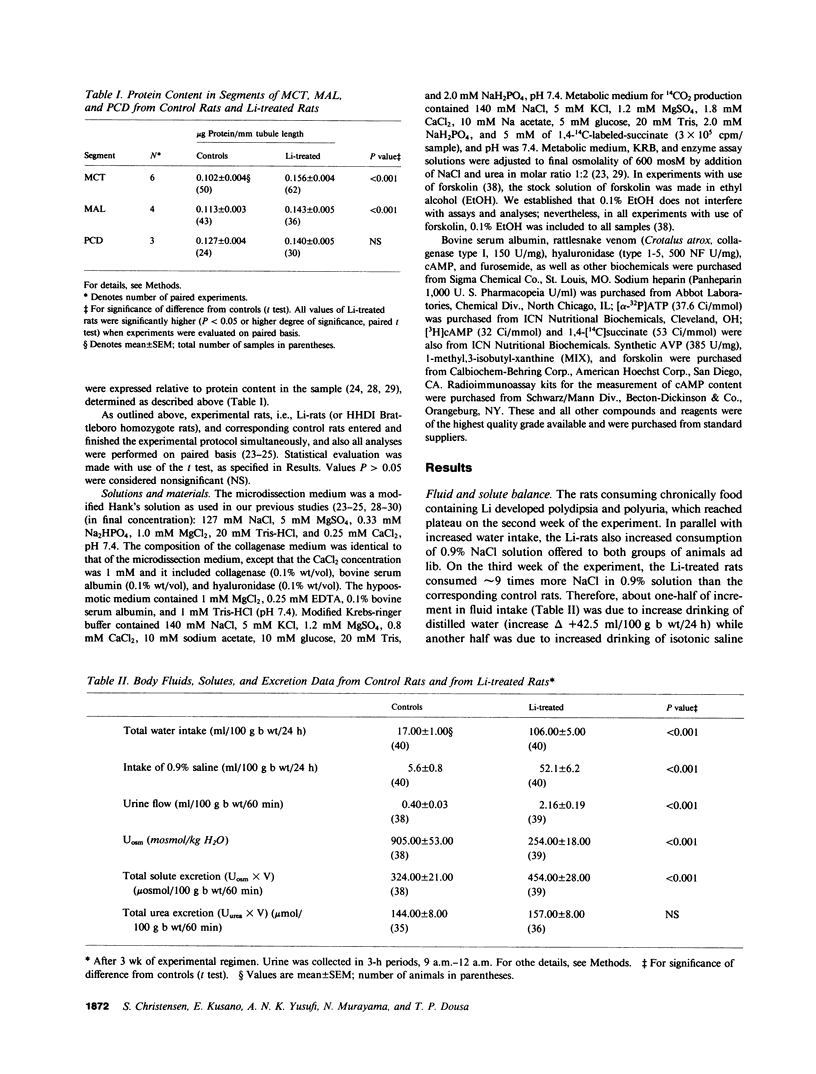
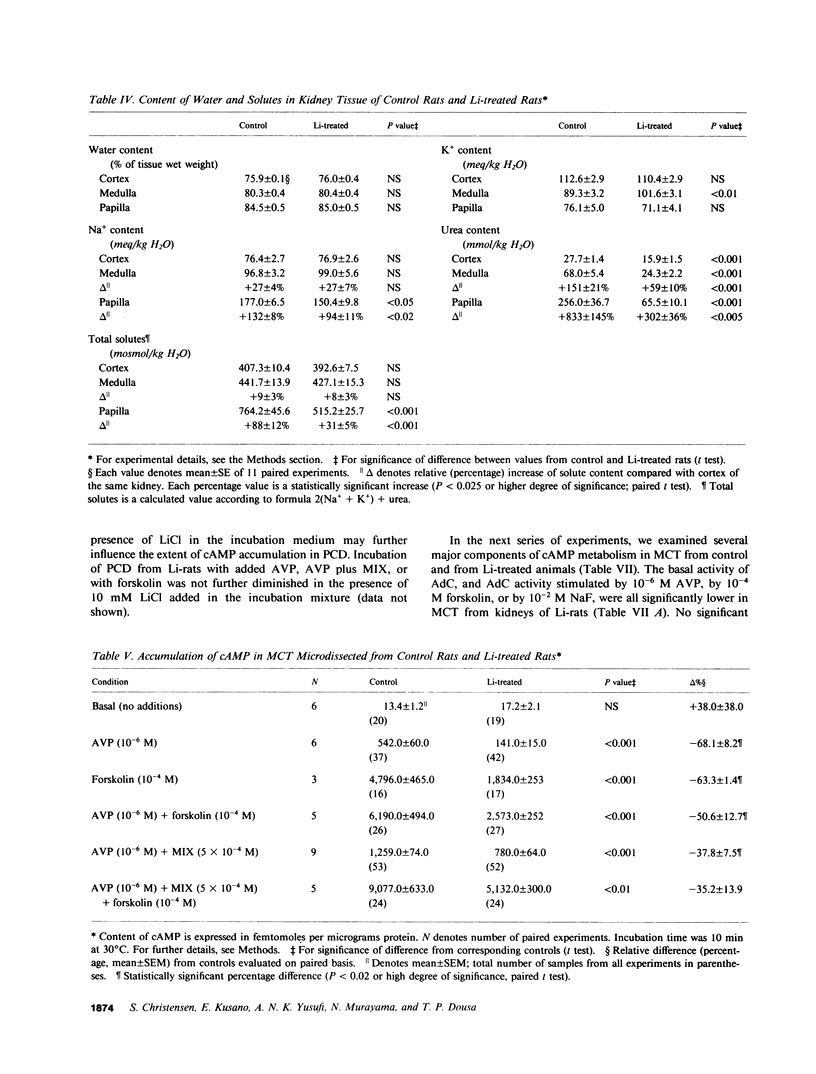
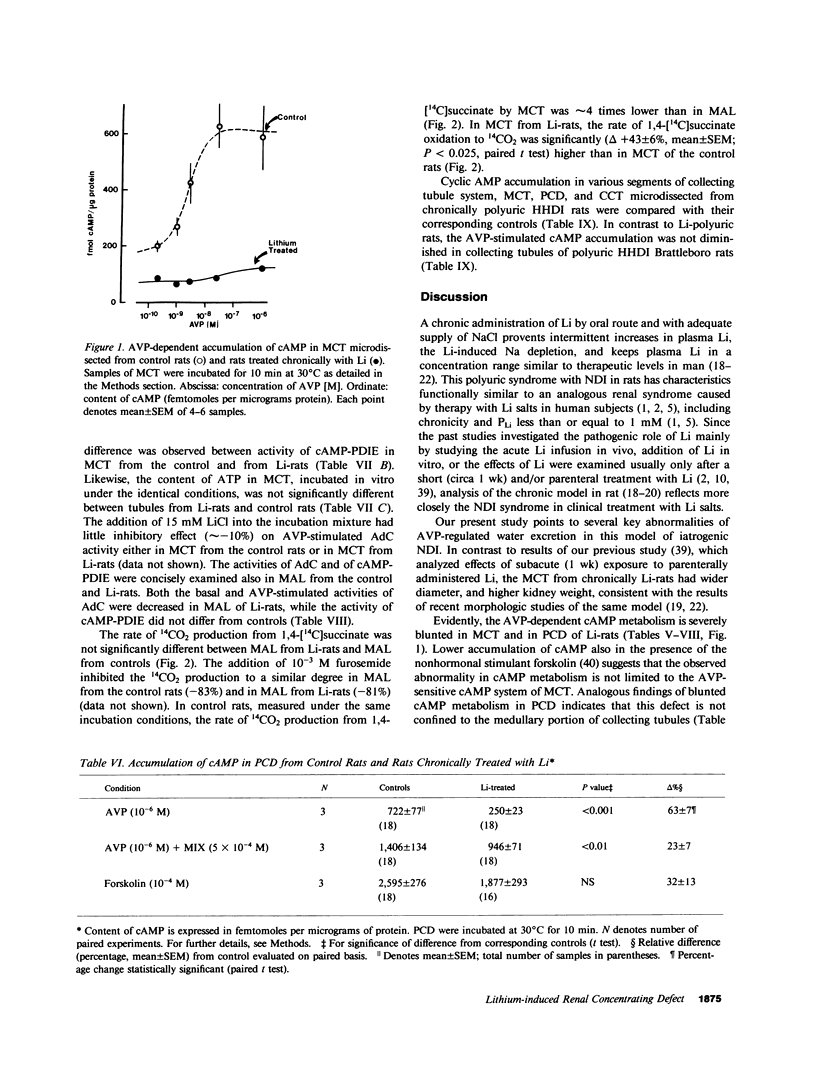
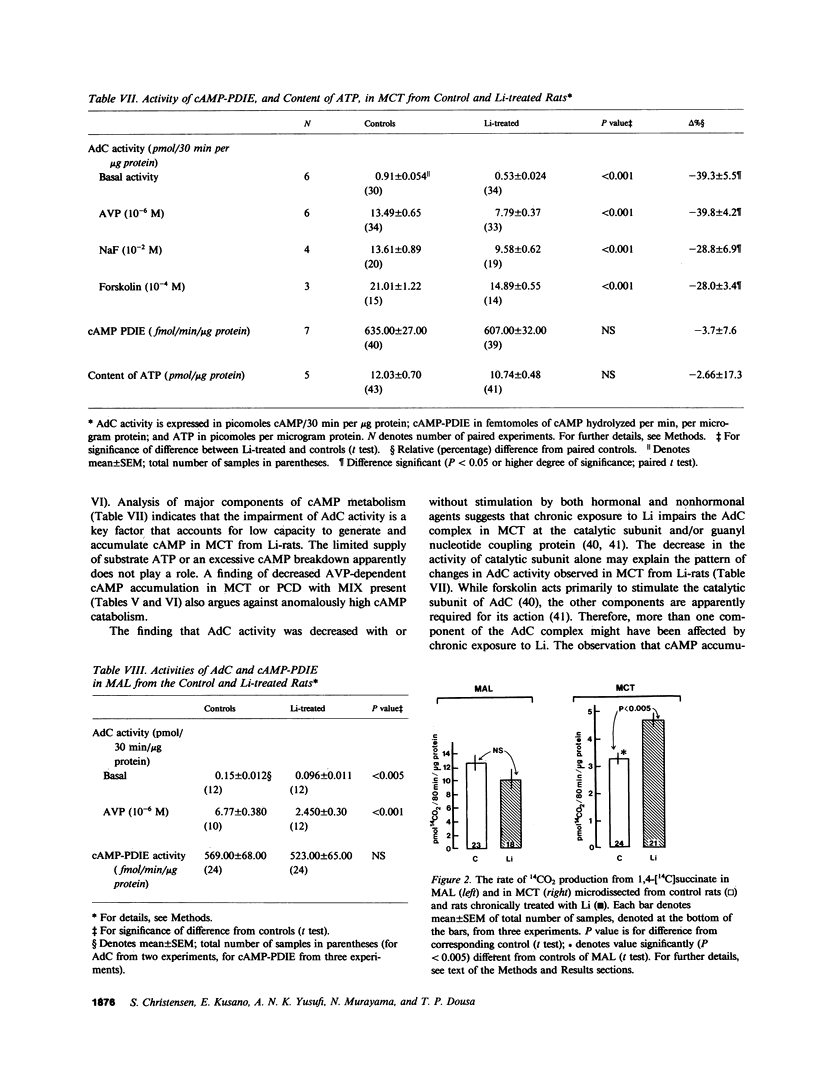
Abstract
A polyuric syndrome with nephrogenic diabetes insipidus (NDI) is a frequent consequence of prolonged administration of lithium (Li) salts. Studies in the past, mainly the acute and in vitro experiments, indicated that Li ions can inhibit hydroosmotic effect of [8-arginine]vasopressin (AVP) at the step of cAMP generation in vitro. However, the pathogenesis of the NDI due to chronic oral administration of low therapeutic doses of Li salts is not yet clarified. We conducted a comprehensive study to clarify the mechanism by which Li administered orally for several weeks induces polyuria and NDI in rats. Albino rats consuming a diet which contained Li (60 mmol/kg) for 4 wk developed marked polyuria and polydipsia; at the end of 4 wk the plasma Li was 0.7 +/- 0.09 mM (mean +/- SEM; n = 36). Li-treated rats had a significantly decreased (-33%) tissue osmolality in papilla and greatly reduced cortico-papillary gradient of urea (cortex--43%; medulla--64%; papilla--74%). Plasma urea was significantly (P less than 0.001) lower in Li-treated rats (5.4 +/- 0.2 mM) compared with controls (6.8 +/- 0.3 mM). Medullary collecting tubules (MCT) and papillary collecting ducts (PCD) microdissected from Li-treated animals had higher content of protein than MCT and PCD from the control rats. The cAMP accumulation in response to AVP added in vitro was significantly (delta = -60%) reduced. Also, the cAMP accumulation in MCT and PCD after incubation with forskolin was markedly lower in Li-treated rats. Addition of 0.5 mM 1-methyl,3-isobutyl-xanthine did not restore the cAMP accumulation in response to AVP and forskolin in MCT from Li-treated animals. In collecting tubule segments from polyuric rats with hypothalamic diabetes insipidus (Brattleboro homozygotes) the AVP-dependent cAMP accumulation was not diminished. The activity of adenylate cyclase (AdC) in MCT of Li-treated rats, both the basal and the activity stimulated by AVP, forskolin, or fluoride, was significantly (delta approximately equal to -30%) reduced, while the activity of cAMP phosphodiesterase (cAMP-PDIE) in the same segment showed no significant difference from the controls. Also, the content of ATP in MCT microdissected from Li-treated rats and incubated in vitro did not differ from controls. The rate of [14C]succinate oxidation to 14CO2 in MAL was inhibited (-77%) by 1 mM furosemide, which indicates that this metabolic process is coupled with NaCl cotransport in MAL. The rate of (14)CO(2) production from [14C]succinate in MAL was not significantly different between control and Li-treated rats. In MCT of control rats, the rate of [14C]succinate oxidation was approximately 3 times lower than in MAL. The rate of (14)CO(2) production from [(14)C]succinate in MCT of Li-treated rats was significantly (delta +33%) higher than in MCT dissected from control rats. Based on these results, we conclude that at least two factors play an important role in the pathogenesis of NDI consequent to chronic oral administration of Li: (a) decreased ability of MCT and PCD to generate and accumulate cAMP in response to stimulation by AVP; this defect is primarily due to diminished activity of AdC in these tubular segments caused by prolonged exposure to Li; and (b) lower osmolality of renal papillary tissue, due to primarily to depletion of urea, which decreases osmotic driving force for water reabsorption in collecting tubules. On the other hand, NaCI reabsorption in MAL is apparently not affected by chronic Li treatment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELBOOM J. W., BRODSKY W. A., TUTTLE W. S., DIAMOND I. The freezing point depression of mammalian tissues after sudden heating in boiling distilled water. J Gen Physiol. 1958 Jul 20;41(6):1153–1169. doi: 10.1085/jgp.41.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramow M., Cogan E. Lithium et mouvements d'eau. Agressologie. 1982 Feb;23(B):85–87. [PubMed] [Google Scholar]
- Abramow M., Cogan E. Role of lithium-ADH interaction in lithium-induced polyuria. Adv Nephrol Necker Hosp. 1984;13:29–34. [PubMed] [Google Scholar]
- Atherton J. C., Hai M. A., Thomas S. Effects of water diuresis and osmotic (mannitol) diuresis on urinary solute excretion by the conscious rat. J Physiol. 1968 Jul;197(2):395–410. doi: 10.1113/jphysiol.1968.sp008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J. C., Hai M. A., Thomas S. The time course of changes in renal tissue composition duruig water diuresis in the rat. J Physiol. 1968 Jul;197(2):429–443. doi: 10.1113/jphysiol.1968.sp008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S. Effects of water deprivation in rats with polydipsia and polyuria due to long-term administration of lithium. Acta Pharmacol Toxicol (Copenh) 1974 Sep;35(3):201–211. doi: 10.1111/j.1600-0773.1974.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Christensen S., Hansen B. B., Faarup P. Functional and structural changes in the rat kidney by long-term lithium treatment. Ren Physiol. 1982;5(2):95–104. doi: 10.1159/000172845. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Lithium-induced diuretic effect of antidiuretic hormone in rats. Am J Physiol. 1976 Dec;231(6):1754–1759. doi: 10.1152/ajplegacy.1976.231.6.1754. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Hui Y. S., Barnes L. D. Microtubule assembly in renal medullary slices: effects of vasopressin, vinblastine, and lithium. J Lab Clin Med. 1978 Aug;92(2):252–261. [PubMed] [Google Scholar]
- Dousa T. P. Interaction of lithium with vasopressin-sensitive cyclic AMP system of human renal medulla. Endocrinology. 1974 Nov;95(5):1359–1366. doi: 10.1210/endo-95-5-1359. [DOI] [PubMed] [Google Scholar]
- Dousa T., Hechter O. The effect of NaCl and LiCl on vasopressin-sensitive adenyl cyclase. Life Sci I. 1970 Jul 1;9(13):765–770. doi: 10.1016/0024-3205(70)90286-9. [DOI] [PubMed] [Google Scholar]
- Edwards R. M., Jackson B. A., Dousa T. P. Protein kinase activity in isolated tubules of rat renal medulla. Am J Physiol. 1980 Apr;238(4):F269–F278. doi: 10.1152/ajprenal.1980.238.4.F269. [DOI] [PubMed] [Google Scholar]
- Forrest J. N., Jr, Cohen A. D., Torretti J., Himmelhoch J. M., Epstein F. H. On the mechanism of lithium-induced diabetes insipidus in man and the rat. J Clin Invest. 1974 Apr;53(4):1115–1123. doi: 10.1172/JCI107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler A., Wraae O., Olesen O. V. Adenyl cyclase activity in kidneys of rats with lithium-induced polyuria. Acta Pharmacol Toxicol (Copenh) 1972;31(3):203–208. doi: 10.1111/j.1600-0773.1972.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Jenner F. A. Some aspects of the inhibition of the action of antidiuretic hormone by lithium ions in the rat kidney and bladder of the toad Bufo marinus. Br J Pharmacol. 1972 Feb;44(2):223–232. [PMC free article] [PubMed] [Google Scholar]
- Jackson B. A., Braun-Werness J. L., Kusano E., Dousa T. P. Concentrating defect in the adrenalectomized rat. Abnormal vasopressin-sensitive cyclic adenosine monophosphate metabolism in the papillary collecting duct. J Clin Invest. 1983 Sep;72(3):997–1004. doi: 10.1172/JCI111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Lithium-induced polyuria: effect of lithium on adenylate cyclase and adenosine 3',5'-monophosphate phosphodiesterase in medullary ascending limb of Henle's loop and in medullary collecting tubules. Endocrinology. 1980 Dec;107(6):1693–1698. doi: 10.1210/endo-107-6-1693. [DOI] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Measurements of cyclic AMP and cyclic GMP phosphodiesterase activity in isolated tubular segments. Kidney Int. 1980 Oct;18(4):512–518. doi: 10.1038/ki.1980.166. [DOI] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Valtin H., Dousa T. P. Cellular action of vasopressin in medullary tubules of mice with hereditary nephrogenic diabetes insipidus. J Clin Invest. 1980 Jul;66(1):110–122. doi: 10.1172/JCI109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen N. O., Olesen O. V., Thomsen K., Ottosen P. D., Olsen S. Early changes in renal distal convoluted tubules and collecting ducts of lithium-treated rats: light microscopy, enzyme histochemistry, and 3H-thymidine autoradiography. Lab Invest. 1982 Mar;46(3):298–305. [PubMed] [Google Scholar]
- Jamison R. L., Oliver R. E. Disorders of urinary concentration and dilution. Am J Med. 1982 Feb;72(2):308–322. doi: 10.1016/0002-9343(82)90823-3. [DOI] [PubMed] [Google Scholar]
- Kiebzak G. M., Yusufi A. N., Kusano E., Braun-Werness J., Dousa T. P. ATP and cAMP system in the in vitro response of microdissected cortical tubules to PTH. Am J Physiol. 1985 Jan;248(1 Pt 2):F152–F159. doi: 10.1152/ajprenal.1985.248.1.F152. [DOI] [PubMed] [Google Scholar]
- Kusano E., Braun-Werness J. L., Vick D. J., Keller M. J., Dousa T. P. Chlorpropamide action on renal concentrating mechanism in rats with hypothalamic diabetes insipidus. J Clin Invest. 1983 Oct;72(4):1298–1313. doi: 10.1172/JCI111086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murayama N., Werness J. L., Kusano E., Christensen S., Dousa T. P. Interaction of forskolin with vasopressin-sensitive cyclic AMP system in renal medullary tubules. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):427–433. [PubMed] [Google Scholar]
- Plenge P., Mellerup E. T., Nørgaard T. Functional and structural rat kidney changes caused by peroral or parenteral lithium treatment. Acta Psychiatr Scand. 1981 Apr;63(4):303–313. doi: 10.1111/j.1600-0447.1981.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Ray C., Morgan T., Carney S. The mechanism of polyuria in rats pretreated with lithium studies by in vitro microperfusion. Clin Exp Pharmacol Physiol. 1983 Mar-Apr;10(2):153–160. doi: 10.1111/j.1440-1681.1983.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Singer I. Lithium and the kidney. Kidney Int. 1981 Feb;19(2):374–387. doi: 10.1038/ki.1981.28. [DOI] [PubMed] [Google Scholar]
- Singer I., Rotenberg D., Puschett J. B. Lithium-induced nephrogenic diabetes insipidus: in vivo and in vitro studies. J Clin Invest. 1972 May;51(5):1081–1091. doi: 10.1172/JCI106900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel D., Guenet L., Desmier M., Insel P., Hanoune J. Forskolin requires more than the catalytic unit to activate adenylate cyclase. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):681–690. doi: 10.1016/0303-7207(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Thomsen K. The effect of sodium chloride on kidney function in rats with lithium intoxication. Acta Pharmacol Toxicol (Copenh) 1973;33(2):92–102. doi: 10.1111/j.1600-0773.1973.tb01512.x. [DOI] [PubMed] [Google Scholar]
- Valtin H. Sequestration of urea and nonurea solutes in renal tissues of rats with hereditary hypothalamic diabetes insipidus: effect of vasopressin and dehydration on the countercurrent mechanism. J Clin Invest. 1966 Mar;45(3):337–345. doi: 10.1172/JCI105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraae O., Geisler A., Olesen O. V. The relation between vasopressin stimulation of renal adenyl cyclase and lithium-induced polyuria in rats. Acta Pharmacol Toxicol (Copenh) 1972;31(4):314–317. doi: 10.1111/j.1600-0773.1972.tb00687.x. [DOI] [PubMed] [Google Scholar]


