognitive impairment [77]. Multiple studies have also demonstrated cognitive deficits in retired NFL players on neurological and neuropsychological examination [63, 87, 157] that in some studies correlated with white matter pathology on DTI and FLAIR imaging as well as alterations in regional cerebral blood flow [84, 87]. Retired NFL players with only modest behavioral performance deficits on an executive task showed pronounced hyperactivation and hypoconnectivity of the dorsolateral frontal and frontopolar cortices using an fMRI-optimized neuropsycho-logical test of executive function [84]. A study looking at the cause of death in retired NFL players who played for 5 years or more also found elevated rates of death due to AD and amyotrophic lateral sclerosis (ALS). Although the death certificates indicated AD or ALS, there was no neuropathological verification and the actual diagnoses might well have included CTE and CTE-MND [109]. Clinical and functional impairments in cognition have also been correlated with the frequency of impacts to the head in high school and collegiate football players wearing helmet-mounted accelerometers [16, 121, 180], although not all helmet sensor studies have supported this relationship [81].
Brain trauma experienced in sport has also been linked to disturbances in mood. The survey of retired professional football players conducted by Guskiewicz and colleagues found that athletes who experienced three or more concussions had a threefold increase in diagnosed depression [78]. A follow-up survey administered 9 years later provided further evidence for a dose–response relationship between self-reported concussions and depressive symptoms later in life. Neuropsychological assessment in former professional football players has confirmed the relationship between increased self-reported concussions and depression [52]. Depression in these athletes is also associated with increased fractional anisotropy on DTI, as well as white matter abnormalities on structural imaging [87, 176].
Clinical symptoms in CTE
In 1928, Harrison Martland, a New Jersey pathologist, outlined a symptom complex well recognized in professional pugilists that appeared to result from repeated sublethal blows to the head [118]. In his monograph, ‘Punch Drunk’, Martland described unsteadiness of gait, mental confusion, and slowing of muscular movements, occasionally combined with hesitancy in speech, tremors of the hands, and nodding of the head. This condition was referred to as “dementia pugilistica”, “traumatic progressive encephalopathy”, and “chronic traumatic encephalopathy” (CTE) to highlight its chronic and progressive nature [43, 44]. The clinical symptoms associated with this disease were examined in case summaries throughout the twentieth century; however, the disease presentation described in these reports was inconsistent [38, 65, 92, 100, 126]. In 2013, the largest single cohort of neuropathologically confirmed cases of CTE was reported [127], and a subset of these cases without co-morbid neurologic disease was supplemented with eight additional cases to identify clinical features distinctive for CTE [175]. Specifically, two presentations of CTE were found, one that consisted of behavioral and mood symptoms usually appearing in the third decade of life (mean age at onset of 35 years) and another consisting of cognitive impairment and memory loss developing in the fifth decade (mean age at onset of 59 years). The majority of subjects (86 %) who presented with initial behavior or mood symptoms progressed to have cognitive symptoms and memory disturbances before passing away (mean age at death of 51 years), whereas behavioral and mood disorders less commonly developed (46 %) in the cohort presenting with cognitive impairment (mean age at death of 69 years). The earlier CTE literature also suggests two differing disease presentations, but proper validation will require additional longitudinal clinical studies. It will also be important to determine if other factors account for the difference in presentations, such as genetics, other environmental exposures such as use of performance-enhancing drugs, and evolving changes in the play of modern American football compared to football played decades ago.
Neuropathology of CTE
Although isolated neuropathological reports of CTE appeared in the literature in the 1950s, it was not until 1973, with the detailed description of the clinical and neuropathological features of 15 retired boxers by Corsellis, Bruton and Freeman-Browne [38], that a relatively stereotyped pathological pattern of structural brain abnormalities began to emerge. These changes included cerebral atrophy with enlargement of the lateral and third ventricles, thinning of the corpus callosum, cavum septum pellucidum with fen-estrations, and cerebellar scarring. Neuronal loss was noted in the cerebellar tonsils and substantia nigra with Cresyl violet stain, and there was neurofibrillary degeneration of the substantia nigra and cerebral cortex using Von Braunmühl’s silver stain. Senile plaques were found in 27 % of cases. The observation that PHF-tau immunoreactive NFTs in CTE are preferentially distributed to the superficial layers of the cerebral cortex (layers II and upper third of layer III) was made by Hof, who noted the similarity between the superficial distribution of NFTs in CTE and two other environmentally triggered tauopathies, post-encephalitic Parkinson disease and Guamanian ALS parkinsonism/dementia [91]. In addition to a different topography of NFTs in CTE compared to AD, the size of individual NFTs in CTE is generally larger and the neurites are more dot like and spindle shaped [128]. The conspicuous perivascular nature of the neurofibrillary pathology and its tendency to be irregularly concentrated at the sulcal depths of the cortex in CTE were first noted by Geddes in her description of the neuropathological alterations of five young men, 23– 28 years old, including two boxers, one soccer player, one developmentally challenged individual with a long history of head banging, and a poorly controlled epileptic patient who frequently hit his head during seizures [65, 66]. Geddes described argyrophilic, PHF-tau positive neocortical NFTs and neurites, strikingly arranged in groups around small intracortical blood vessels, in the absence of Ab [65], a feature that has recently been found to distinguish even early stages of CTE from other tauopathies.
Phosphorylated tau pathology
The focal collections of perivascular NFTs in the depths of the cortical sulci in CTE [65, 66, 126–128] differ substantially from PHF-tau pathology of the Alzheimer type reported in some cognitively normal young adults [15], opiate abusers [4], and non-demented elderly subjects such as those in the Framingham Heart Study [6] or Honolulu Asian Aging Study [67, 194]. Additionally, medial temporal lobe PHF-tau pathology is not found in early stages of CTE, while it is considered the hallmark of emerging or preclinical AD pathology.
CTE is similar to AD in its tau isoform profile and phosphorylation state [165], and neuronal tau pathology is immunoreactive to both 3R and 4R tau [128]. Astrocytic PHF-tau pathology in CTE is predominantly 4R tau immunopositive, but more widely distributed than that reported in the medial temporal lobe in aging and AD [103, 114]. Characteristically, the locus coeruleus shows neurofibrillary PHF-tau pathology in early stages of CTE, which can be substantial even in individuals under the age of 30. Although some studies have interpreted phosphorylated tau abnormalities in the locus coeruleus to be an early stage of AD pathology [15], young control subjects were not screened for a history of exposure to mTBI despite the fact that mTBI is common among young individuals and that 17 % of the control subjects died from acute trauma or accidental death.
The patchy, perivascular location of the PHF-tau pathology in sulcal depths, features prominent in early CTE, is most likely related to the physics of traumatic injury and shear deformation of the brain. Stress and resultant axonal injury are greatest at the interface of two tissues with differing viscoelastic properties (such as between blood vessels and brain, or between gray and white matter) and the depths of the cortical sulci are areas of stress concentration [11, 28, 33]. In addition, the local distribution of TAI to the subcortical white matter at the sulcal depths correlates with the distribution of PHF-tau pathology in the overlying cortex [72]. The early and predominant involvement of PHF-tau pathology in the superior and dorsolateral frontal lobes in football players parallels the high frequency of impacts to the top of the head compared to those to the front, back, and side of the head in football [79], as well as fMRI data showing activation impairment in dorsolateral prefrontal cortex that is associated with significantly higher numbers of head collisions to the top-front of the head [180].
Many factors might influence the initiation and accelerate the propagation of PHF-tau pathology in CTE, including age at initial traumatic exposure, gender, physiological stress, or environmental influences such as alcohol, opiates, or performance-enhancing drugs. Rodent studies have shown that glucocorticoids and stress increase tau phosphorylation and cognitive deficits [75, 144, 173], and PHF-tau immunoreactivity in opiate user brains is significantly higher than in age-matched controls [4]. In addition, the developing brain may be more susceptible to poor outcomes following TBI. Studies have suggested that children and adolescents experience prolonged recovery rates after TBI compared to adults [61] and poorer outcome [70]. Factors that might contribute to increased vulnerability to trauma in the developing brain include age-specific differences in myelination rates, synaptic pruning, neurotrophic factor levels, brain water content, cerebral blood flow, and glucocorticoid receptor expression [69, 76, 183].
Ab in CTE
In early reports of dementia pugilistica or CTE using Ab immunohistochemistry, it was determined that 95 % of cases of CTE showed widespread diffuse Ab deposits. This, coincident with the demonstration of Ab deposits in up to 30 % of cases of acute severe trauma and APP abnormalities after axonal injury [98, 145, 159, 169], provoked a surge of interest in the role of Ab and APP in CTE pathogenesis. However, despite evidence for Ab deposition in moderate to severe TBI [94], Ab plaques have not been found in early or mild stages of CTE in young individuals [65, 66, 72, 91, 126] but occur in older subjects with CTE in association with increased age at death [128]. Nonetheless, it is possible that APP or some form of Ab, such as oligomeric Ab, plays a role in CTE pathogenesis, although to date it has not been demonstrated.
Staging system
Provisional criteria for the pathological diagnosis of CTE and four pathological stages of severity have been proposed based on the topography of PHF-tau pathology [128]; formal consensus validation of these diagnostic and staging criteria by a large group of neuropathologists is currently in progress (National Institute of Neurological Diseases and Stroke, 1UO1NS086659–01). The contributions of other pathologies to the diagnostic and staging criteria, including TDP-43 immunoreactivity, axonal and neuronal loss, remain to be established.
Stages of CTE pathology
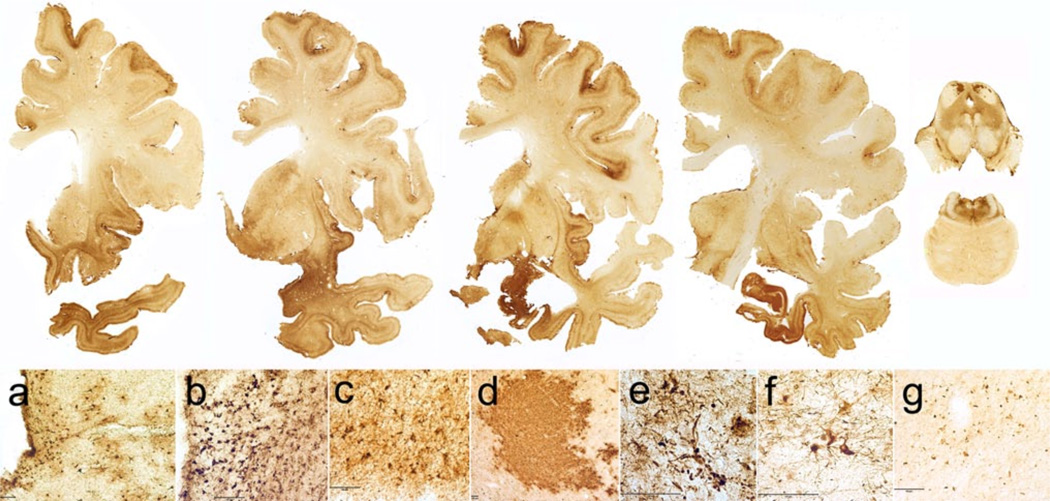
Stage I Brains with Stage I CTE are grossly unremarkable. Microscopically, there are one or two isolated perivascular foci of PHF-tau NFTs and dot-like neuropil threads, most commonly found at the depths of cerebral sulci of the frontal cortex (Fig. 2) and usually in association with subpial PHF-tau positive astrocytes. NFTs are also commonly found in the locus coeruleus. About half of Stage I cases will also have abnormal TDP-43 inclusions within the sub-cortical frontal white matter and fornix [128]. Ab plaques or vascular amyloid deposits are not found.
Fig. 2.
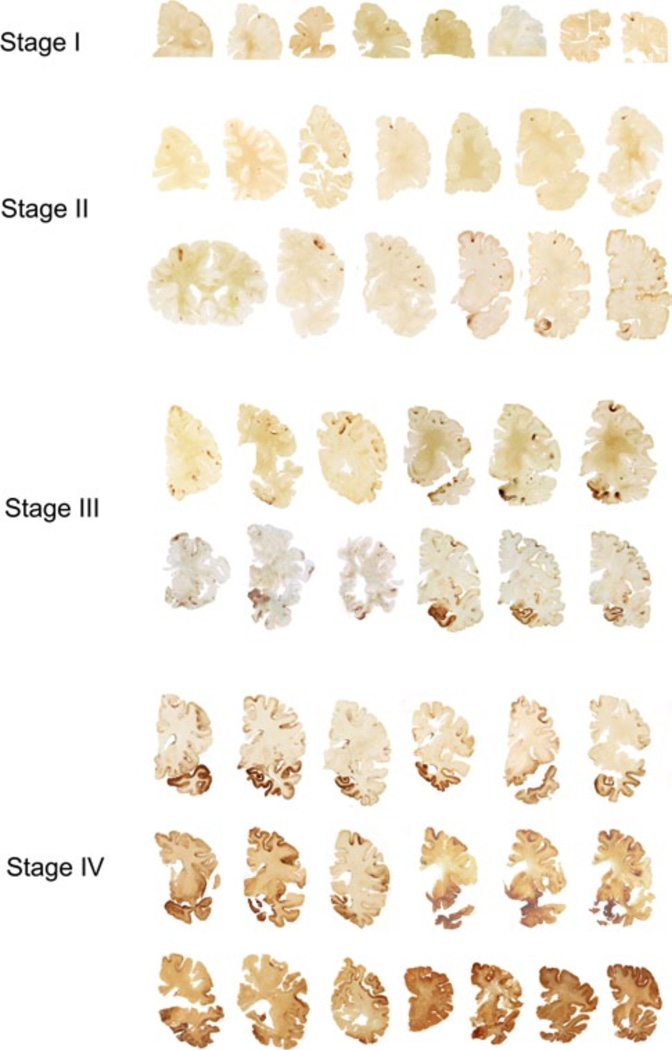
The four stages of chronic traumatic encephalopathy. In Stage I CTE, PHF-tau pathology is restricted to discrete foci in the cerebral cortex, most commonly in the superior, dorsolateral or lateral frontal cortices, and typically around small vessels at the depths of sulci. In Stage II CTE, there are multiple epicenters at the depths of the cerebral sulci and spread of neurofibrillary pathology to the superficial layers of adjacent cortex. The medial temporal lobe is spared neurofibrillary PHF-tau pathology in Stage II CTE, although it becomes progressively more involved as disease severity increases. In Stage III, PHF-tau pathology is widespread; the frontal, insular, temporal, and parietal cortices, amygdala, hippocampus, and entorhinal cortex show widespread neurofibrillary pathology. In Stage IV CTE, there is widespread severe PHF-tau pathology affecting most regions of the cerebral cortex and the medial temporal lobe, sparing calcarine cortex in all, but the most severe cases. All images, CP-13 immunostained 50 m tissue sections
This stage is most likely preclinical, although some subjects with Stage I pathology report vague and non-specific symptoms such as headache, loss of attention and concentration, short-term memory difficulties, and depression.
Stage II Half of the cases with Stage II CTE show subtle macroscopic changes including mild enlargement of the lateral and third ventricles, cavum septum pellucidum, and pallor of the locus coeruleus and substantia nigra. Microscopically, multiple foci of PHF-tau pathology are found at the depths of the sulci, commonly in the frontal, temporal, parietal, insular, and septal cortices (Figs. 2, 3, 6). NFTs are also found in superficial layers of the adjacent cerebral cortex, locus coeruleus and substantia innominata, but the medial temporal lobe structures are spared. Deep structures such as the substantia nigra, dorsal and median raphe, and thalamus show mild neurofibrillary degeneration. TDP-43 pathology consists of rare neuropil threads and inclusions within cerebral subcortical white matter, medial temporal lobe, and brainstem. In Stage II CTE in our series, Ab plaques were found only in an 87-year-old former NFL player, and vascular Ab was found only in a 28-year-old former NHL player.
Fig. 3.
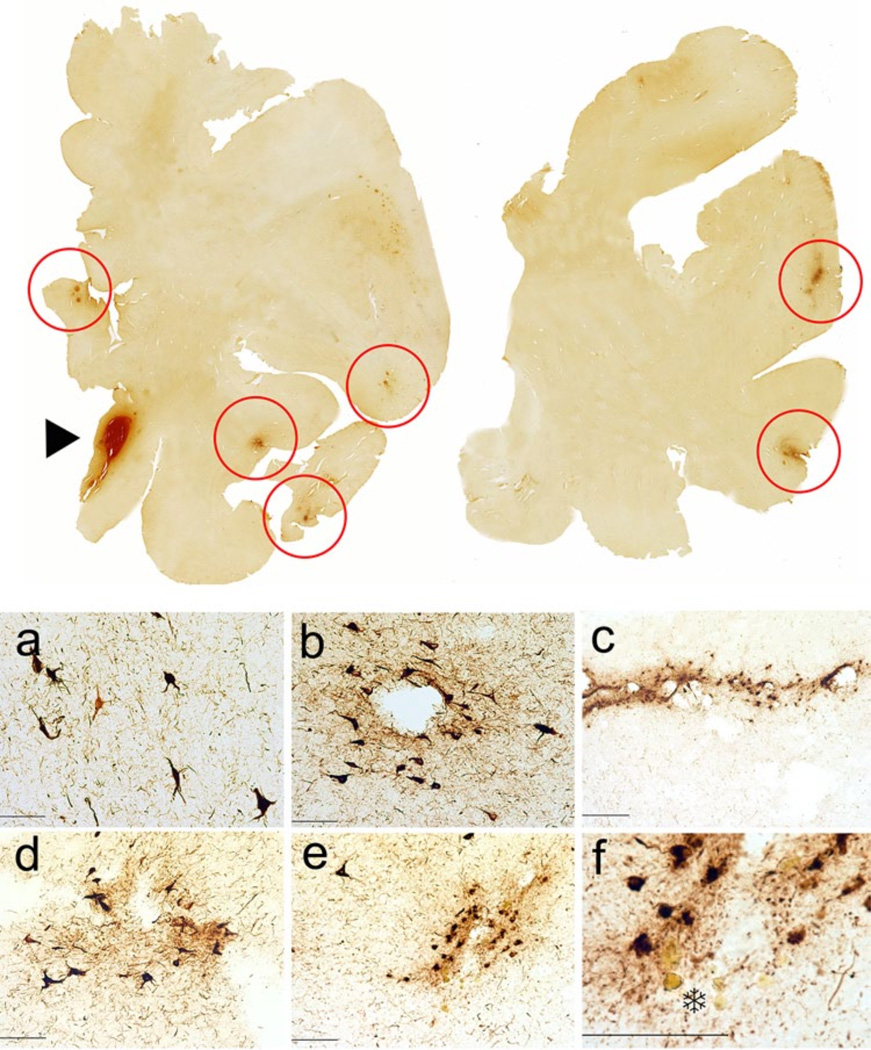
CTE Stage II in a 36-year-old former Major League baseball player. To p row whole mount sections of occipital and frontal cortex; CP-13 immunostained 50 m tissue sections. Red circles indicate clusters of neurofibrillary pathology, which are primarily perivascular and concentrated at the sulcal depths. The arrowhead shows an area of acute hemorrhage secondary to the fatal gunshot. a–e Neurofibrillary pathology in the frontal and occipital cortices, showing a tendency for the PHF-tau pathology to be perivascular, subpial, and more concentrated at the depths of the cerebral sulci. CP-13 immunostain, 50 mm free-floating sections. f A focus of PHF-tau neurofibrillary pathology surrounding a small blood vessel. There are hematoidin-laden macrophages around the vessel (asterisk) and focal neuronal loss, changes indicative of a remote micro-bleed. CP-13 immunostain, 50 mm free-floating section. All magnification bars 100 mm
Fig. 6.
CTE Stage II with motor neuron disease in a 29-year-old soccer player who developed fatigue and weakness of his lower extremities and hands at age 27 and 3 months later was diagnosed with ALS. 21 months after the onset of his symptoms, he died of respiratory insufficiency at age 29. He had played soccer since the age of 3, and started heading the ball at age 5. He played soccer for 12 years in public school, 4 years in college, and 2.5 years as a semi-professional. a Whole mount sections showing clusters of perivascular PHF-tau immunoreactivity preferentially at the sulcal depths of the frontal, temporal, and parietal cortices; CP-13 immunostain. b Sections of lower medulla, cervical thoracic, and lumbar spinal cord show extreme loss of lateral (asterisks) and ventral corticospinal tracts; luxol fast blue, and hematoxylin and eosin stain. c Extreme loss of axons, myelin with macrophage infiltration in lateral corticospinal tracts; luxol fast blue, and hematoxylin and eosin stain. d Severe loss of anterior horn cells in the ventral horn of spinal cord; luxol fast blue, and hematoxylin and eosin stain. e–g Perivascular clusters of PHF-tau immunoreactive NFTs and neurites in cortex; CP-13 immunostain, 50 mm free-floating sections. h Axonal swellings and distorted axons and hemosiderin-laden macrophages around small blood vessel in cortical white matter; SMI-34 immunostain, 10 m paraffin section. i FUS immunoreactive intracytoplasmic inclusions, both rounded and fibrillar; FUS immunostain, 10 m paraffin section. j Ubiquilin immunoreactive nucleus; ubiquilin immunostain, 10 m-paraffin section. k PTDP-43 neurites in frontal cortex; phospho-rylated-TDP-43 immunostain, 50-mm free-floating section. l PTDP-43 neurites in Rolandic cortex; phosphorylated-TDP-43 immunostain, 50-mm free-floating section. m PTDP43 intraneuronal fibrillar inclusions and neurites in lumbar spinal cord; phosphorylated-TDP-43 immunostain, 50 mm free-floating section. n PTDP-43 immuno-reactivity in axon in lumbar spinal cord; phosphorylated-TDP-43 immunostain, 50-mm free-floating section. o PTDP immunoreactive intracytoplasmic inclusions and neurites; phosphorylated-TDP-43 immunostain, 10 m-paraffin section. p PHF-tau immunoreactive intra-cytoplasmic granular inclusions; AT8 immunostain, 10 m-paraffin section
Symptoms in Stage II CTE are variable, but in some instances there are prominent behavioral and personality changes, paranoia, irritability, depression, headaches, and short-term memory loss. In our autopsy series, suicide claimed the life of 33 % NFL players with Stage II CTE and suicide combined with drug or alcohol abuse accounted for 55 % of deaths (Table 2).
Table 2.
Causes of death in former NFL athletes with CTE (without other pathological diagnoses)
| n | Age | Cause of death | |
|---|---|---|---|
| Stage I CTE | 3 | 37.7–16.8 years | 1 cardiac, 1 suicide, 1 malignancy |
| Stage II CTE | 9 | 48.1–20.0 years | 3 cardiac, 3 suicide, 1 alcohol-related, 1 drug use, 1 malignancy |
| Stage III CTE | 9 | 59.9–11.5 years | 2 cardiac, 3 suicide, 2 overdose, 1 accidental gunshot wound, 1 malignancy |
| Stage IV CTE | 8 | 81.5–7.3 years | 2 cardiac, 2 respiratory, 3 FTT, 1 malignancy, |
| Overall | 27 | 63.9–17.4 years |
CTE chronic traumatic encephalopathy, FTT failure to thrive secondary to dementia, NFL National Football League
Stage III In Stage III CTE, most brains show reduced brain weight, mild frontal and temporal atrophy, and enlargement of the lateral and third ventricles. Septal abnormalities are common (50 %), including cavum septum pellucidum or septal fenestrations. There is often pallor of the locus coeruleus and substantia nigra, atrophy of the mammillary bodies, thalamus and hypothalamus, and thinning of the corpus callosum. Microscopically, NFTs are present diffusely in the frontal, temporal, and parietal cortices and are most concentrated around small vessels and at the depths of sulci. The hippocampus, entorhinal cortex, amygdala, nucleus basalis of Meynert, and locus coeruleus show extensive NFTs. NFTs are also present in hypothalamus, mammillary bodies, substantia nigra, and dorsal and median raphe nuclei. In about one-third of cases, NFTs are also found in the dentate nucleus of the cerebellum and spinal cord. The majority of cases show TDP-43-positive neurites and inclusions in cerebral cortex, medial temporal lobe, diencephalon, and brainstem. Ab deposition is found in 13 % of cases as sparse diffuse and neuritic Ab plaques and vascular amyloid.
Symptoms in Stage III CTE include memory loss, executive dysfunction, explosivity, difficulty with attention and concentration, and aggression. 75 % of subjects in our series were considered cognitively impaired. As in Stage II, 33 % of former NFL players with Stage III CTE committed suicide, and suicide combined with drug or alcohol abuse led to 55 % of deaths in Stage III CTE in our series (Table 2).
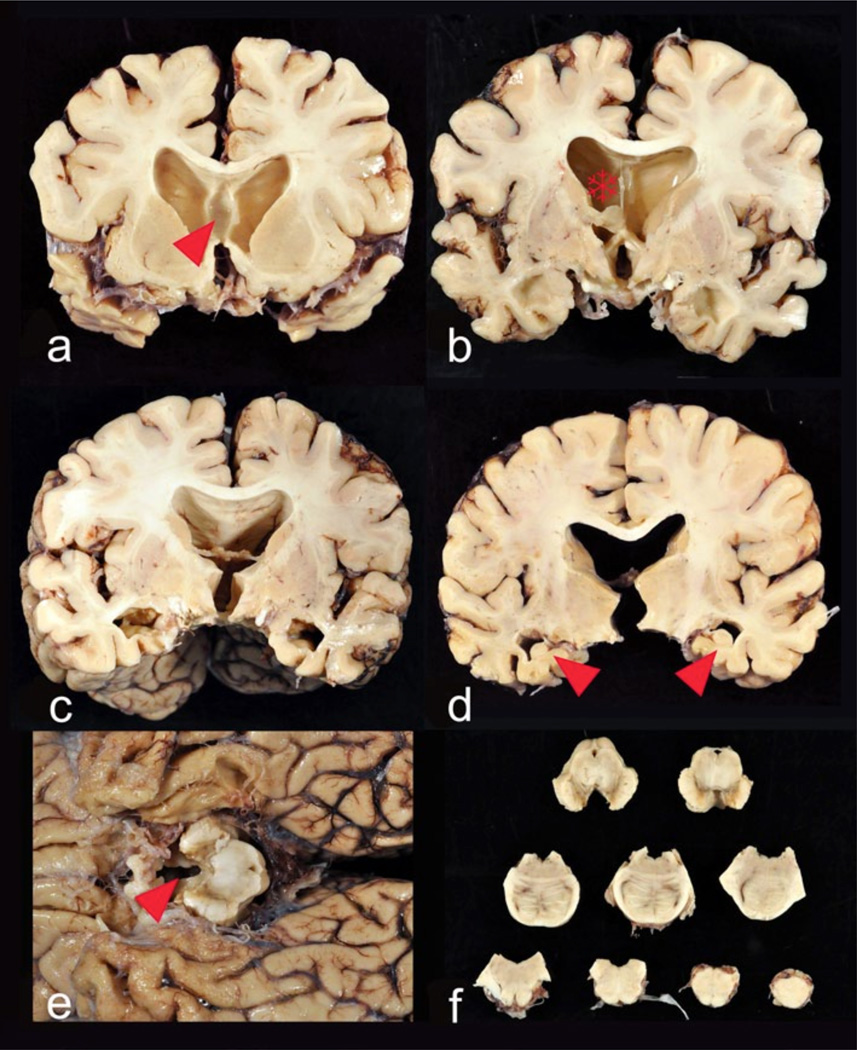
Stage IV Brains with Stage IV CTE usually show significant reduction of brain weight (Fig. 4), with pronounced atrophy of the frontal and temporal lobes, medial temporal lobe, and anterior thalamus. The hypothalamic floor is thinned, the mammillary bodies are darkly discolored and atrophied, and there is marked enlargement of the lateral and third ventricles. Approximately, 2/3 of subjects will have septal abnormalities including cavum septum pellucidum, fenestrations, or absence. There is generalized atrophy of the white matter, and the posterior body of the corpus callosum is disproportionately thin. The locus coeruleus and substantia nigra are pale.
Fig. 4.
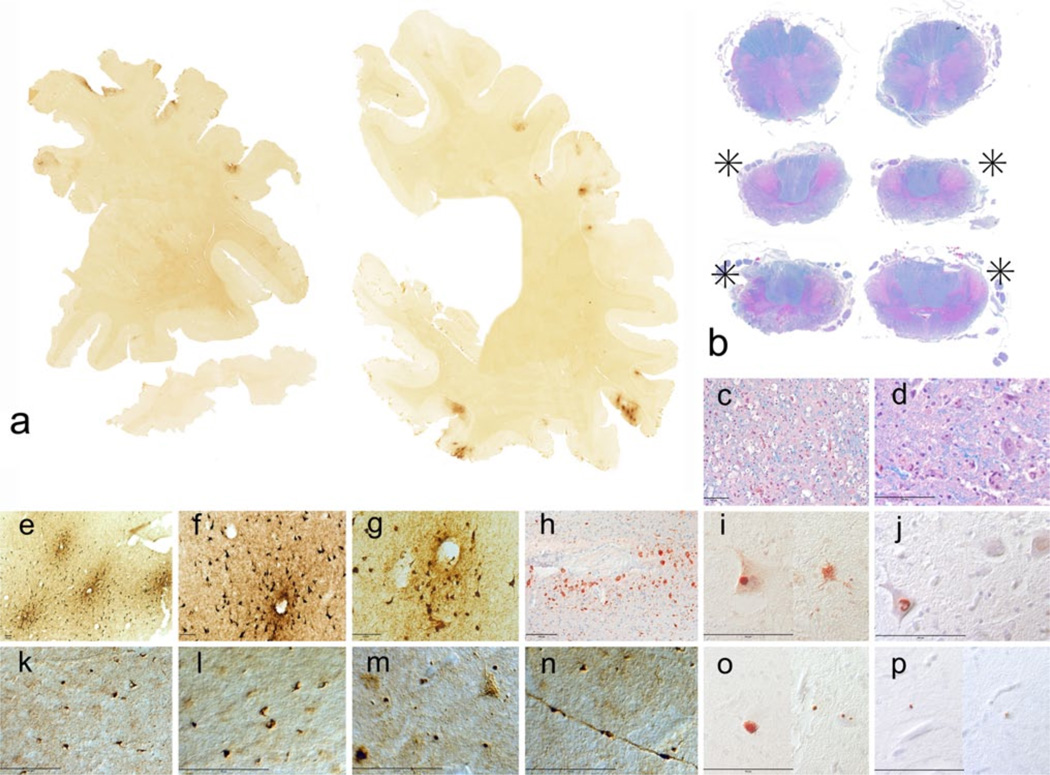
Gross neuropathological findings in a 77-year-old former Australian Rules rugby player who died with severe dementia and Stage IV CTE. Cognitive problems, memory loss, attention difficulties, and executive dysfunction were first noted in his mid-50s, followed by depression and anxiety, worsening explosivity and impulsivity. By his mid-60s, he was physically and verbally abusive, paranoid, and severely demented. He began playing rugby at age 13, and played for 19 years in U21 and senior leagues. a At autopsy, the brain weighed 1,030 g and showed severe atrophy and ventricular enlargement with a prominent cavum septum pellucidum (arrowhead). b–c The mid-portion of the septum pellucidum (asterisk) is reduced to a thin filament with severe atrophy of the fornix, thalamus, hypothalamus, mammillary bodies, amygdala, anterior hippocampus, and entorhinal cortex. d There is bilateral hippocampal atrophy (arrowheads). e The floor of the hypo-thalamus is severely thinned and the mammillary bodies are severely atrophic (arrowhead). f Brainstem sections show pallor of the pars compacta of the substantia nigra and locus coeruleus, with discoloration of the frontal tracts of the cerebral peduncle
Microscopically, there is severe spongiosus of layer 2 of the cerebral cortex and widespread neuronal loss. Neurons in the substantia nigra are severely depleted. There is prominent, patchy, widespread myelin loss and astrocytosis of the white matter of the cerebral hemispheres with perivascular macrophage deposition. There is also severe PHF-tau deposition as clusters of glial tangles and small NFTs in a patchy irregular distribution throughout the frontal, temporal, and parietal cortices (Fig. 5). Neurofibrillary degeneration is extremely severe in insula, septal area, temporal cortex, amygdala, hippocampus, entorhinal cortex, substantia nigra, and locus coeruleus. The calcarine cortex is relatively spared, though 39 % of cases will show some PHF-tau pathology. In Stage IV CTE, NFTs are found widely distributed throughout the hippocampal formation including the dentate gyrus, CA3, CA2, and CA4. CA1 is typically severely sclerotic, with few remaining neurons, numerous ghost tangles and PHF-tau immunoreactive astrocytes. In Stage IV CTE, PHF-tau pathology also generally involves the cerebellum, including the dentate nucleus and granule cell layer and the medial lemniscus and inferior olives of the medulla. There is marked loss and distortion of axons throughout the cerebral and cerebellar white matter. TDP-43 deposition is severe and widespread with dense accumulations of dot-like and thread-like inclusions in neurites and intra-neuronal cytoplasmic inclusions in all cases.
Fig. 5.
Microscopic neuropathological findings in a 77-year-old former Australian Rules rugby player with Stage IV CTE. To p row whole mount coronal sections of the brain and brainstem; CP-13 immunostained 50 m tissue sections. There is widespread PHF-tau immunoreactive neurofibrillary pathology. a–f Microscopic sections show extreme neuronal loss and PHF-tau pathology; CP-13 immunostaining, 50 mm free-floating sections. a. Frontal cortex. b Periventricular caudate. c Temporal cortex. d Superior colliculus. e Substantia nigra pars compacta. f Locus coeruleus. g P-TDP-43 immunoreactive inclusions and neurites in the frontal cortex; pTDP-43 immunostaining, 50 mm free-floating sections. All magni cation bars 100 mm
Symptoms in Stage IV CTE include severe executive dysfunction and memory loss with dementia. Most subjects also show profound loss of attention and concentration, language difficulties, explosivity, aggressive tendencies, paranoia, depression, and gait and visuospatial difficulties. In our autopsy series, none of the athletes diagnosed with Stage IV CTE died from suicide or drug overdose (Table 2).
TDP-43 pathology
Abnormal TDP-43 pathology is found in all stages of CTE. In early stages the inclusions consist of neuritic threads and dot-like inclusions in subpial, perivascular, and periventricular regions; cytoplasmic neuronal inclusions are first seen in Stage II disease and are characteristic of late stages where they partially co-localize with PHF-tau inclusions [128].
Axonal pathology in CTE
In addition to PHF-tau pathology, axonal pathology is present at all stages of CTE and becomes more severe as CTE stage advances [72, 128]. In early CTE, distorted axonal profiles are found in the cortex, subcortical white matter, and deep white matter tracts of the diencephalon. By stages III and IV, there is severe axonal loss and pathological profiles throughout the subcortical white matter, particularly the frontal and temporal lobes.
Chronic traumatic encephalopathy with co-morbid degenerative disease
CTE is associated with the development of other neurode-generations, notably Lewy body disease (LBD), AD, fron-totemporal lobar degeneration (FTLD), and motor neuron disease (MND) [127, 128]. In our current experience of 103 neuropathologically confirmed cases of CTE, co-existent LBD was found in 12 (12 %) cases, MND in 13 (13 %), AD in 15 (15 %), and FTLD in 6 (6 %), suggesting that either repetitive trauma or the accumulation of tau pathology in CTE provokes the deposition of other abnormal proteins involved in neurodegeneration [127, 128].
CTE as an acquired frontotemporal lobar degeneration
TDP-43 is an RNA-binding protein that regulates RNA metabolism, including mRNA splicing, stability, and transport [167]. After acute traumatic injury in animal models, TDP-43 expression is upregulated and TDP-43 relocates from the neuronal nucleus to accumulate in the neuronal cytoplasm [134, 135] TDP-43 binds to many cellular transcripts including tau and alpha synuclein, and its dysregulation may underlie some of the pathologies seen with these proteins [162]. In particular, TDP-43 may influence tau iso-form expression [136]. There is also evidence that alteration in tau protein metabolism including hyperphosphorylation, tau phosphatase resistance, and deposition of PHF-tau intracellular aggregates may be found in diseases characterized by abnormal TDP-43 metabolism, such as ALS [177]. As CTE is accompanied by a range of symptoms reflective of frontotemporal dysfunction, including behavioral and cognitive deficits and a dysexecutive syndrome, and is associated with frontotemporal lobar atrophy, superficial spongiosus, neuronal loss, deposition of tau and TDP-43, and MND, it is increasingly considered an acquired fronto-temporal lobar degeneration (FTLD).
CTE with motor neuron disease
Some data suggests that trauma and athletic exposure are risk factors for developing ALS [30, 31, 166], although there are conflicting reports regarding single versus repetitive head injury [24]. Recent data on American football players who played professionally for more than five seasons show the risk of dying from ALS is more than four times greater than age- and gender-matched controls [109]. In our current experience of 103 neuropathologically confirmed cases of CTE, there have been 13 individuals who developed a progressive motor neuron disease (MND) (13 %) [128]. Most subjects (63 %) presented with motor weakness, atrophy, and fasciculations indistinguishable from sporadic ALS and developed mild cognitive and behavioral symptoms several years after the onset of motor symptoms. Individuals who present with motor symptoms of MND have milder CTE at death (Stages II–III), a reflection of their shortened life span, whereas those who present with cognitive symptoms die with advanced CTE (Stage III and IV). In all cases, there is a distinct TDP-43 proteinopathy affecting the brain and spinal cord (Fig. 6) [128].
CTE in sports
Although CTE was first identified in association with boxing, CTE has been diagnosed neuropathologically in American football players, professional and amateur boxers, ice hockey players, professional wrestlers, soccer players, rugby players, and a professional baseball player (Figs. 3, 4, 5, 6) [38, 65, 66, 88, 91, 127, 128, 146–148].
American football
American football is the most common sport associated with CTE, accounting for more than half of reported cases; 75 % of reported football players with CTE played at the professional level. In different autopsy series, the percentage of former professional American football players who have died and donated their brains to research varies from 50 % [88], 80 % [146], to 97 %) [128]. While many football players had a history of repeated concussions, some did not, suggesting that exposure to football even in the absence of symptomatic or reported concussions is associated with CTE. Among American football players, the stage of CTE at death significantly correlates with age at death, number of years playing football, and number of years after retirement from football [128].
Soccer
There have been reports in the lay press about soccer players with CTE [138] and early changes of CTE were by noted by Geddes in an amateur soccer player [65]. We have also confirmed CTE-MND in a 29-year-old semi-professional soccer player (Fig. 6). In soccer players, it is unclear what role heading of the ball and cervical spine injury play in the development of CTE and MND; this determination has considerable importance, as heading is considered a feature of soccer potentially amenable to restriction or elimination. Recently, soccer players who head the ball more than 1,800 times per year were found to have microstructural abnormalities in the temporo-occipital white matter on DTI that correlated with poorer memory scores [111]. ALS incidence and mortality are also reported to be unusually high among professional soccer players in Italy [30, 31]. The 29-year-old soccer player with CTE-MND in our series played soccer since the age of 3 and frequently headed the ball; at autopsy, PHF-tau neu-rofibrillary changes were extensive in both the frontal and posterior temporo-occipital regions. The demonstration of CTE- MND in a young soccer player, college and professional football players, and boxers raises the possibility that the neuropathological substrate of MND in some athletes might be CTE-MND.
Rugby
The demonstration of CTE in a professional rugby player (Figs. 4, 5), as well as a semi-professional soccer player (Fig. 6) indicate that CTE can affect players of non-hel-meted sports. These findings have important implications on potential rule changes in helmeted sports, as it has been argued that risk compensation in helmeted sports has increased the incidence of brain injury [82]. The diagnosis of CTE in non-helmeted sports suggests that risk compensation alone is insufficient in reducing brain trauma burden below the threshold of developing disease. However, whether the risk of developing CTE in rugby players and athletes in other non-helmeted sports is as high as players of helmeted sports remains to be determined and will require future epidemiologic studies of CTE.
Ice hockey
In our series, CTE was found in the brains of five hockey players, including four former NHL players. Three of the hockey players were enforcers, which likely contributed to their overall exposure to repetitive brain trauma. Hockey players experience a very different profile of linear and rotational head impacts compared to football players as measured by helmet sensor studies [42, 195]. Elucidating the relationship between the nature of these exposures and the resulting neurodegenerative disease may uncover the type of impacts responsible for the pathogenesis of CTE.
Trauma exposure
The sequence of neuropathological changes in CTE—first as a single focus and later as multiple perivascular foci of PHF-tau at the depths of the cerebral sulci—and their association with acute axonal injury and microbleeds suggest that an initial trauma may induce a focal change in PHF-tau and that continued exposure to repetitive trauma may induce multifocal, more widespread PHF-tau changes (Nowinski, personal communication). Whether an isolated PHF-tau lesion advances to involve adjacent brain parenchyma may depend on additional factors, including age, gender, exposure to other toxins, and genetic susceptibilities. It is possible, even likely, that some individuals develop a single isolated focus of PHF-tau that never progresses. The progression of CTE from a multifocal state (Stage II) to widespread disease (Stage III) might represent a period of exponential increase in PHF-tau accumulation, hypothetically from such mechanisms as protein templating or other modes of interneuronal spreading [128]. Stage II and III disease correlate with the onset of overt symptoms and mental distress, including depression and death due to suicide, alcohol, or drug overdose. Stage IV disease, characterized by widespread neuronal loss and neurodegen-eration, correlates with advanced memory loss, executive dysfunction, and dementia. The degree of aggregated PHF-tau and TDP-43 protein deposition, neuronal and axonal loss, neuroinflammation, cerebral atrophy and ventricular enlargement, as well as clinical symptoms, all appear to increase with longer survival.
Conclusion
The benefits of regular exercise, physical fitness, and sports participation are irrefutable. Physical activity reduces the risk for cardiovascular disease, type 2 diabetes, hypertension, obesity and stroke, and enhances psychological health. Exercise also reduces age-related loss of brain volume, improves cognition, and reduces the risk of developing dementia. Nonetheless, the play of sports is also associated with some risks, including risks for TBI, catastrophic traumatic injury and death. There is also growing awareness that repetitive minor TBIs may lead to persistent cognitive, behavioral, and psychiatric problems and, rarely, to the development of CTE. CTE has been reported most frequently in American football players and boxers, but players of other sports are also vulnerable, including those participating in ice hockey, professional wrestling, soccer, rugby, and baseball. The incidence and prevalence of CTE are not currently known and their precise determination will require longitudinal clinical studies and the capability to accurately diagnose CTE during life. All reported cases of CTE have had a history of repetitive mTBI, yet there continues to be vigorous debate surrounding the concept that a chronic neurodegeneration might be triggered by the traumatic impacts experienced in popular sports, a debate reminiscent of the controversies surrounding boxing in the last century [124, 125, 157]. Nevertheless, the risk of mTBI in sports is increasingly recognized, and changes in the play and management of popular sports have started to begin. Resistance is perhaps understandable, as accepting trauma as the primary cause for CTE would have enormous financial repercussions, as well as necessitate major changes in the play or management of many popular sports and constitute a paradigm shift in the science of neurode-generation. Further understanding of the precise relationship between traumatic exposure and the development of CTE will be greatly facilitated by efforts to develop a highly specific and sensitive way to diagnose CTE in living persons, such as with new PET tau ligands [29, 119, 198, 201], as well as prospective longitudinal studies with accurate measures of cumulative traumatic exposures. Neuropathological evaluation of the brains of athletes who have experienced repetitive brain trauma has already provided important information on the acute and long-term consequences of neurotrauma experienced in sport and will continue to be important for confirming clinical diagnoses, understanding pathogenetic mechanisms, developing biomarkers, and evaluating potential therapies.
Acknowledgments
We gratefully acknowledge the extraordinary help of Christopher Nowinski, Lisa McHale, Dr. Robert Stern, Dr. Robert Cantu, and all other members of the Center for the Study of Traumatic Encephalopathy at Boston University and Boston VA , as well as the individuals and families whose participation and contributions made this work possible. We also gratefully acknowledge the use of resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA) and Dr. Peter Davies for antibodies. This work was supported by the Department of Veterans Affairs; Veterans Affairs Biorepository (CSP 501); Translational Research Center for Traumatic Brain Injury and Stress Disorders (TRACTS); Veterans Affairs Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B6796-C); Sports Legacy Institute; National Institute of Aging Boston University Alzheimer’s Disease Center [P30AG13846; supplement 0572063345–5; National Institute of Aging Boston University Framingham Heart Study R01 [AG1649]; National Operating Committee on Standards for Athletic Equipment. This work was also supported by unrestricted gifts from the National Football League, the Andlinger Foundation, and Worldwide Wrestling Entertainment.
Contributor Information
Ann C. McKee, VA Boston HealthCare System, 150 South Huntington Ave, Boston, MA 02130, USA, ann.mckee@va.gov; amckee@bu.edu Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA, USA; Departments of Neurology, Boston University School of Medicine, Boston, MA, USA; Alzheimer Disease Center, Boston University School of Medicine, Boston, MA, USA; Center for the Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, MA, USA.
Daniel H. Daneshvar, Departments of Neurology, Boston University School of Medicine, Boston, MA, USA Center for the Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, MA, USA.
Victor E. Alvarez, Departments of Neurology, Boston University School of Medicine, Boston, MA, USA Center for the Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, MA, USA.
Thor D. Stein, VA Boston HealthCare System, 150 South Huntington Ave, Boston, MA 02130, USA Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA, USA; Alzheimer Disease Center, Boston University School of Medicine, Boston, MA, USA; Center for the Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, MA, USA.
References
- 1.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113(1):186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14(24):2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- 4.Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133(12):3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83(9):870–876. doi: 10.1136/jnnp-2012-302742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, Auerbach S, Green RC, Wolf PA, McKee AC. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9(6):673–686. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber BK, Erickson LD. Adolescent social initiative: antecedents in the ecology of social connections. J Adolesc Res. 2001;16:326–354. [Google Scholar]
- 8.Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56(9):1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartholomew J, Ciccolo JT. Exercise, depression, and cognition. In: Spirduso W, Poon L, Chodzko-Zajko W, editors. Exercise and its mediating effects on cognition. Human Kinetics, Champaign; 2008. [Google Scholar]
- 10.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344(8929):1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 13.Boone E, Leadbeater B. Game on: diminishing risks for depressive symptoms in early adolescence through positive involvement in team sports. J Res Adolesc. 2006;16(1):79–90. [Google Scholar]
- 14.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol (Berl) 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 16.Breedlove EL, Robinson M, Talavage TM, Morigaki KE, Yoruk U, O’Keefe K, King J, Leverenz LJ, Gilger JW, Nauman EA. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J Biomech. 2012;45(7):1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantu R, Gean A. Second impact syndrome in a small SDH: an uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. J Neurotrauma. 2010 doi: 10.1089/neu.2010.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- 21.Cantu RC, Guskiewicz K, Register-Mihalik JK. A retrospective clinical analysis of moderate to severe athletic concussions. PM R. 2010;2(12):1088–1093. doi: 10.1016/j.pmrj.2010.07.483. [DOI] [PubMed] [Google Scholar]
- 22.Cantu RC, Herring SA, Putukian M. Concussion. N Engl J Med. 2007;356(17):1787. doi: 10.1056/NEJMc070289. author reply 1789. [DOI] [PubMed] [Google Scholar]
- 23.Cantu RC, Mueller FO. The prevention of catastrophic head and spine injuries in high school and college sports. Br J Sports Med. 2009;43(13):981–986. doi: 10.1136/bjsm.2009.067728. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166(7):810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, Chuang JI, Wu FS, Liao PC, Jen CJ. Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89(4):489–496. doi: 10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen JK, Johnston KM, Petrides M, Ptito A. Recovery from mild head injury in sports: evidence from serial functional magnetic resonance imaging studies in male athletes. Clin J Sport Med. 2008;18(3):241–247. doi: 10.1097/JSM.0b013e318170b59d. [DOI] [PubMed] [Google Scholar]
- 27.Chen MF, Huang TY, Kuo YM, Yu L, Chen HI, Jen CJ. Early postinjury exercise reverses memory deficits and retards the progression of closed-head injury in mice. J Physiol. 2013;591(4):985–1000. doi: 10.1113/jphysiol.2012.241125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165(2):357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Shankle WR, Elizarov A, Kolb HC. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 30.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(3):472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 31.Chio A, Calvo A, Dossena M, Ghiglione P, Mutani R, Mora G. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler. 2009;10(4):205–209. doi: 10.1080/17482960902721634. [DOI] [PubMed] [Google Scholar]
- 32.Clifton GL, Ziegler MG, Grossman RG. Circulating cat-echolamines and sympathetic activity after head injury. Neuro-surgery. 1981;8(1):10–14. doi: 10.1227/00006123-198101000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Cloots RJ, Gervaise HM, van Dommelen JA, Geers MG. Biomechanics of traumatic brain injury: influences of the morphologic heterogeneities of the cerebral cortex. Ann Biomed Eng. 2008;36(7):1203–1215. doi: 10.1007/s10439-008-9510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 35.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins M, Lovell MR, Iverson GL, Ide T, Maroon J. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three-year prospective cohort study. Neurosurgery. 2006;58(2):275–286. doi: 10.1227/01.NEU.0000200441.92742.46. discussion 275–286. [DOI] [PubMed] [Google Scholar]
- 37.Concussion (mild traumatic brain injury) and the team physician: a consensus statement. Med Sci Sports Exerc. 2006;38(2):395–399. doi: 10.1249/01.mss.0000202025.48774.31. [DOI] [PubMed] [Google Scholar]
- 38.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 39.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Covassin T, Swanik CB, Sachs ML. Epidemiological considerations of concussions among intercollegiate athletes. Appl Neuropsychol. 2003;10(1):12–22. doi: 10.1207/S15324826AN1001_3. [DOI] [PubMed] [Google Scholar]
- 41.Crisco JJ, Fiore R, Beckwith JG, Chu JJ, Brolinson PG, Duma S, McAllister TW, Duhaime AC, Greenwald RM. Frequency and location of head impact exposures in individual collegiate football players. J Athl Train. 2010;45(6):549–559. doi: 10.4085/1062-6050-45.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crisco JJ, Wilcox BJ, Beckwith JG, Chu JJ, Duhaime AC, Rowson S, Duma SM, Maerlender AC, McAllister TW, Greenwald RM. Head impact exposure in collegiate football players. J Biomech. 2011;44(15):2673–2678. doi: 10.1016/j.jbiomech.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Critchley M. Hommage a Clovis Vincent. Paris: Maloine; 1949. Punch-drunk syndromes: the chronic traumatic encephalopathy of boxers. [Google Scholar]
- 44.Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. 1957;1(5015):357–362. doi: 10.1136/bmj.1.5015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28(2):189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. The Cochrane Database Syst Rev. 2013;5:CD005229. doi: 10.1002/14651858.CD005229.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. 2011;30(1):1–17. doi: 10.1016/j.csm.2010.08.006. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daneshvar DH, Riley DO, Nowinski CJ, McKee AC, Stern RA, Cantu RC. Long-term consequences: effects on normal development profile after concussion. Phys Med Rehabil Clin N Am. 2011;22(4):683–700. doi: 10.1016/j.pmr.2011.08.009. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006;42(4):273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 50.De Moor MH, Stubbe JH, Boomsma DI, De Geus EJ. Exercise participation and self-rated health: do common genes explain the association? Eur J Epidemiol. 2007;22(1):27–32. doi: 10.1007/s10654-006-9088-8. [DOI] [PubMed] [Google Scholar]
- 51.Dean PJ, Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Frontiers Hum Neurosci. 2013;7:30. doi: 10.3389/fnhum.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Didehbani N, Munro Cullum C, Mansinghani S, Conover H, Hart J., Jr Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28(5):418–424. doi: 10.1093/arclin/act028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 54.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 55.Eime RM, Young JA, Harvey JT, Charity MJ, Payne WR. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Activity. 2013;10:98. doi: 10.1186/1479-5868-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chad-dock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mai-ley E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43(8):615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague- Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 60.Ferron C, Narring F, Cauderay M, Michaud PA. Sport activity in adolescence: associations with health perceptions and experimental behaviours. Health Educ Res. 1999;14(2):225–233. doi: 10.1093/her/14.2.225. [DOI] [PubMed] [Google Scholar]
- 61.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 62.Fogelman D, Zafonte R. Exercise to enhance neurocognitive function after traumatic brain injury. PM R. 2012;4(11):908–913. doi: 10.1016/j.pmrj.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 63.Ford JH, Giovanello KS, Guskiewicz KM. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J Neurotrauma. 2013 doi: 10.1089/neu.2012.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67(1):80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol (Berl) 1999;98(2):171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 66.Geddes JF, Vowles GH, Robinson SF, Sutcliffe JC. Neurofibrillary tangles, but not Alzheimer-type pathology, in a young boxer. Neuropathol Appl Neurobiol. 1996;22(1):12–16. [PubMed] [Google Scholar]
- 67.Gelber RP, Petrovitch H, Masaki KH, Abbott RD, Ross GW, Launer LJ, White LR. Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc. 2012;60(1):118–123. doi: 10.1111/j.1532-5415.2011.03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495–503. [PMC free article] [PubMed] [Google Scholar]
- 69.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 70.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157(1):11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 72.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Bud-son AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gore S, Farrell F, Gordon J. Sports involvement as protection against depressed mood. J Res Adolesc. 2001;11:119–130. [Google Scholar]
- 74.Gosselin N, Bottari C, Chen JK, Petrides M, Tinawi S, de Guise E, Ptito A. Electrophysiology and functional MRI in post-acute mild traumatic brain injury. J Neurotrauma. 2011;28(3):329–341. doi: 10.1089/neu.2010.1493. [DOI] [PubMed] [Google Scholar]
- 75.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grieve SM, Korgaonkar MS, Clark CR, Williams LM. Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55(3):868–879. doi: 10.1016/j.neuroimage.2010.12.087. [DOI] [PubMed] [Google Scholar]
- 77.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–726. [DOI] [PubMed] [Google Scholar]
- 78.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 79.Guskiewicz KM, Mihalik JP, Shankar V, Marshall SW, Crow-ell DH, Oliaro SM, Ciocca MF, Hooker DN. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery. 2007;61(6):1244–1252. doi: 10.1227/01.neu.0000306103.68635.1a. discussion 1252–1243. [DOI] [PubMed] [Google Scholar]
- 80.Guskiewicz KM, Valovich McLeod TC. Pediatric sports-related concussion. PM R. 2011;3(4):353–364. doi: 10.1016/j.pmrj.2010.12.006. quiz 364. [DOI] [PubMed] [Google Scholar]
- 81.Gysland SM, Mihalik JP, Register-Mihalik JK, Trulock SC, Shields EW, Guskiewicz KM. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann Biomed Eng. 2012;40(1):14–22. doi: 10.1007/s10439-011-0421-3. [DOI] [PubMed] [Google Scholar]
- 82.Hagel B, Meeuwisse W. Risk compensation: a “side effect” of sport injury prevention? Clin J Sport Med. 2004;14(4):193–196. doi: 10.1097/00042752-200407000-00001. pii:00042752-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 83.Hamer M, Chida Y. Physical activity and risk of neurode-generative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 84.Hampshire A, Macdonald A, Owen AM. Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep. 2013;3:2972. doi: 10.1038/srep02972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanlon EM, Bir CA. Real-time head acceleration measurement in girls’ youth soccer. Med Sci Sports Exerc. 2012;44(6):1102–1108. doi: 10.1249/MSS.0b013e3182444d7d. [DOI] [PubMed] [Google Scholar]
- 86.Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Hal-stead M, Herring SA, Kutcher JS, Pana A, Putukian M, Roberts WO. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 87.Hart J, Jr, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, Conover H, Mansinghani S, Lu H, Cullum CM. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70(3):326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hazrati LN, Tartaglia MC, Diamandis P, Davis KD, Green RE, Wennberg R, Wong JC, Ezerins L, Tator CH. Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Frontiers Hum Neurosci. 2013;7:222. doi: 10.3389/fnhum.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 90.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 91.Hof PR, Bouras C, Buee L, Delacourte A, Perl DP, Morrison JH. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol (Berl) 1992;85(1):23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- 92.Hof PR, Knabe R, Bovier P, Bouras C. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol (Berl) 1991;82(4):321–326. doi: 10.1007/BF00308819. [DOI] [PubMed] [Google Scholar]
- 93.Hollis SJ, Stevenson MR, McIntosh AS, Shores EA, Collins MW, Taylor CB. Incidence, risk, and protective factors of mild traumatic brain injury in a cohort of Australian nonprofessional male rugby players. Am J Sports Med. 2009;37(12):2328–2333. doi: 10.1177/0363546509341032. [DOI] [PubMed] [Google Scholar]
- 94.Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, Pimlott S, Hutchinson PJ, Tavares A, Canales R, Mathis CA, Klunk WE, Aigbirhio FI, Coles JP, Baron JC, Pickard JD, Fryer TD, Stewart W, Menon DK. Amyloid Imaging With Carbon 11-Labeled Pittsburgh Compound B for Traumatic Brain Injury. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 96.Hu S, Ying Z, Gomez-Pinilla F, Frautschy SA. Exercise can increase small heat shock proteins (sHSP) and pre-and post-synaptic proteins in the hippocampus. Brain Res. 2009;1249:191–201. doi: 10.1016/j.brainres.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113(7):803–811. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 98.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Tro-janowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190(1):192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 100.Jordan BD, Kanik AB, Horwich MS, Sweeney D, Relkin NR, Petito CK, Gandy S. Apolipoprotein E epsilon 4 and fatal cerebral amyloid angiopathy associated with dementia pugilistica. Ann Neurol. 1995;38(4):698–699. doi: 10.1002/ana.410380429. [DOI] [PubMed] [Google Scholar]
- 101.Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, Love S, van den Maagdenberg AM, Haan J, Frants RR, Ferrari MD. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49(6):753–760. doi: 10.1002/ana.1031. [DOI] [PubMed] [Google Scholar]
- 102.Kunz M. Big count: 265 million playing football. Fifa Mag. 2007;7:10–15. [Google Scholar]
- 103.Lace G, Ince PG, Brayne C, Savva GM, Matthews FE, de Silva R, Simpson JE, Wharton SB. Mesial temporal astrocyte tau pathology in the MRC-CFAS ageing brain cohort. Dement Geriatr Cogn Disord. 2012;34(1):15–24. doi: 10.1159/000341581. [DOI] [PubMed] [Google Scholar]
- 104.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Applied physiology, nutrition, and metabolism. Physiologie appliquee, nutrition et metabolisme. 2007;32(1):76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 105.Lam JM, Hsiang JN, Poon WS. Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J Neurosurg. 1997;86(3):438–445. doi: 10.3171/jns.1997.86.3.0438. [DOI] [PubMed] [Google Scholar]
- 106.Langburt W, Cohen B, Akhthar N, O’Neill K, Lee JC. Incidence of concussion in high school football players of Ohio and Pennsylvania. J Child Neurol. 2001;16(2):83–85. doi: 10.1177/088307380101600203. [DOI] [PubMed] [Google Scholar]
- 107.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 108.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bock-xmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 109.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neuro-degenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (Part 1) Sports Med. 2008;38(12):1009–1024. doi: 10.2165/00007256-200838120-00005. [DOI] [PubMed] [Google Scholar]
- 111.Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, Lipton RB. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268(3):850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, Liao PC, Jen CJ. Upregulation of hippocampal TrkB and syn-aptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90(1):81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 113.Logan SM, Bell GW, Leonard JC. Acute subdural hematoma in a high school football player after 2 unreported episodes of head trauma: a case report. J Athl Train. 2001;36(4):433–436. [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Gonzalez I, Carmona M, Blanco R, Luna-Munoz J, Martinez-Mandonado A, Mena R, Ferrer I. Characterization of thorn-shaped astrocytes in white matter of temporal lobe in Alzheimer’s disease brains. Brain Pathol. 2013;23(2):144–153. doi: 10.1111/j.1750-3639.2012.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lovell MR, Pardini JE, Welling J, Collins MW, Bakal J, Lazar N, Roush R, Eddy WF, Becker JT. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2):352–359. doi: 10.1227/01.NEU.0000279985.94168.7F. discussion 359–360. [DOI] [PubMed] [Google Scholar]
- 116.Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747–755. doi: 10.1177/0363546511435626. [DOI] [PubMed] [Google Scholar]
- 117.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 118.Martland HS. Punch drunk. JAMA. 1928;91(15):1103–1107. [Google Scholar]
- 119.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski JQ, Lee VM, Ono M, Masamoto K, Takano H, Sahara N, Iwata N, Okamura N, Furumoto S, Kudo Y, Chang Q, Saido TC, Takashima A, Lewis J, Jang MK, Aoki I, Ito H, Higuchi M. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79(6):1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32(11):1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McAllister TW, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Tosteson TD, Crisco JJ, Brolinson PG, Duma SM, Duhaime AC, Grove MR, Turco JH. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78(22):1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53(6):1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 123.McCrory P. Does second impact syndrome exist? Clin J Sport Med. 2001;11(3):144–149. doi: 10.1097/00042752-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 124.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion in sport-the 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16(6):755–763. doi: 10.1016/j.jocn.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 125.McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, Sills A, Benson BW, Davis GA, Ellenbogen RG, Guskiewicz K, Herring SA, Iverson GL, Jordan BD, Kissick J, McCrea M, McIntosh AS, Maddocks D, Makdissi M, Purcell L, Putukian M, Schneider K, Tator CH, Turner M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 126.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Bud-son AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010 doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McQuillen JB, McQuillen EN, Morrow P. Trauma, sport, and malignant cerebral edema. Am J Forensic Med Pathol. 1988;9(1):12–15. doi: 10.1097/00000433-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Meaney DF, Smith DH, Shreiber DI, Bain AC, Miller RT, Ross DT, Gennarelli TA. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. 1995;12(4):689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- 131.Meehan WP3rd, Bachur RG. Sport-related concussion. Pediatrics. 2009;123(1):114–123. doi: 10.1542/peds.2008-0309. [DOI] [PubMed] [Google Scholar]
- 132.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58(7):1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miele VJ, Carson L, Carr A, Bailes JE. Acute on chronic subdural hematoma in a female boxer: a case report. Med Sci Sports Exerc. 2004;36(11):1852–1855. doi: 10.1249/01.mss.0000145470.16938.7a. [DOI] [PubMed] [Google Scholar]
- 134.Moisse K, Mepham J, Volkening K, Welch I, Hill T, Strong MJ. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL −/− mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1296:176–186. doi: 10.1016/j.brainres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 135.Moisse K, Volkening K, Leystra-Lantz C, Welch I, Hill T, Strong MJ. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: implications for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1249:202–211. doi: 10.1016/j.brainres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 136.Morales R, Green KM, Soto C. Cross currents in protein misfolding disorders: interactions and therapy. CNS Neurol Disord: Drug Targets. 2009;8(5):363–371. doi: 10.2174/187152709789541998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mori T, Katayama Y, Kawamata T. Acute hemispheric swelling associated with thin subdural hematomas: pathophysiology of repetitive head injury in sports. Acta Neurochirurgica Supplement. 2006;96:40–43. doi: 10.1007/3-211-30714-1_10. [DOI] [PubMed] [Google Scholar]
- 138.Morris S. Heading the ball killed striker. 2002 The Guardian, November 11. Retrieved from http://www.theguardian.com/uk/2002/nov/12/football.stevenmorris.
- 139.Mueller FO, Cantu RC. Catastrophic football injuries annual report. National Center for Catastrophic Injury Research. 2009 [Google Scholar]
- 140.Mueller FO, Cantu RC. Catastrophic sport injury research 26th annual report: fall 1982-Spring 2003. National Center for Catastrophic Injury Research. 2003 Spring. [Google Scholar]
- 141.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63(1):71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 142.Naunheim RS, Standeven J, Richter C, Lewis LM. Comparison of impact data in hockey, football, and soccer. J Trauma. 2000;48(5):938–941. doi: 10.1097/00005373-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 143.Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28(7):1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 144.Okawa Y, Ishiguro K, Fujita SC. Stress-induced hyperphosphorylation of tau in the mouse brain. FEBS Lett. 2003;535(1–3):183–189. doi: 10.1016/s0014-5793(02)03883-8. [DOI] [PubMed] [Google Scholar]
- 145.Olsson A, Csajbok L, Ost M, Hoglund K, Nylen K, Rosengren L, Nellgard B, Blennow K. Marked increase of beta-amyloid(1–42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251(7):870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 146.Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69(1):173–183. doi: 10.1227/NEU.0b013e318212bc7b. discussion 183. [DOI] [PubMed] [Google Scholar]
- 147.Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 1092–1083. [DOI] [PubMed] [Google Scholar]
- 148.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–134. [DOI] [PubMed] [Google Scholar]
- 149.Oni MB, Wilde EA, Bigler ED, McCauley SR, Wu TC, Yallampalli R, Chu Z, Li X, Hunter JV, Vasquez AC, Levin HS. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. J Child Neurol. 2010;25(8):976–984. doi: 10.1177/0883073809356034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oppenheimer DR. Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry. 1968;31(4):299–306. doi: 10.1136/jnnp.31.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pedersen S, Seidman E. Team sports achievement and self-esteem development among urban adolescent girls. Psychol Women Q. 2004;28(4):412–422. [Google Scholar]
- 152.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 153.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Physical activity guidelines advisory committee report. 2008 Available from http://www.health.gov/paguidelines/report/
- 155.Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2(1):1–12. [PubMed] [Google Scholar]
- 156.Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42(3):225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 157.Randolph C, Karantzoulis S, Guskiewicz K. Prevalence and characterization of mild cognitive impairment in retired national football league players. J Int Neuropsychol Soc. 2013;19(8):873–880. doi: 10.1017/S1355617713000805. [DOI] [PubMed] [Google Scholar]
- 158.Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196(1):126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 159.Roberts GW, Gentleman SM, Lynch A, Graham DI. Beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1433. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 160.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264(1):49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sa MJ. Exercise therapy and multiple sclerosis: a systematic review. J Neurol. 2013 doi: 10.1007/s00415-013-7183-9. [DOI] [PubMed] [Google Scholar]
- 162.Sato T, Takeuchi S, Saito A, Ding W, Bamba H, Matsuura H, Hisa Y, Tooyama I, Urushitani M. Axonal ligation induces transient redistribution of TDP-43 in brainstem motor neurons. Neuroscience. 2009;164(4):1565–1578. doi: 10.1016/j.neuroscience.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 163.Sattler C, Erickson KI, Toro P, Schroder J. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis. 2011;26(4):709–718. doi: 10.3233/JAD-2011-110548. [DOI] [PubMed] [Google Scholar]
- 164.Saunders RL, Harbaugh RE. The second impact in catastrophic contact-sports head trauma. JAMA. 1984;252(4):538–539. [PubMed] [Google Scholar]
- 165.Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanow-ski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol (Berl) 2001;101(5):518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- 166.Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291(1–2):22–29. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sephton CF, Cenik B, Cenik BK, Herz J, Yu G. TDP-43 in central nervous system development and function: clues to TDP-43-associated neurodegeneration. Biol Chem. 2012;393(7):589–594. doi: 10.1515/hsz-2012-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202(2):341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003;98(5):1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 170.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A. Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Hum Brain Mapp. 2009;30(9):2789–2803. doi: 10.1002/hbm.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Snyder AR, Martinez JC, Bay RC, Parsons JT, Sauers EL, Valovich McLeod TC. Health-related quality of life differs between adolescent athletes and adolescent nonathletes. J Sport Rehabil. 2010;19(3):237–248. doi: 10.1123/jsr.19.3.237. [DOI] [PubMed] [Google Scholar]
- 173.Sotiropoulos I, Catania C, Riedemann T, Fry JP, Breen KC, Michaelidis TM, Almeida OF. Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human tau. J Neurochem. 2008;107(2):385–397. doi: 10.1111/j.1471-4159.2008.05613.x. [DOI] [PubMed] [Google Scholar]
- 174.Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 175.Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Mon-tenigro PH, Riley DO, Fritts NG, Stamm JM, Robbins CA, McHale LIS, Stein TDVA, Goldstein LE, Budson AE, Kowall NW, Nowinski CJ, Cantu RC, McKee AC. Clinical presentation of neuropathologically-confirmed chronic traumatic encephalopathy in athletes. Neurology. in Press doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA, Hart J, Jr, Womack KB. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. 2013;81(1):25–32. doi: 10.1212/WNL.0b013e318299ccf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strong MJ, Yang W. The frontotemporal syndromes of ALS. Clinicopathological correlates. J Mol Neurosci. 2011;45(3):648–655. doi: 10.1007/s12031-011-9609-0. [DOI] [PubMed] [Google Scholar]
- 178.Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O’Keefe JH, Milani RV, Blair SN, Church TS. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J Off J Jpn Circ Soc. 2013;77(2):281–292. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 180.Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, Leverenz LJ. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma. 2013 doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thomas M, Haas TS, Doerer JJ, Hodges JS, Aicher BO, Garberich RF, Mueller FO, Cantu RC, Maron BJ. Epidemiology of sudden death in young, competitive athletes due to blunt trauma. Pediatrics. 2011;128(1):e1–e8. doi: 10.1542/peds.2010-2743. [DOI] [PubMed] [Google Scholar]
- 182.Tysvaer AT, Storli OV. Soccer injuries to the brain. A neurologic and electroencephalographic study of active football players. Am J Sports Med. 1989;17(4):573–578. doi: 10.1177/036354658901700421. [DOI] [PubMed] [Google Scholar]
- 183.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziale S, Zoccatelli G, Tavazzi B, Del Bol-gia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 185.Valois RF, Zullig KJ, Huebner ES, Drane JW. Physical activity behaviors and perceived life satisfaction among public high school adolescents. J Sch Health. 2004;74(2):59–65. doi: 10.1111/j.1746-1561.2004.tb04201.x. [DOI] [PubMed] [Google Scholar]
- 186.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84(4):699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- 189.Voelcker-Rehage C, Godde B, Staudinger UM. Physical and motor fitness are both related to cognition in old age. Eur J Neurosci. 2010;31(1):167–176. doi: 10.1111/j.1460-9568.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 190.Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev. 2013;37(9 Pt B):2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 191.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wojcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Frontiers Aging Neurosci. 2013;5:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grod-stein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 194.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18(3):713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 195.Wilcox BJ, Beckwith JG, Greenwald RM, Chu JJ, McAllister TW, Flashman LA, Maerlender AC, Duhaime AC, Crisco JJ. Head impact exposure in male and female collegiate ice hockey players. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 197.Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1319–1323. doi: 10.1016/j.apmr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 198.Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Su H, Szardenings AK, Walsh JC, Wang E, Yu C, Zhang W, Zhao T, Kolb HC. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 199.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 200.Yung LM, Laher I, Yao X, Chen ZY, Huang Y, Leung FP. Exercise, vascular wall and cardiovascular diseases: an update (part 2) Sports Med. 2009;39(1):45–63. doi: 10.2165/00007256-200939010-00004. [DOI] [PubMed] [Google Scholar]
- 201.Zhang W, Arteaga J, Cashion DK, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Szardenings AK, Wang E, Walsh JC, Xia C, Yu C, Zhao T, Kolb HC. A highly selective and specific PET tracer for imaging of Tau pathologies. J Alzheimers Dis. 2012;31(3):601–612. doi: 10.3233/JAD-2012-120712. [DOI] [PubMed] [Google Scholar]