Abstract
Background and objectives
Patients with CKD have a high prevalence of cardiovascular disease associated with or exacerbated by inactivity. This randomized, controlled study investigated whether a renal rehabilitation exercise program for patients with stages 3 or 4 CKD would improve their physical function and quality of life.
Design, setting, participants, & measurements
In total, 119 adults with CKD stages 3 and 4 were randomized, and 107 of these patients proceeded to usual care or the renal rehabilitation exercise intervention consisting of usual care plus guided exercise two times per week for 12 weeks (24 sessions). Physical function was determined by three well established performance-based tests: 6-minute walk test, sit-to-stand test, and gait-speed test. Health-related quality of life was assessed by the RAND 36-Item Short Form Health Survey.
Results
At baseline, no differences in self-reported level of activity, 6-minute walk test, and sit-to-stand test scores were observed between the usual care (n=48) and renal rehabilitation exercise (n=59) groups, although baseline gait-speed test score was higher in the renal rehabilitation exercise group (P<0.001). At follow-up, the renal rehabilitation exercise group but not the usual care group showed significant improvements in the 6-minute walk test (+210.4±266.0 ft [19% improvement] versus −10±219.9 ft; P<0.001), the sit-to-stand test (+26.9±27% of age prediction [29% improvement] versus +0.7±12.1% of age prediction; P<0.001), and the RAND-36 physical measures of role functioning (P<0.01), physical functioning (P<0.01), energy/fatigue levels (P=0.01), and general health (P=0.03) and mental measure of pain scale (P=0.04). The renal rehabilitation exercise regimen was generally well tolerated.
Conclusions
A 12-week/24-session renal rehabilitation exercise program improved physical capacity and quality of life in patients with CKD stages 3 and 4. Longer follow-up is needed to determine if these findings will translate into decreased mortality rates.
Keywords: CKD, exercise training, physical function, quality of life, renal rehabilitation exercise
Introduction
Cardiovascular disease (CVD) is the major source of morbidity and mortality in patients with CKD (1). The overall burden of CVD in the CKD population is nearly two times as great as that of patients without CKD (82.1% versus 45.2%) (1). This high prevalence is associated with and exacerbated by physical inactivity (2–4).
Cardiac rehabilitation in patients with CVD improves exercise capacity and overall quality of life and exerts beneficial effects on various CVD risk factors (2,5). Pooled data from randomized studies and population-based analyses indicate that cardiac rehabilitation significantly reduces major coronary events, cardiac mortality, and all-cause mortality (5,6).
Exercise training in patients with ESRD undergoing dialysis, studied for over 30 years, has been shown to improve physical functioning, cardiorespiratory fitness, cardiovascular risk, and health-related quality of life (7,8). In addition, patients physically active at the time of dialysis initiation survive longer compared with their sedentary counterparts (9). A recent observational study in patients with CKD found an association between physical performance and all-cause mortality (10). However, the effects of exercise training on patients with predialysis CKD are less well described. This study examined whether a renal rehabilitation exercise (RRE) program would improve physical function and health-related quality of life in patients with CKD stages 3 and 4.
Materials and Methods
Study Design
This single-center, randomized, controlled trial randomized 119 participants, of whom 107 participants received usual CKD clinic care alone (usual care [UC] group) or usual CKD care plus RRE for 12 weeks (RRE group). The study was conducted at Maine Nephrology Associates, a practice comprising 10 board-certified nephrologists also affiliated with the Maine Medical Center, in Portland, Maine. The Maine Medical Center Institutional Review Board approved the study, and it was conducted with adherence to the Declaration of Helsinki. The study is registered at ClinicalTrials.gov (Identifier NCT00792454).
Participants
The study included men and women 18 years of age or older with CKD stages 3 or 4 as calculated by the Modification of Diet in Renal Disease equation (GFR=30–59 or 15–29 ml/min per 1.73 m2, respectively) (11). This population was identified through International Classification of Diseases, 9th Edition, codes and screened consecutively by the principal investigator and study coordinator to determine additional eligibility of potential participants on the basis of predefined exclusion criteria.
The study excluded patients with angina pectoris, chronic lung disease resulting in significant shortness of breath or oxygen desaturation at rest, cerebral vascular disease manifested by transient ischemic attacks, active musculoskeletal conditions, lower-extremity amputation with no prosthesis, orthopedic disorder severely exacerbated by activity, metastatic carcinoma, or inability to follow directions because of language barrier or decreased mental capacity. In addition, the primary care nephrologist and primary care physician were required to give permission for their patient to enter the study after the potential intervention was described to them on the basis of their independent assessment of whether it was safe for their patient to participate in the event that they were randomized to the exercise intervention.
All patients with CKD stages 3 or 4 who met entry criteria were invited to participate in the trial. All participants provided written informed consent, at which time demographic data and vital signs were obtained. A glucose finger stick was performed on participants with diabetes as well. Baseline physical functioning and quality of life outcomes data were obtained at the next scheduled office visit after randomization to the study group but before the intervention was initiated.
Randomization
Participants were stratified by age (≤70 or >70 years of age) and CKD stage (3 or 4) and randomized to one of two study arms using computer-generated random numbers. Given the nature of the intervention, it was not possible to blind participants or their CKD providers to study group assignments.
Intervention
All patients received standard CKD clinic care according to the Kidney Disease Improving Global Outcomes clinical practice guidelines for management of CKD (12) as provided by their primary nephrologists within the group practice. In addition, those randomized to the RRE intervention were asked to participate in guided exercise two times per week for 12 weeks at selected physical therapy or cardiac rehabilitation facilities in the greater Portland area, where staff had been trained to implement an established exercise regimen. Participants with a cardiac history were sent to the cardiac rehabilitation facility; all others were free to choose among the facilities offered. Pre- and postassessments were conducted at the same facility for each patient.
Exercise sessions at these centers were conducted individually or in a group setting and consisted of cardiovascular, weight training (resistance), and stretching exercises. An exercise physiologist or physical therapist assessed cardiovascular and strength capabilities at the initial session according to the perceived level of exertion (PLE) scale (13) to prescribe the appropriate exercise regimen. The regimen limited participants to a PLE≤11, corresponding to a 60%–65% predicted maximal heart rate.
Cardiovascular exercises included treadmill walking and/or stationary cycling. Participants were instructed to increase the duration of cardiovascular exercises by 2–3 minutes each session (according to PLE) and enhance the intensity by increasing bicycle freewheel tension or treadmill speed or elevation. The goal of cycling or walking was to achieve 60 minutes of continuous exercise. Patients were also encouraged to exercise on their own and walk 5000–10,000 steps per day as measured on pedometers provided for this purpose.
Weight training consisted of upper and lower extremity extensions and flexions with free weights. Strengthening and stretching exercises, described in the Dialysis Patients’ Guide to Exercise (14), were started at one set of 10 repetitions of each exercise using 1- to 10-lb weights (according to tolerance) and increased to three sets of 15 repetitions, after which time weight was further increased.
Some participants experienced difficulties attending all exercise sessions in a consecutive fashion, and therefore, it was determined that the exercise period could be extended as needed to reasonably complete 24 exercise sessions.
Outcomes
Physical function and health-related quality of life were measured at baseline and on completion of the course of guided exercise in the RRE group and at comparable time periods in the UC group.
Physical Function Testing.
Three well established performance-based tests were used to assess changes in physical function. The 6-minute walk test (6MWT) measured the distance (in feet) that patients covered in 6 minutes while traversing a scored hallway (14–17). The sit-to-stand test (STST) measured lower-extremity muscle strength as a function of the time that patients needed to stand up from a seated position and sit back down 10 times from a chair of standardized height (18). The STST results were standardized as a percentage of age-predicted value using prediction formulas developed by Csuka and McCarty (19). Gait speed was measured as the time needed to travel 20 ft at a comfortable pace expressed in centimeters per second for comparison with normative values presented by Bohannon (20).
Self-reported level of activity was measured at baseline using a scale of 1–4 as described by Painter et al. (7): (1) activities of daily living only; (2) stretching or strengthening activity; (3) low cardiovascular exercise (walking or cycling less than three times weekly and/or <20 minutes per session); and (4) recommended cardiovascular exercise (at least three times per week for ≥20 minutes per session).
Health-Related Quality of Life.
The RAND 36-Item Short Form Health Survey (RAND-36) questionnaire was used to evaluate self-reported domains of health status (21). Questionnaires were self-administered at the study center by those participants capable of doing so independently. The research coordinator administered the questionnaire to patients unable to complete the survey on their own.
Adherence, Tolerability, and Adverse Events
All adverse events were recorded in patient charts. The number of exercise sessions attended and the total time span required to complete the guided exercise program were noted, and means were tabulated.
Statistical Analyses
Baseline characteristics were compared by chi-squared tests for categorical variables and t tests for continuous variables. When data distribution was not normal, a Mann–Whitney U test was used. The mean change from baseline was compared between groups using a two-sample t test if normally distributed or a Mann–Whitney U test if not normally distributed. Because we were interested in any change from baseline, we were only able to compare such changes in those participants who had a follow-up measurement. Because our losses to follow-up were fairly high and to have a conservative estimate of the effect size, we performed a secondary analysis of our data using the last-observation-carried-forward method for those participants who did not have a follow-up measurement. The correlation between the number of sessions attended and improvement in physical function was evaluated using the Spearmen correlation coefficient. A P value ≤0.05 was considered statistically significant. Calculations were done with SAS, version 9.2 and STATA 11.1 software.
Statistical Power
Our power calculation was on the basis of the ability to detect differences between groups in RAND-36 scales. Relying on the formulas published by Cohen (22) and variance estimates from studies of the general United States population, Ware (23) estimated sample size for experimentation comparison between two randomly formed groups with comparisons between repeated assessments over time. These estimates assume a nondirectional hypothesis (two tailed) with a false rejection rate of 5% and a statistical power of 80%. On the basis of these calculations, a sample size of 60 patients per group will detect a 10-point difference between postintervention scores of the two treatment groups.
Results
Demographics
Among 404 participants screened, 285 participants were excluded, because they either did not meet eligibility criteria or lacked interest in study participation (Figure 1). Of 119 participants randomized, six participants per group did not receive allocated intervention, most commonly for not showing up in the UC group and lack of medical clearance in the RRE group. In total, 107 participants entered the study: 48 (mean age=69±12 years) participants in the UC group and 59 (mean age=68±12 years) participants in the RRE group (Table 1). The mean time between baseline and follow-up testing was 99±19.4 days for the UC group versus 125.5±46.9 days for the RRE group (P<0.001). Although mean ages were similar in the two groups, the RRE group had a lower proportion of men (39% versus 69%), was more likely to be taking oral hypoglycemic agents (27% versus 6%), and was less likely to have arrhythmias (5% versus 17%). The UC group had higher systolic BP (140.5±20.2 versus 126.3±18.7 mmHg). Approximately 74% of patients had stage 3 CKD, and 26% of patients had stage 4 CKD; there were no meaningful differences in CKD stage by treatment group assignment. The length of time between allocation and start of RRE was a median of 25 days (interquartile range [IQR]=16.5–46 days).
Figure 1.
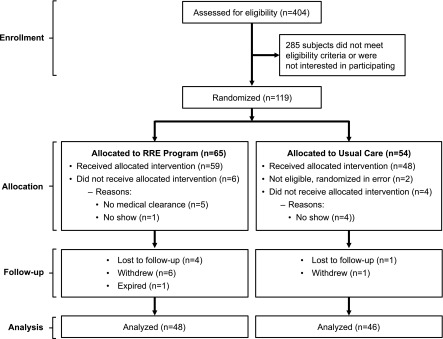
Flow chart: distribution of patients from assessment of eligibility to completion of study period. RRE, renal rehabilitation exercise.
Table 1.
Baseline characteristics by study group
| Characteristics | UC Group (n=48)a | RRE Group (n=59)a |
|---|---|---|
| Sex, n (% men) | 33 (68.8) | 23 (39) |
| Age (yr), mean ± SD | 69.2±12.4 | 67.7±12.4 |
| CKD stage, n (%) | ||
| 3 | 35 (74.5) | 42 (72.4) |
| 4 | 12 (25.5) | 16 (27.6) |
| Comorbidities, n (%) | ||
| Diabetes mellitus | 17 (35.4) | 27 (47.4) |
| Coronary artery disease | 10 (22) | 17 (30) |
| Congestive heart failure | 1 (2) | 3 (5) |
| Peripheral artery disease | 4 (9) | 4 (7) |
| Arrhythmia | 8 (17) | 3 (5) |
| BP (mmHg), mean±SD | ||
| Systolic | 140.5±20.2 | 126.3±18.7 |
| Diastolic | 71.8±10.5 | 69.1±9.7 |
| Body mass index (kg/m2) | 30.7±8.7 | 32.2±7.3 |
| Medications, n (%) | ||
| ACE inhibitor | 22 (48) | 24 (44) |
| ARB | 10 (22) | 17 (31) |
| β-Blocker | 27 (59) | 31 (55) |
| Epoetin therapy | 12 (25) | 11 (19.3) |
| Insulin | 8 (17) | 11 (20) |
| Oral hypoglycemic agents | 3 (6) | 15 (27) |
| Vitamin D supplementation | 23 (47.9) | 23 (40.3) |
| Tobacco use, n (%) | 2 (4.9) | 2 (4.3) |
| Self-reported health, n (%) | ||
| Excellent | 3 (6.4) | 4 (6.9) |
| Very good | 5 (10.6) | 9 (15.5) |
| Good | 27 (57.5) | 33 (56.9) |
| Fair | 11 (23.4) | 10 (17.2) |
| Poor | 1 (2.1) | 2 (3.5) |
| Self-reported level of activity (scale of 1–4), mean±SD | 1.65±1.1 | 1.76±1.1 |
| 6-min walk test (ft), mean±SD | 1080±296 | 1091±340 |
| Prediction for healthy individual, %b | 61.5 | 62.2 |
| Sit-to-stand test (% age predicted), mean±SD | 62.5±19.2 | 67.8±21.4 |
| Gait-speed test (cm/s), median (interquartile range) | 102.5 (81–123) | 130 (102.5–180) |
| Prediction for healthy individual, %b | 90.5 | 131.6 |
Physical Function
Baseline physical function was generally similar between the groups (Table 1). The UC and RRE groups had comparable self-reported levels of activity (1.65±1.1 versus 1.76±1.1; P=0.59), 6MWT scores (1080±296 versus 1091±340 ft; P=0.86), and STST scores (62.5±19.2% versus 67.8±21.4% of age predicted; P=0.19) at baseline. However, patients assigned to the RRE group had significantly higher gait-speed test (GST) at baseline than those in the UC group (median=130 versus 102.5 cm/s; P<0.001).
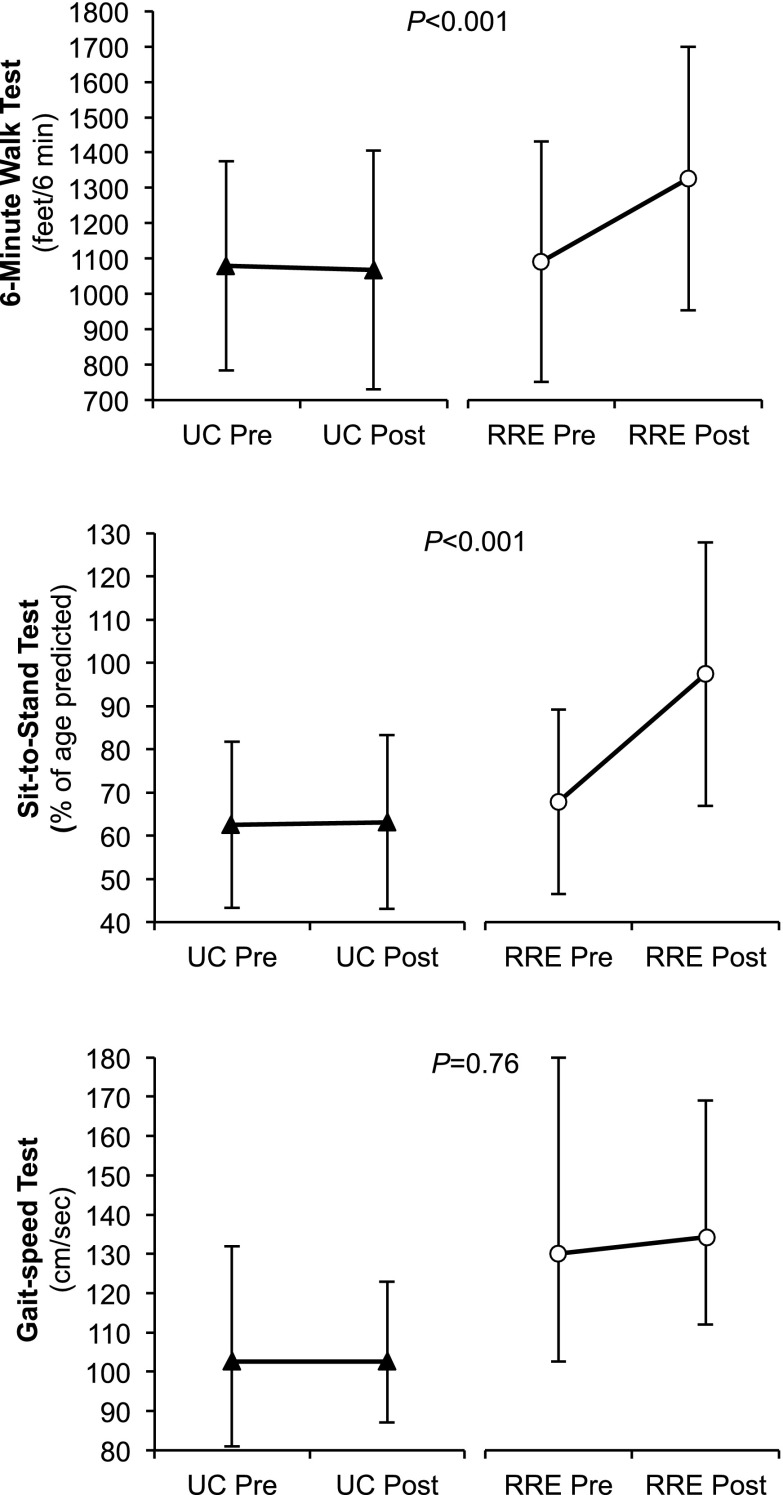
Patients in the RRE group realized significant improvements from baseline in physical function according to 6MWT and STST measures (Figure 2). A 12-week/24-session course of guided exercise produced a 19% increase in 6MWT scores in patients with CKD stages 3 or 4 (+210±266 versus −10±220 ft for the UC group; P<0.001) and a 29% improvement in the STST scores (+27±27% of age predicted for the RRE group versus +0.7±12% of age predicted for the UC group; P<0.001). No significant differences between groups were observed for GST (median change=0; IQR=−9 to +13 cm/s in the UC group versus median change=+9.5; IQR=−36.4 to +34 cm/s in the RRE group; P=0.76) (Figure 2). The effect size was slightly smaller but remained statistically significant according to the last observation carried forward analysis as well (Supplemental Table A).
Figure 2.
Physical function: baseline and follow-up measures by group. (Top) Six-minute walk test; (middle) sit-to-stand test; (bottom) gait-speed test. Values are means±SDs for the 6-minute walk and sit-to-stand tests and medians with 25%–75% interquartile ranges for gait-speed test. UC, usual care.
The addition of RRE to the usual CKD care produced significant beneficial effects, regardless of CKD stage (P values for the interaction terms=0.65 for 6MWT; P=0.59 for STST), sex (P=0.43 for 6MWT; P=0.19 for STST), and age ≤70 versus >70 years (P=0.64 for 6MWT; P=0.19 for STST).
Health-Related Quality of Life
Patients in the RRE group reported significant improvements in the physical measures of the RAND-36 compared with patients in the UC group (Table 2), including role functioning/physical (mean change=−8.9 in the UC group versus mean change=19.0 in the RRE group; P<0.001), physical functioning (mean change=−0.7 in the UC group versus mean change=11.1 in the RRE group; P=0.004), energy/fatigue levels (mean change=0.5 in the UC group versus mean change=9.8 in the RRE group; P=0.01), and general health (mean change=−1.2 in the UC group versus mean change=4.9 in the RRE group; P=0.03). The RRE group also reported significant improvement in the pain scale compared with the UC group (mean change=−3.8 in the UC group versus mean change=5.7 in the RRE group; P=0.04). Neither study group experienced significant changes in other mental measures of the RAND-36 (Table 2).
Table 2.
Health-related quality of life: mean change from baseline (SD) by group
| Measure | UC Group (n=46)a | RRE Group (n=48)a | P |
|---|---|---|---|
| Physical measures | |||
| Role functioning/physical | −8.9 (38.4) | 19.0 (31.7) | <0.001 |
| Physical functioning | −0.7 (18.7) | 11.1 (19.3) | 0.004 |
| Energy/fatigue | 0.5 (18.0) | 9.8 (17.6) | 0.01 |
| General health | −1.2 (11.5) | 4.9 (15.3) | 0.03 |
| Mental measures | |||
| Pain | −3.8 (24.4) | 5.7 (20.0) | 0.04 |
| Emotional wellbeing | −0.4 (17.1) | 4.2 (16.9) | 0.20 |
| Social functioning | 1.6 (22.6) | 4.2 (20.8) | 0.57 |
| Role functioning/emotional | 1.9 (29.2) | 6.9 (24.5) | 0.38 |
Patients with missing data on either measure are not included; not all patients have values for all measures.
Adherence, Tolerability, and Adverse Events
This guided exercise program in patients with CKD stages 3 or 4 was generally well tolerated. No exercise-related adverse events were observed. Not all participants in the RRE group followed the protocol of exercising two times per week for 12 consecutive weeks. Of 48 participants included in the analysis, 35 (72.9%) participants attended all 24 sessions, completing the exercise program in a mean of 15.1 weeks; the remaining 13 participants attended a mean of 13 sessions in 14.5 weeks.
Eleven patients allocated to the RRE group (18.6%) did not complete the RRE program: four patients were lost to follow-up, six patients withdrew from the study early, and one patient suddenly died, which was not related to exercise (Table 3). These 11 patients attended an average of 9.5 exercise sessions. Chronic pain and time conflict were among the reasons why patients withdrew from the study (Table 3). Only two (4.2%) patients in the UC group did not complete the study; one patient withdrew early citing a lack of interest, and one patient was lost to follow-up.
Table 3.
Reasons for study discontinuation
| Reasons for Study Discontinuation | UC Group (n=48) | RRE Group (n=59) |
|---|---|---|
| Early withdrawal | ||
| Time conflict | 0 | 2 |
| Chronic joint pain | 0 | 2 |
| Lack of interest | 1 | 0 |
| Bell’s palsy | 0 | 1 |
| No explanation | 0 | 1 |
| Lost to follow-up | 1 | 4 |
| Death | 0 | 1 |
| Total | 2 (4.2%) | 11 (18.6%)a |
P=0.03.
Discussion
Guided RRE can improve the physical function of patients with stages 3 or 4 CKD. This study shows that a 12-week/24-session program of guided exercise, conducted at a physical therapy or cardiac rehabilitation center and accompanied by distribution of a pedometer and encouragement to use it, affords measureable significant improvements in physical function testing, like the 6MWT and the STST, and enhances health-related quality of life, including perception of energy, physical functioning, general health, and pain in patients with substantially compromised renal function. Our findings corroborate results from smaller exercise studies that included 8–30 participants with predialysis CKD (24) as well as the recently published randomized Australian LANDMARK III trial that examined the effects of lifestyle intervention, including aerobic and resistance exercise training, for 12 months on cardiorespiratory fitness in a subset of 83 patients with stages 3 or 4 CKD (25). An observational study by Roshanravan et al. (10) found that impaired physical performance of the lower extremities in patients with CKD was strongly associated with all-cause mortality, with timed-up-and-go and gait speed more strongly predictive of 3-year mortality than kidney function or commonly measured serum biomarkers and 6MWT having the greatest receiver-operating characteristic curve for 3-year mortality. However, to our knowledge, this is the first randomized, controlled trial that reported a substantial improvement in the physical measures of the RAND-36 in this patient population undergoing RRE, and it is the largest prospective, randomized study to date to show a significant improvement in physical function in patients with stages 3 or 4 CKD receiving an exercise intervention.
This study proves that RRE programs can be integrated into standard CKD clinic care. Given the multiple comorbidities of the CKD population, the implementation of a successful exercise program requires a multidisciplinary approach, including clearance from a primary care provider, cardiologist, or surgeon before beginning RRE and collaboration with the exercise or cardiac rehabilitation facility staff. We found that, from time to time, exercise staff were uncomfortable with the burden of comorbidities of some of the patients with stages 3 or 4 CKD. The exercise trainers required significant teaching and reassurance to allow them to confidently coach this patient population at increased risk for cardiovascular morbidity and mortality. Additionally, 18.6% of participants in the exercise arm did not complete the program, and not all participants in the RRE group followed the protocol of exercising two times per week for 12 consecutive weeks, revealing the difficulty inherent in motivating sustained health-related behavioral change. For 48 participants included in the analysis of the number of sessions attended, the mean number attended was 19.3 sessions, with 35 participants attending all 24 sessions. Although this incompletion rate may seem to be high, it should be considered in the context of other studies of lifestyle intervention in patients with CKD (25) and other patients with multiple comorbidities (26,27). In the LANDMARK III substudy, in which 12% of patients with CKD in the intervention group dropped out, those remaining attended 70% of supervised gym-based sessions (25). In more general studies not restricted to patients with CKD, such as the Lifestyle Interventions and Independence for Elders Pilot, in which Fielding et al. (26) assessed activity adherence in 424 sedentary older adults with functional limitations, and the Diabetes Prevention Program analysis by Crandall et al. (27), which looked at the influence of age on the effects of lifestyle modifications and metformin to prevent diabetes, adherence rates ranged from 34% to 76%.
Our study has several limitations. Given the nature of the intervention, neither participants nor investigators were blinded to the intervention arm. In addition, randomization at the baseline assessment might have been preferable; it may have avoided that our intent to treat analysis does not include those patients who were randomized but withdrew from the study before the period when baseline data were collected. Despite randomization, the sex distribution was not balanced at baseline, with more women in the RRE arm and more men in the UC arm. Therefore, we also analyzed the main outcomes by sex. No sex differences were found in the results for the UC group. In the RRE group, although men had a greater qualitative improvement in the 6MWT and the STST, this effect was not statistically significant when the interaction between sex and treatment group was analyzed. It is also important to note that patients had to be able to travel to an exercise facility. This might have introduced an overall selection bias in both groups, because people who consented to be in the study might have been more physically active than those who declined to participate. Finally, baseline GST scores were higher in the RRE group, despite randomization, raising the possibility that, by chance, this group was more physically fit than the UC group. However, we believe that this result is unlikely, because all other measures of physical capacity were similar in both groups at baseline, including the self-reported level of activity. Because our hypothesis test was of improvement, using change from baseline as its measure accounted somewhat for baseline imbalances.
In summary, a 12-week RRE program was effective at improving the physical capacity and quality of life of patients with CKD stages 3 and 4. Overall, exercise training in this particular study setting and patient population was well tolerated, with no exercise-related adverse events observed. Larger studies and longer follow-up are needed to determine if these findings will translate into decreased mortality rates and slow the progression of CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was funded by a Maine Medical Center Research Institute Grant.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11791113/-/DCSupplemental.
See related editorial, “Exercise to Improve Physical Function and Quality of Life in CKD,” on pages 2023–2024.
References
- 1.USRDS : 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, US Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2012 [Google Scholar]
- 2.Venkataraman R, Sanderson B, Bittner V: Outcomes in patients with chronic kidney disease undergoing cardiac rehabilitation. Am Heart J 150: 1140–1146, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 4.O’Hare AM, Tawney K, Bacchetti P, Johansen KL: Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41: 447–454, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, Reeder GS, Roger VL: Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol 44: 988–996, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N: Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med 116: 682–692, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Painter P, Carlson L, Carey S, Paul SM, Myll J: Low-functioning hemodialysis patients improve with exercise training. Am J Kidney Dis 36: 600–608, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Cheema BS, Singh MA: Exercise training in patients receiving maintenance hemodialysis: A systematic review of clinical trials. Am J Nephrol 25: 352–364, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL: Exercise in the end-stage renal disease population. J Am Soc Nephrol 18: 1845–1854, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, Ikizler TA, Himmelfarb J, Katzel LI, Kestenbaum B, Seliger S: Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 24: 822–830, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Borg G: Borg's Perceived Exertion and Pain Scales, Champaign, IL, Human Kinetics, 1998 [Google Scholar]
- 14.Painter P, Blagg C, Moore GE: Exercise for the Dialysis Patient: A Comprehensive Program, Madison, WI, Medical Education Institute, 1995 [Google Scholar]
- 15.Fitts SS, Guthrie MR: Six-minute walk by people with chronic renal failure. Assessment of effort by perceived exertion. Am J Phys Med Rehabil 74: 54–58, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG: The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 110: 325–332, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Sherrill DL: Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158: 1384–1387, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Bohannon RW: Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills 80: 163–166, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Csuka M, McCarty DJ: Simple method for measurement of lower extremity muscle strength. Am J Med 78: 77–81, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Bohannon RW: Comfortable and maximum walking speed of adults aged 20-79 years: Reference values and determinants. Age Ageing 26: 15–19, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hays RD, Sherbourne CD, Mazel RM: The RAND 36-Item Health Survey 1.0. Health Econ 2: 217–227, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Cohen J: Statistical Power for the Behavioral Sciences, Hillsdale, NJ, Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 23.Ware JE: SF-36 Health Survey: Manual and Interpretation Guide, Boston, The Health Institute, New England Medical Center, 1993 [Google Scholar]
- 24.Johansen KL, Painter P: Exercise in individuals with CKD. Am J Kidney Dis 59: 126–134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH: Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding RA, Katula J, Miller ME, Abbott-Pillola K, Jordan A, Glynn NW, Goodpaster B, Walkup MP, King AC, Rejeski WJ, Life Study Investigators : Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc 39: 1997–2004, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R, Diabetes Prevention Program Research Group : The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 61: 1075–1081, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.