Abstract
Tuberculosis, caused by the intracellular pathogen Mycobacterium tuberculosis, is the world's leading cause of death in humans from a single infectious agent. A safe and effective vaccine against this scourge is urgently needed. This study demonstrates that immunization with the 30-kDa major secretory protein, alone or in combination with other abundant extracellular proteins of M. tuberculosis, induces strong cell-mediated immune responses and substantial protective immunity against aerosol challenge with virulent M. tuberculosis bacilli in the highly susceptible guinea pig model of pulmonary tuberculosis. Protection is manifested by decreased clinical illness including decreased weight loss, reduced mortality, and decreased growth of M. tuberculosis in the lungs and spleens of immunized animals compared with sham-immunized controls. This study demonstrates that purified major extracellular proteins of M. tuberculosis are candidate components of a subunit vaccine against tuberculosis and provides compelling support for the concept that extracellular proteins of intracellular pathogens are key immunoprotective molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994 Jun;62(6):2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Major cytoplasmic membrane protein of Legionella pneumophila, a genus common antigen and member of the hsp 60 family of heat shock proteins, induces protective immunity in a guinea pig model of Legionnaires' disease. J Clin Invest. 1993 Feb;91(2):717–723. doi: 10.1172/JCI116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella induces humoral and cell-mediated immune responses and protective immunity across different serogroups of Legionella pneumophila and different species of Legionella. J Immunol. 1991 Jul 1;147(1):285–291. [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J Exp Med. 1989 Mar 1;169(3):691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995 Jan 1;181(1):257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. K., Bartow R. A., Mintzer C. L., McMurray D. N. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect Immun. 1987 Feb;55(2):314–319. doi: 10.1128/iai.55.2.314-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle. 1985 Dec;66(4):267–276. doi: 10.1016/0041-3879(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Fine P. E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- Fok J. S., Ho R. S., Arora P. K., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. V. Lack of hematogenous dissemination of Mycobacterium tuberculosis to the lungs in animals vaccinated with Bacille Calmette-Guérin. J Infect Dis. 1976 Feb;133(2):137–144. doi: 10.1093/infdis/133.2.137. [DOI] [PubMed] [Google Scholar]
- Harth G., Clemens D. L., Horwitz M. A. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlert A., Young D. B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989 Feb;3(2):125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
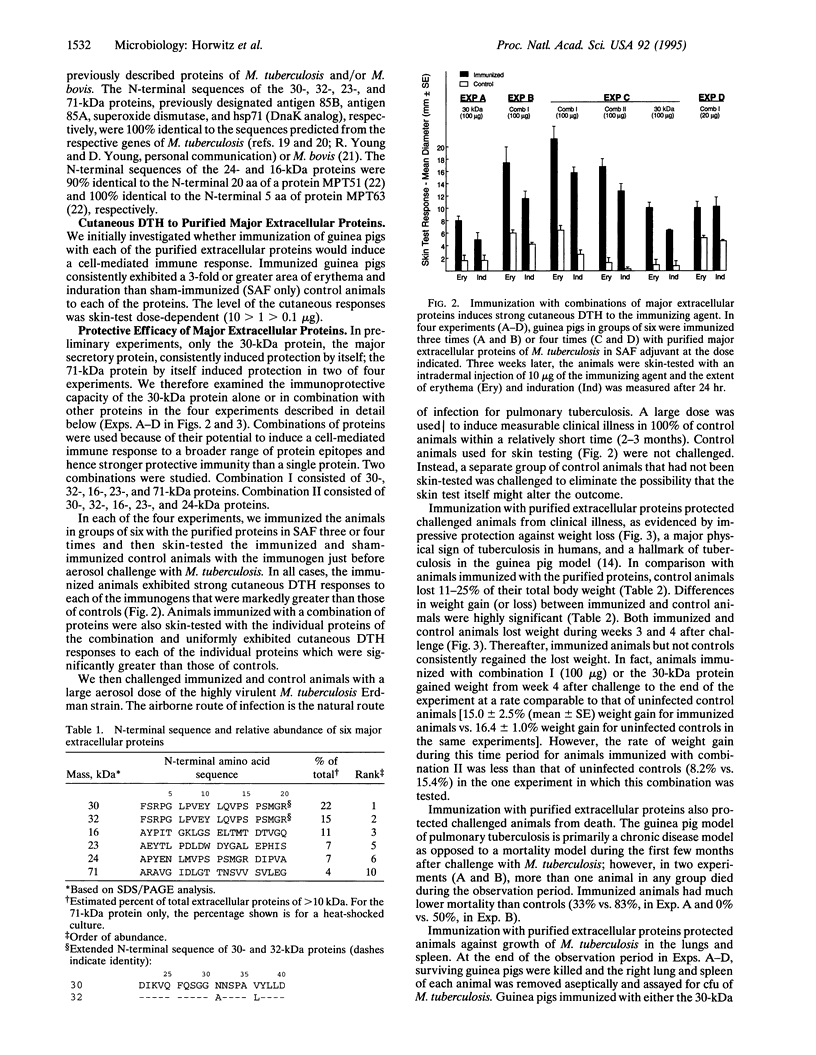
- Pal P. G., Horwitz M. A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992 Nov;60(11):4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C. Interactions of the human immunodeficiency virus and tuberculosis and the implications for BCG vaccination. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S379–S384. doi: 10.1093/clinids/11.supplement_2.s379. [DOI] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Schlesinger L. S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993 Apr 1;150(7):2920–2930. [PubMed] [Google Scholar]
- Smith D. W., McMurray D. N., Wiegeshaus E. H., Grover A. A., Harding G. E. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis. 1970 Dec;102(6):937–949. doi: 10.1164/arrd.1970.102.6.937. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Wiegeshaus E., Navalkar R., Grover A. A. Host-parasite relationships in experimental airborne tuberculosis. I. Preliminary studies in BCG-vaccinated and nonvaccinated animals. J Bacteriol. 1966 Feb;91(2):718–724. doi: 10.1128/jb.91.2.718-724.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]




