Abstract
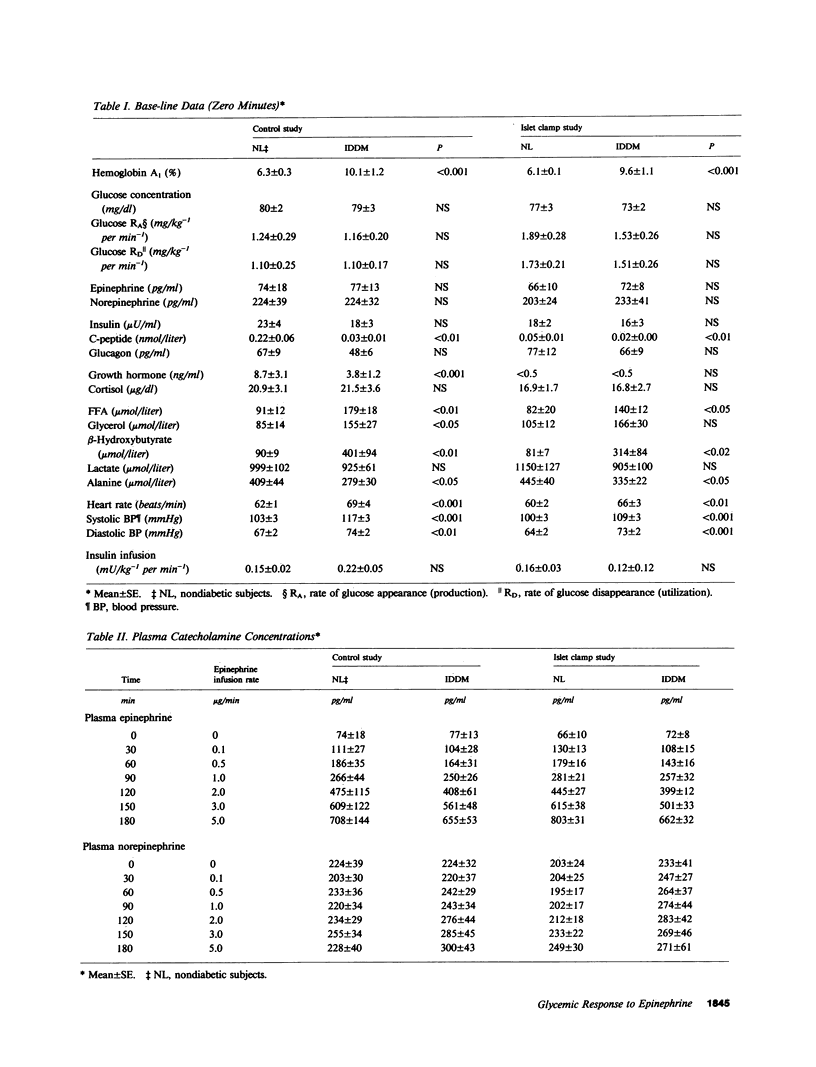
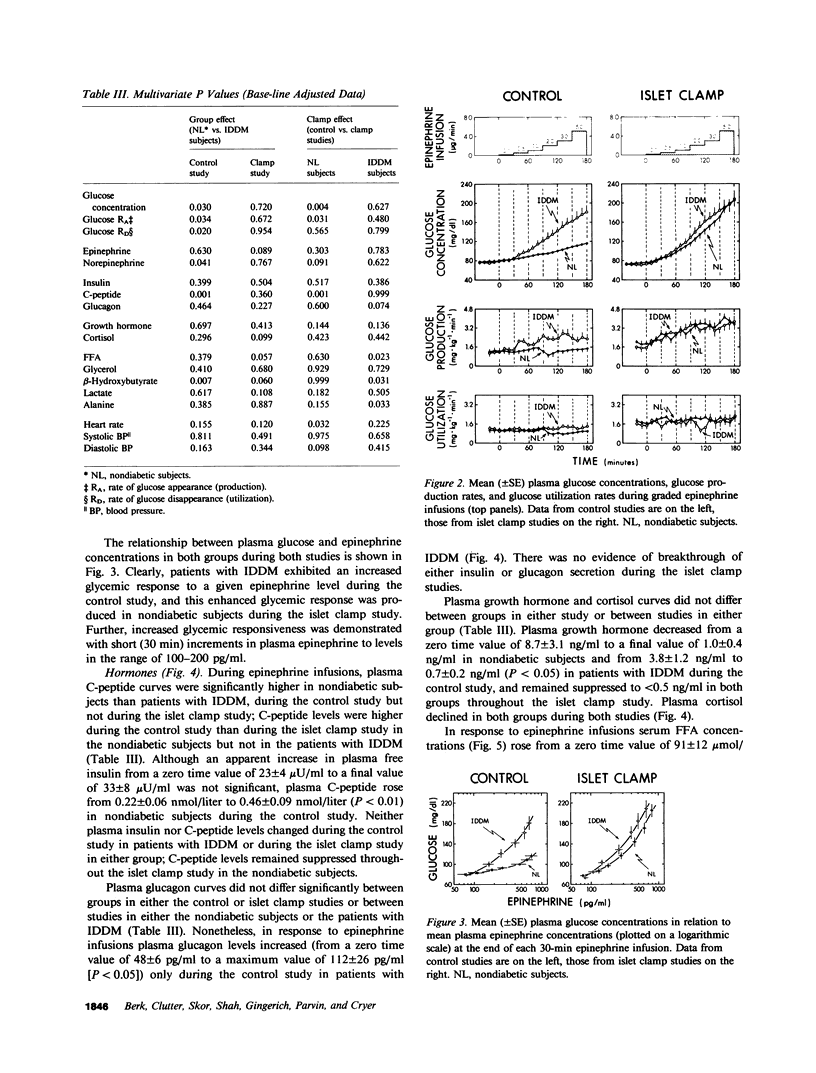
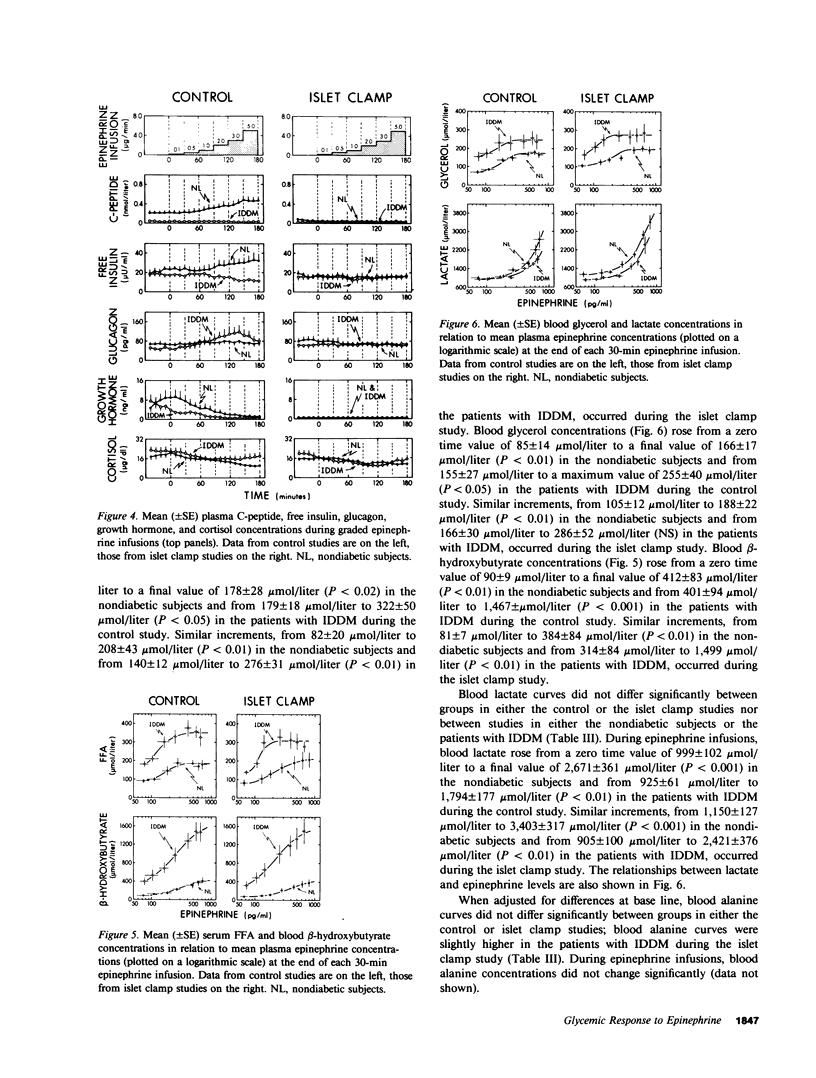
To determine if the enhanced glycemic response to epinephrine in patients with insulin-dependent diabetes mellitus (IDDM) is the result of increased adrenergic sensitivity per se, increased glucagon secretion, decreased insulin secretion, or a combination of these, plasma epinephrine concentration-response curves were determined in insulin-infused (initially euglycemic) patients with IDDM and nondiabetic subjects on two occasions: once when insulin and glucagon were free to change (control study), and again when insulin and glucagon were held constant (islet clamp study). During the control study, plasma C-peptide doubled, and glucagon did not change in the nondiabetic subjects, whereas plasma C-peptide did not change but glucagon increased in the patients. The patients with IDDM exhibited threefold greater increments in plasma glucose, largely the result of greater increments in glucose production. This enhanced glycemic response was apparent with 30-min increments in epinephrine to plasma concentrations as low as 100-200 pg/ml, levels that occur commonly under physiologic conditions. During the islet clamp study (somatostatin infusion with insulin and glucagon replacement at fixed rates), the heightened glycemic response was unaltered in the patients with IDDM, but the nondiabetic subjects exhibited an enhanced glycemic response to epinephrine indistinguishable from that of patients with IDDM. In contrast, the FFA, glycerol, and beta-hydroxybutyrate responses were unaltered. Thus, we conclude the following: Short, physiologic increments in plasma epinephrine cause greater increments in plasma glucose in patients with IDDM than in nondiabetic subjects, a finding likely to be relevant to glycemic control during the daily lives of such patients as well as during the stress of intercurrent illness. Enhanced glycemic responsiveness of patients with IDDM to epinephrine is not the result of increased sensitivity of adrenergic receptor-effector mechanisms per se nor of their increased glucagon secretory response; rather, it is the result of their inability to augment insulin secretion. Augmented insulin secretion, albeit restrained, normally limits the glycemic response, but not the lipolytic or ketogenic responses, to epinephrine in humans.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L., Kaye R., Haque N. Metabolic homeostasis in juvenile diabetes mellitus. II. Increased ketone responsiveness to epinephrine. Diabetes. 1969 Jun;18(6):421–427. doi: 10.2337/diab.18.6.421. [DOI] [PubMed] [Google Scholar]
- Benson J. W., Jr, Johnson D. G., Palmer J. P., Werner P. L., Ensinck J. W. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab. 1977 Mar;44(3):459–464. doi: 10.1210/jcem-44-3-459. [DOI] [PubMed] [Google Scholar]
- Beretta-Piccoli C., Weidmann P. Exaggerated pressor responsiveness to norepinephrine in nonazotemic diabetes mellitus. Am J Med. 1981 Nov;71(5):829–835. doi: 10.1016/0002-9343(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Chiasson J. L., Liljenquist J. E., Lacy W. W., Park C. R. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochem Soc Symp. 1978;(43):31–45. [PubMed] [Google Scholar]
- Cherrington A. D., Fuchs H., Stevenson R. W., Williams P. E., Alberti K. G., Steiner K. E. Effect of epinephrine on glycogenolysis and gluconeogenesis in conscious overnight-fasted dogs. Am J Physiol. 1984 Aug;247(2 Pt 1):E137–E144. doi: 10.1152/ajpendo.1984.247.2.E137. [DOI] [PubMed] [Google Scholar]
- Clutter W. E., Bier D. M., Shah S. D., Cryer P. E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980 Jul;66(1):94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer P. E. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980 Aug 21;303(8):436–444. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Deibert D. C., DeFronzo R. A. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980 Mar;65(3):717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing S. E., Lee J. C., Fripp R. R. Enhanced sensitivity of diabetic hearts to alpha-adrenoceptor stimulation. Am J Physiol. 1983 Nov;245(5 Pt 1):H808–H813. doi: 10.1152/ajpheart.1983.245.5.H808. [DOI] [PubMed] [Google Scholar]
- Engfeldt P., Arner P., Bolinder J., Ostman J. Phosphodiesterase activity in human subcutaneous adipose tissue in insulin- and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1982 Nov;55(5):983–988. doi: 10.1210/jcem-55-5-983. [DOI] [PubMed] [Google Scholar]
- Farmer R. W., Pierce C. E. Plasma cortisol determination: radioimmunoassay and competitive protein binding compared. Clin Chem. 1974 Apr;20(4):411–414. [PubMed] [Google Scholar]
- Foster D. W., McGarry J. D. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med. 1983 Jul 21;309(3):159–169. doi: 10.1056/NEJM198307213090307. [DOI] [PubMed] [Google Scholar]
- Galster A. D., Clutter W. E., Cryer P. E., Collins J. A., Bier D. M. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981 Jun;67(6):1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Tsalikian E., Karam J. H. Studies on the mechanism of epinephrine-induced hyperglycemia in man. Evidence for participation of pancreatic glucagon secretion. Diabetes. 1976 Jan;25(1):65–71. doi: 10.2337/diab.25.1.65. [DOI] [PubMed] [Google Scholar]
- Gray D. E., Lickley H. L., Vranic M. Physiologic effects of epinephrine on glucose turnover and plasma free fatty acid concentrations mediated independently of glucagon. Diabetes. 1980 Aug;29(8):600–608. doi: 10.2337/diab.29.8.600. [DOI] [PubMed] [Google Scholar]
- Gøtzsche O. The adrenergic beta-receptor adenylate cyclase system in heart and lymphocytes from streptozotocin-diabetic rats. In vivo and in vitro evidence for a desensitized myocardial beta-receptor. Diabetes. 1983 Dec;32(12):1110–1116. doi: 10.2337/diab.32.12.1110. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebretsen C. G., Hawelu-Johnson C., Ingebretsen W. R., Jr Alloxan-induced diabetes reduces beta-adrenergic receptor number without affecting adenylate cyclase in rat ventricular membranes. J Cardiovasc Pharmacol. 1983 May-Jun;5(3):454–461. doi: 10.1097/00005344-198305000-00018. [DOI] [PubMed] [Google Scholar]
- Kemmer F. W., Lickley H. L., Gray D. E., Perez G., Vranic M. State of metabolic control determines role of epinephrine-glucagon interaction in glucoregulation in diabetes. Am J Physiol. 1982 Jun;242(6):E428–E436. doi: 10.1152/ajpendo.1982.242.6.E428. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lacasa D., Agli B., Giudicelli Y. Effects of experimental insulin-dependent diabetes on the beta-adrenergic-receptor-coupled adenylate-cyclase system and lipolysis in fat cells of the rat. Eur J Biochem. 1983 Feb 15;130(3):457–464. doi: 10.1111/j.1432-1033.1983.tb07172.x. [DOI] [PubMed] [Google Scholar]
- MacLeod K. M., McNeill J. H. Alpha adrenoceptor mediated responses in aorta from three month streptozotocin diabetic rats. Proc West Pharmacol Soc. 1982;25:245–247. [PubMed] [Google Scholar]
- Martin M. J., Robbins D. C., Bergenstal R., LaGrange B., Rubenstein A. H. Absence of exercise-induced hypoglycaemia in type i (insulin-dependent) diabetic patients during maintenance of normoglycaemia by short-term, open-loop insulin infusion. Diabetologia. 1982 Oct;23(4):336–342. doi: 10.1007/BF00253741. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Rizza R. A., Haymond M. W., Gerich J. E. Effects of acute insulin deficiency on glucose and ketone body turnover in man: evidence for the primacy of overproduction of glucose and ketone bodies in the genesis of diabetic ketoacidosis. Diabetes. 1980 Nov;29(11):926–930. doi: 10.2337/diab.29.11.926. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Nosadini R., Noy G. A., Nattrass M., Alberti K. G., Johnston D. G., Home P. D., Orskov H. The metabolic and hormonal response to acute normoglycaemia in type 1 (insulin-dependent) diabetes: studies with a glucose controlled insulin infusion system (artificial endocrine pancreas). Diabetologia. 1982 Sep;23(3):220–228. doi: 10.1007/BF00252845. [DOI] [PubMed] [Google Scholar]
- Perez G., Kemmer F. W., Lickley H. L., Vranic M. Importance of glucagon in mediating epinephrine-induced hyperglycemia in alloxan-diabetic dogs. Am J Physiol. 1981 Oct;241(4):E328–E335. doi: 10.1152/ajpendo.1981.241.4.E328. [DOI] [PubMed] [Google Scholar]
- Pickup J. C., Keen H., Parsons J. A., Alberti K. G., Rowe A. S. Continuous subcutaneous insulin infusion: improved blood-glucose and intermediary-metabolite control in diabetics. Lancet. 1979 Jun 16;1(8129):1255–1257. doi: 10.1016/s0140-6736(79)92225-6. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Haymond M. W., Miles J. M., Verdonk C. A., Cryer P. E., Gerich J. E. Effect of alpha-adrenergic stimulation and its blockade on glucose turnover in man. Am J Physiol. 1980 May;238(5):E467–E472. doi: 10.1152/ajpendo.1980.238.5.E467. [DOI] [PubMed] [Google Scholar]
- Rizza R., Haymond M., Cryer P., Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol. 1979 Oct;237(4):E356–E362. doi: 10.1152/ajpendo.1979.237.4.E356. [DOI] [PubMed] [Google Scholar]
- Rosen S. G., Clutter W. E., Shah S. D., Miller J. P., Bier D. M., Cryer P. E. Direct alpha-adrenergic stimulation of hepatic glucose production in human subjects. Am J Physiol. 1983 Dec;245(6):E616–E626. doi: 10.1152/ajpendo.1983.245.6.E616. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clarke W. L., Shah S. D., Cryer P. E. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980 Oct;51(4):877–883. doi: 10.1210/jcem-51-4-877. [DOI] [PubMed] [Google Scholar]
- Savarese J. J., Berkowitz B. A. beta-Adrenergic receptor decrease in diabetic rat hearts. Life Sci. 1979 Dec 10;25(24-25):2075–2078. doi: 10.1016/0024-3205(79)90200-5. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Altered responsiveness to cortisol, epinephrine, and glucagon in insulin-infused juvenile-onset diabetics. A mechanism for diabetic instability. Diabetes. 1980 Apr;29(4):284–291. doi: 10.2337/diab.29.4.284. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Sherwin R. Beta-adrenergic blockade is more effective in suppressing adrenaline-induced glucose production in Type 1 (insulin-dependent) diabetes. Diabetologia. 1984 Mar;26(3):183–189. doi: 10.1007/BF00252404. [DOI] [PubMed] [Google Scholar]
- Sumi S., Mineo I., Kono N., Shimizu T., Nonaka K., Tarui S. Decreases in hepatic fructose-2,6-bisphosphate level and fructose-6-phosphate,2-kinase activity in diabetic mice: a close relationship to the development of ketosis. Biochem Biophys Res Commun. 1984 Apr 16;120(1):103–108. doi: 10.1016/0006-291x(84)91419-0. [DOI] [PubMed] [Google Scholar]
- Sundaresan P. R., Sharma V. K., Gingold S. I., Banerjee S. P. Decreased beta-adrenergic receptors in rat heart in streptozotocin-induced diabetes: role of thyroid hormones. Endocrinology. 1984 Apr;114(4):1358–1363. doi: 10.1210/endo-114-4-1358. [DOI] [PubMed] [Google Scholar]
- Sérusclat P., Rosen S. G., Smith E. B., Shah S. D., Clutter W. E., Cryer P. E. Mononuclear leukocyte beta 2-adrenergic receptors and adenylate cyclase sensitivity in insulin-dependent diabetes mellitus. Diabetes. 1983 Sep;32(9):825–829. doi: 10.2337/diab.32.9.825. [DOI] [PubMed] [Google Scholar]
- Valverde I., Dobbs R., Unger R. H. Heterogeneity of plasma glucagon immunoreactivity in normal, depancreatized, and alloxan-diabetic dogs. Metabolism. 1975 Sep;24(9):1021–1028. doi: 10.1016/0026-0495(75)90095-5. [DOI] [PubMed] [Google Scholar]
- Weiss M., Keller U., Stauffacher W. Effect of epinephrine and somatostatin-induced insulin deficiency on ketone body kinetics and lipolysis in man. Diabetes. 1984 Aug;33(8):738–744. doi: 10.2337/diab.33.8.738. [DOI] [PubMed] [Google Scholar]
- White N. H., Skor D., Santiago J. V. Practical closed-loop insulin delivery. A system for the maintenance of overnight euglycemia and the calculation of basal insulin requirements in insulin-dependent diabetics. Ann Intern Med. 1982 Aug;97(2):210–213. doi: 10.7326/0003-4819-97-2-210. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Schaible T. F., Scheuer J., Kennedy R. Effects of experimental diabetes on adrenergic and cholinergic receptors of rat myocardium. Diabetes. 1983 Oct;32(10):881–886. doi: 10.2337/diab.32.10.881. [DOI] [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Zumstein P., Froesch E. R. Increased sensitivity to epinephrine of the cyclic AMP--protein kinase system in adipose tissue of diabetic rats. FEBS Lett. 1978 Oct 1;94(1):43–46. doi: 10.1016/0014-5793(78)80902-8. [DOI] [PubMed] [Google Scholar]
- Zinman B., Stokes E. F., Albisser A. M., Hanna A. K., Minuk H. L., Stein A. N., Leibel B. S., Marliss E. B. The metabolic response to glycemic control by the artificial pancreas in diabetic man. Metabolism. 1979 May;28(5):511–518. doi: 10.1016/0026-0495(79)90190-2. [DOI] [PubMed] [Google Scholar]
- von Schenck H., Grubb A. O. Interference of immunoglobulins in two glucagon radioimmunoassays. Clin Chem. 1982 May;28(5):1103–1107. [PubMed] [Google Scholar]