Abstract
Species of Pestalotiopsis occur commonly as plant pathogens, and represent a fungal group known to produce a wide range of chemically novel, diverse metabolites. In the present study, we investigated 91 Pestalotiopsis isolates from the CBS-KNAW Fungal Biodiversity Centre (CBS) culture collection. The phylogeny of the Amphisphaeriaceae was constructed based on analysis of 28S nrRNA gene (LSU) sequence data, and taxonomic changes are proposed to reflect more natural groupings. We combined morphological and DNA data, and segregated two novel genera from Pestalotiopsis, namely Neopestalotiopsis and Pseudopestalotiopsis. The three genera are easily distinguishable on the basis of their conidiogenous cells and colour of their median conidial cells. We coupled morphological and combined sequence data of internal transcribed spacer (ITS), partial β-tubulin (TUB) and partial translation elongation factor 1-alpha (TEF) gene regions, which revealed 30 clades in Neopestalotiopsis and 43 clades in Pestalotiopsis. Based on these data, 11 new species are introduced in Neopestalotiopsis, 24 in Pestalotiopsis, and two in Pseudopestalotiopsis. Several new combinations are proposed to emend monophyly of Neopestalotiopsis, Pestalotiopsis and Pseudopestalotiopsis.
Key words: Amphisphaeriaceae, New species, Pestalosphaeria, Pestalotia, Phylogeny, Taxonomy
Introduction
History of Pestalotia, Pestalotiopsis and Truncatella
Based on the conidial forms, Steyaert (1949) split Pestalotia into three genera, namely Pestalotia, Pestalotiopsis and Truncatella. Pestalotia pezizoides is the generic type of Pestalotia, which was described from leaves and stems of Vitis vinifera collected in Italy, and is presently not known from culture nor DNA sequence. Characteristics of the species include 6-celled conidia with four olivaceous-brown median cells, distoseptate, hyaline terminal cells and simple or branched appendages arising from the apex of the apical cell (Fig. 1). Pestalotiopsis was introduced for species with 5-celled conidia, and Truncatella for those with 4-celled conidia. Pestalotia was retained as a monotypic genus with a single 6-celled species, P. pezizoides. Steyaert (1949) subsequently divided Pestalotiopsis into additional sections, namely Monosetulatae, Bisetulatae, Trisetulatae and Multisetulatae, based on the number of apical appendages. These sections were further divided into subdivisions based on concolourous (for those possessing equally pigmented median cells) or versicolourous conidia (two upper median cells darker than lowest median cell), fusoid or claviform conidia, branched or unbranched apical appendages and spatulate or non-spatulate apical appendages. Steyaert (1949) did not retain Monochaetia as a distinct genus, and placed species with single apical appendages in section Monosetulatae of Pestalotiopsis, or in Truncatella. Steyaert (1949) provided descriptions of 46 species and Pestalotiopsis guepinii was considered to be the type species of the newly introduced genus. Steyaert's (1949) introduction of the genus Pestalotiopsis to accommodate the 5-celled conidial forms of Pestalotia resulted in appreciable controversy from Moreau (1949) and Guba (1956, 1961). All expressed disapproval of Steyaert's classification, which resulted in three different genera instead of the single genus Pestalotia.
Fig. 1.

Pestalotia pezizoides (BPI0406483). A–B. Conidiomata on stems of Vitis vinifera. C. Conidiogenous cells. D–E. Conidia. Scale bars = 10 μm.
A major revision of Pestalotia sensu lato was published by Guba (1961) in his “Monograph of Monochaetia and Pestalotia” in which he described 220 species. Guba (1961) separated Pestalotia into the sections quadriloculate (4-celled conidia), quinqueloculatae (5-celled conidia) and sexloculatae (6-celled conidia). He further subdivided the sections into different categories, mainly on the basis of conidial form, colour, and the position, and nature of the setulae. Monochaetia was retained as a distinct genus, but the two novel genera (Pestalotiopsis and Truncatella) proposed by Steyaert (1949) were synonymised under Pestalotia. In his support of a single genus Guba (1956) emphasised that there is no justification for other genera based on fruiting structure and there was no point in assembling species with similar numbers of conidial septa into distinct genera. These characters might be useful only for defining species. Furthermore, Dube & Bilgrami (1965) favoured Guba's opinion and pointed out that there is no clear differentiation in conidial morphology of Pestalotia, Pestalotiopsis and Truncatella. Therefore, Dube & Bilgrami (1965) considered it to be more reasonable to retain all species in Pestalotia, instead of three different genera, which were introduced mainly on the basis of cell number.
Steyaert (1953a,b, 1961, 1963), however, provided further evidence in support of splitting Pestalotia, arguing that retention of Monochaetia as a separate genus based on a solitary character, a single apical appendage, was unwise, while Pestalotiopsis, Truncatella and Pestalotia were distinguished from each other based on a set of characters. Steyaert (1963) opined that Monochaetia was an artificial genus, which is incompatible with modern views of fungal systematics. Sutton (1980) accepted most of the genera discussed here (Pestalotia, Pestalotiopsis, Truncatella) which fitted into fairly well-defined groups and are characterised by acervuli, most with pigmented conidia, with annellidic conidiogenous cells. Sutton (1980) cited the electron microscope investigation of Griffiths & Swart (1974a,b), which examined the conidial wall of Pestalotia pezizoides and two species of Pestalotiopsis (P. funerea and P. triseta) to support Steyaert's division of Pestalotiopsis. Griffiths & Swart (1974a,b) regarded the conidial wall of P. pezizoides as being composed of three zones (based on electron density and melanisation) and in Pestalotiopsis of 2-layered zones. Until an evaluation of the 5-celled Pestalotia species in culture is made, Sutton (1969) preferred to regard Pestalotia as a monotypic genus. According to the revisions of Steyaert (1949) and Sutton (1969, 1980), all earlier designated Pestalotia species, except P. pezizoides, have been transferred to other genera, many to Pestalotiopsis. Pestalotia valdiviana, P. cornu-cervae, and P. corni were also included in Pestalotia section sexloculatae (Guba 1961). In his revision of Pestalotia, Sutton (1969) considered P. valdiviana as a nomen dubium, P. cornu-cervae was maintained as the type and only species of Labridella, and P. corni was transferred to Seiridium. Sutton (1980) identified several problems with the taxonomy of Pestalotiopsis. Although Steyaert (1949) treated Pestalotia as a monotypic genus, more than 600 species still remain in the genus and need reassignment to Monochaetia, Pestalotiopsis or Truncatella (Sutton 1980). Furthermore, identification of species from culture and the application of names based on herbarium material as designated by Guba (1961) and Steyaert (1949, 1953a,b, 1955, 1956, 1961), present a confusing situation.
Nag Raj (1985, 1993) found it necessary to reassign many species described in Pestalotia to other genera. However Nag Raj (1985, 1993) preferred to adopt a broader concept for Pestalotiopsis to include 3-septate conidial forms. Pestalotiopsis besseyi, P. casuarinae, P. citrina, P. eupyrena, P. gastrolobi, P. jacksoniae, P. moorie, P. pestalozzioides, P. puyae, P. stevensoniii and P. torrendiii are 3-celled conidial forms Nag Raj (1993) placed in Pestalotiopsis but which actually belong in Truncatella. Therefore, his view of Pestalotiopsis was far broader than the actual concept of Steyaert (1949) (Jeewon et al. 2003). Pestalotiopsis guepinii, the type species of Pestalotiopsis, was described from stems and leaves of Camellia japonica collected in France, and is characterised by 5-celled conidia with three concolourous median cells, hyaline terminal cells and simple or unbranched appendages arising from the apex of the apical cell (Steyaert 1949). However, Nag Raj (1985) pointed out that it is essential to re-examine the type material of Pestalotiopsis and related genera and also consider the contentious placement of P. guepinii as the generic type of Pestalotiopsis. Nag Raj (1985) redescribed Pestalotiopsis maculans and considered it as the generic type of Pestalotiopsis, with P. guepinii as synonym. Hughes (1958) introduced a new combination for P. maculans, which was originally described by Corda (1839) as Sporocadus maculans. However, the new combination introduced by Hughes (1958) lacked a detailed description of the fungus. Furthermore, there was no reference to this binomial in the monograph of Guba (1961), other than reference to a collection of S. maculans listed under P. guepinii. Nag Raj (1985) observed the holotype specimen of S. maculans (PR 155665), which was isolated from Camellia japonica in Prague, Czech Republic, and clarified that the morphology of the fungus exactly matched the generic concept of Pestalotiopsis. Furthermore he observed the isotype specimen of P. guepinii in BPI, which he compared with S. maculans and found them to be identical. Therefore Nag Raj (1985) regarded P. maculans as the correct, older name for P. guepinii, and the type species of Pestalotiopsis. Based on morphology and phylogeny, Jeewon et al. (2003) also pointed out that (based on ITS sequences) P. maculans clusters with species having concolourous median cells, and that P. karstenii might be a synonym of P. maculans.
Biology of Pestalotiopsis species
Pestalotiopsis is a species-rich asexual genus with appendage-bearing conidia in the Amphisphaeriaceae (Barr 1975, 1990, Kang et al. 1999, Lee et al. 2006), and is widely distributed throughout tropical and temperate regions (Bate-Smith & Metcalfe 1957). Most species in the genus lack sexual morphs, and presently only 13 sexual morphs have been recorded in literature, which were previously treated as species of Pestalosphaeria (Maharachchikumbura et al. 2011). Pestalotiopsis species are common phytopathogens that cause a variety of diseases, including canker lesions, shoot dieback, leaf spots, needle blight, tip blight, grey blight, scabby canker, severe chlorosis, fruit rots and various post-harvest diseases (Fig. 2) (Crous et al. 2011, Maharachchikumbura et al. 2012, 2013a,b, Zhang et al. 2012a, 2013). Pestalotiopsis species also reduce production and cause economic loss in apple, blueberry, coconut, chestnut, ginger, grapevine, guava, hazelnut, lychee, mango, orchid, peach, rambutan, tea and wax apple due to disease (Sun & Cao 1990, Sangchote et al. 1998, Xu et al. 1999, Keith et al. 2006, Joshi et al. 2009, Keith & Zee 2010, Chen et al. 2011, Evidente et al. 2012, Ismail et al. 2013, Maharachchikumbura et al. 2013a,b,c, Ren et al. 2013).
Fig. 2.
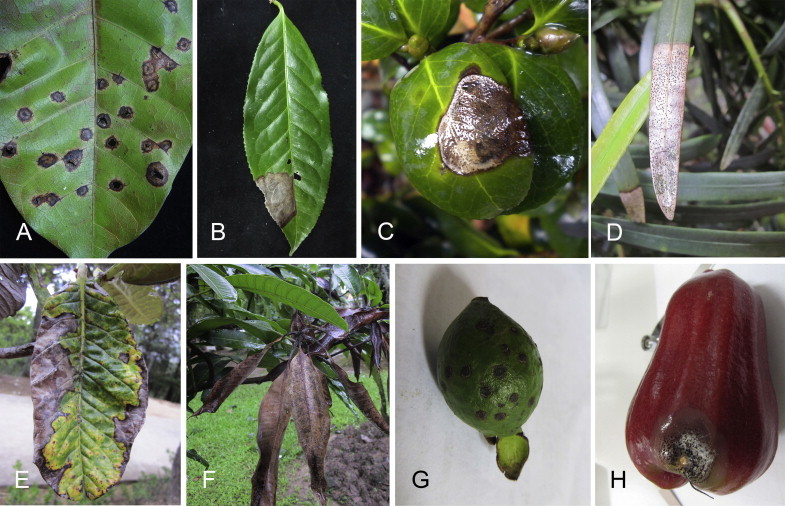
Disease symptoms associated with various species of Pestalotiopsis. A. Leaf spots on Mangifera indica. B. Grey blight on camellia sinensis. C. Leaf blight on camellia japonica. D. Tip blight on Podocarpus macrophyllus. E. Leaf blotch on Rhododendron sinogrande. F. Shoot dieback on Mangifera indica. G. Guava scab on Psidium guajava. H. Fruit rot on Syzygium samarangense.
Pestalotiopsis species are also commonly isolated as endophytes (Watanabe et al. 2010, Maharachchikumbura et al. 2012, Debbab et al. 2013) and there are numerous reports that these endophytes produce novel compounds with medicinal, agricultural and industrial applications (Aly et al. 2010, Xu et al. 2010, 2014). Species of Pestalotiopsis are thought to be a rich source for bioprospecting compared to other fungal genera, and Xu et al. (2010, 2014) reviewed 130 and 160 different compounds respectively, isolated from species of Pestalotiopsis. Due to their ability to switch nutritional-modes, many endophytic and plant pathogenic Pestalotiopsis species persist as saprobes (Hu et al. 2007, Maharachchikumbura et al. 2012), and have been isolated from dead leaves, bark and twigs (Ellis & Ellis 1997, Maharachchikumbura et al. 2013d). Several species have been recovered from soil, polluted stream water, wood, paper, fabrics, and wool (Guba 1961). Some species have been associated with human and animal infections (Sutton 1999, Monden et al. 2013) and others (e.g. Pestalotiopsis guepinii and P. microspora) have also been isolated from extreme environments (Strobel et al. 1996, Tejesvi et al. 2007).
Naming Pestalotiopsis species
Pestalotiopsis species were historically named according to the host from which they were first observed. In spite of this practise, many argued that Pestalotiopsis species are generally not host-specific and are found on a wide range of hosts and substrates (Jeewon et al. 2004, Lee et al. 2006). Therefore, many of the traditional host-based species may be spurious. However, species of Pestalotiopsis display considerable diversity in phenotype, and group together based on similarities in conidial morphology (Jeewon et al. 2003, Maharachchikumbura et al. 2012, 2013d). Conidial characters such as conidial length, width, median cell length, colour of median cells and length of the apical appendages appear to be stable characters within Pestalotiopsis (Jeewon et al. 2003, Hu et al. 2007). Previous phylogenetic studies revealed Pestalotiopsis strains to cluster in three strongly supported clades. These clades corresponded to three conidial types: those with pale brown or olivaceous concolourous median cells, those with versicolourous median cells and those with dark-coloured concolourous median cells (Jeewon et al. 2003, Liu et al. 2010, Maharachchikumbura et al. 2011, 2012). Steyaert (1949) and Guba (1961) had previously grouped species with versicolourous conidia into two groups based on the intensity of colour of the median cells, namely umber-olivaceous (two upper median cells umber and lowest median cell yellow-brown) and fuliginous-olivaceous (two upper median cells fuliginous, usually opaque, and lowest median cell pale brown). However, based on multi-locus DNA sequence analysis, the division of the versicolourous group based on colour intensities of the median conidial cell proved to not be a taxonomically reliable character (Liu et al. 2010, Maharachchikumbura et al. 2011, 2012).
The sexual state of Pesalotiopsis is Pestalosphaeria, which was introduced by Barr (1975) with the type species Pestalosphaeria concentrica. This species was isolated from the grey-brown spots on living leaves of Rhododendron maximum growing on North Carolina, USA. Pestalosphaeria concentrica is characterised by immersed, subglobose ascomata and unitunicate, cylindrical asci with a J+ apical ring; ascospores uniseriate in the ascus, ellipsoid, pale dull brown and 2-septate. The germinated ascospores of Pestalosphaeria concentrica give rise to the Pestalotiopsis conidial state, P. guepini var. macrotricha, which contains three median concolourous conidial cells.
Objectives of study
In the present study we examined 91 Pestalotiopsis strains from the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS), which were isolated from various hosts and geographic origins. Phylogenetic relationships between the strains and other genera in the Amphisphaeriaceae are resolved based on analysis of 28S nrRNA gene (LSU) sequence data. The phylogeny resolved Pestalotiopsis as a distinct clade in Amphisphaeriaceae, with three well-supported groups that correlated with morphology; besides Pestalotiopsis, two new genera, Neopestalotiopsis and Pseudopestalotiopsis are proposed. Various Pestalotiopsis species known from culture are therefore allocated to Neopestalotiopsis and Pseudopestalotiopsis. Phylogenetic analyses of combined sequence data of the internal transcribed spacer regions and intervening 5.8S nrRNA gene (ITS), partial β-tubulin (TUB) and translation elongation factor 1-alpha (TEF) gene regions supplemented with conidial morphology clarify species boundaries in the three genera.
Materials and methods
Isolates
A total of 91 strains were obtained from the CBS culture collection. Freeze-dried strains were revived in 2 mL malt/peptone (50 % / 50 %) and subsequently transferred to Petri dishes containing oatmeal agar (OA) (Crous et al. 2009). Isolates of the CBS collection stored in liquid nitrogen at −80 °C were transferred directly to Petri dishes containing OA.
Morphological analysis
Morphological descriptions were made for isolates grown on 2 % potato dextrose agar (PDA; Crous et al. 2009) under moderate temperatures (∼22 °C) at 12 h daylight. Autoclaved pine needles were placed on synthetic nutrient-poor agar (PNA) (Crous et al. 2009) to observe conidiomatal development. Colony colour on PDA was determined with the colour charts of Rayner (1970). Microscopic preparations were made in distilled water, with 30 measurements per structure as observed under a Nikon SMZ1000 dissecting microscope (DM) or with a Nikon Eclipse 80i compound microscope using differential interference contrast (DIC) illumination. Taxonomic descriptions and nomenclature were deposited in MycoBank (Crous et al. 2004).
PCR and sequencing
The UltraClean Microbial DNA Isolation Kit (MoBio laboratories, Carlsbad, CA, USA) was used to extract genomic DNA from fungal mycelia. For nucleotide sequence comparisons, the nuclear rDNA operon spanning the 3′ end of the 18S nrRNA gene, the first internal transcribed spacer region, the 5.8S nrRNA gene, the second internal transcribed spacer region and the 5′ end of the 28S nrRNA gene (ITS), and the partial β-tubulin (TUB) and partial translation elongation factor 1-alpha (TEF) genes were amplified using primer pairs LR0R/LR5 (Vilgalys & Hester 1990, Rehner & Samuels 1994), ITS5/ITS4 (White et al. 1990), T1/Bt-2b (Glass & Donaldson 1995, O'Donnell & Cigelnik 1997), and EF1-728F/EF-2 (O'Donnell et al. 1998, Carbone & Kohn 1999). Amplification conditions for LSU, ITS and TEF followed Crous et al. (2013) and for TUB, Lee et al. (2004).
Sequencing of the PCR amplicons was conducted using the same primers as those used for the amplification reactions. The sequence products were purified using Sephadex columns (Sephadex G-50 Superfine, Amersham Biosciences, Roosendaal, Netherlands) and analysed with an ABI Prism 3730XL Sequencer (Applied Biosystems) according to the manufacturer's instructions. DNASTAR Lasergene SeqMan Pro v. 8.1.3 was used to obtain consensus sequences from sequences generated from forward and reverse primers and these were subsequently lodged with GenBank (Table 1).
Table 1.
Collection details and GenBank accession numbers of isolates includes in this study.
| Species | Culture accession No.1 | Host/Substrate | Family | Location | GenBank accession2 |
|||
|---|---|---|---|---|---|---|---|---|
| LSU | ITS | TUB | TEF | |||||
| Neopestalotiopsis aotearoa | CBS 367.54; ATCC 11763; QM 381* | Canvas | — | New Zealand | KM116247 | KM199369 | KM199454 | KM199526 |
| N. asiatica | MFLUCC 12-0286; NN0476380* | Unidentified tree | — | China | — | JX398983 | JX399018 | JX399049 |
| N. australis | CBS 114159; STE-U 3017* | Telopea sp. | Proteaceae | Australia: New South Wales | KM116252 | KM199348 | KM199432 | KM199537 |
| N. chrysea | MFLUCC 12-0261; NN042855* | Dead leaves | — | China | — | JX398985 | JX399020 | JX399051 |
| MFLUCC 12-0262; NN047037 | Dead plant | — | China | — | JX398986 | JX399021 | JX399052 | |
| N. clavispora | CBS 447.73 | Decaying wood | — | Sri Lanka | KM116275 | KM199374 | KM199443 | KM199539 |
| MFLUCC 12-0280; NN043011 | Magnolia sp. | Magnoliaceae | China | — | JX398978 | JX399013 | JX399044 | |
| MFLUCC 12-0281; NN043133* | Magnolia sp. | Magnoliaceae | China | — | JX398979 | JX399014 | JX399045 | |
| N. cubana | CBS 600.96; INIFAT C96/44-4* | Leaf litter | — | Cuba | KM116253 | KM199347 | KM199438 | KM199521 |
| N. ellipsospora | CBS 115113; HKUCC 9136 | Ardisia crenata | Myrsinaceae | Hong Kong | KM116269 | KM199343 | KM199450 | KM199544 |
| MFLUCC 12-0283* | Dead plant materials | — | China | — | JX398980 | JX399016 | JX399047 | |
| MFLUCC 12-0284 | Dead plant materials | — | Thailand | — | JX398981 | JX399015 | JX399046 | |
| N. eucalypticola | CBS 264.37; BBA 5300* | Eucalyptus globulus | Myrtaceae | — | KM116256 | KM199376 | KM199431 | KM199551 |
| N. foedans | CGMCC 3.9123* | Mangrove plant | — | China | — | JX398987 | JX399022 | JX399053 |
| CGMCC 3.9178 | Neodypsis decaryi | Arecaceae | China | — | JX398989 | JX399024 | JX399055 | |
| CGMCC 3.9202 | Calliandra haematocephala | Fabaceae | China | — | JX398988 | JX399023 | JX399054 | |
| N. formicarum | CBS 115.83 | Plant debris | — | Cuba | KM116255 | KM199344 | KM199444 | KM199519 |
| CBS 362.72* | Dead Formicidae (ant) | — | Ghana | KM116248 | KM199358 | KM199455 | KM199517 | |
| N. honoluluana | CBS 111535; STE-U 2078 | Telopea sp. | Proteaceae | USA: Hawaii | KM116263 | KM199363 | KM199461 | KM199546 |
| CBS 114495; STE-U 2076* | Telopea sp. | Proteaceae | USA: Hawaii | — | KM199364 | KM199457 | KM199548 | |
| N. javaensis | CBS 257.31* | Cocos nucifera | Arecaceae | Indonesia: Java | — | KM199357 | KM199437 | KM199543 |
| N. magna | MFLUCC 12-652; ICMP 20011* | Pteridium sp. | Dennstaedtiaceae | France | — | KF582795 | KF582793 | KF582791 |
| N. mesopotamica | CBS 299.74 | Eucalyptus sp. | Myrtaceae | Turkey | KM116257 | KM199361 | KM199435 | KM199541 |
| CBS 336.86* | Pinus brutia | Pinaceae | Iraq | KM116271 | KM199362 | KM199441 | KM199555 | |
| CBS 464.69 | Achras sapota | Sapotaceae | India | — | KM199353 | KM199436 | — | |
| N. natalensis | CBS 138.41* | Acacia mollissima | Fabaceae | South Africa | KM116279 | KM199377 | KM199466 | KM199552 |
| N. piceana | CBS 225.30 | Mangifera indica | Anacardiaceae | — | KM116270 | KM199371 | KM199451 | KM199535 |
| CBS 254.32 | Cocos nucifera | Arecaceae | Indonesia: Sulawesi | KM116267 | KM199372 | KM199452 | KM199529 | |
| CBS 394.48* | Picea sp. | Pinaceae | UK | KM116266 | KM199368 | KM199453 | KM199527 | |
| N. protearum | CBS 114178; STE-U 1765* | Leucospermum cuneiforme cv. ‘Sunbird’ | Proteaceae | Zimbabwe | JN712564 | JN712498 | KM199463 | KM199542 |
| N. rosae | CBS 101057* | Rosa sp. | Rosaceae | New Zealand | KM116245 | KM199359 | KM199429 | KM199523 |
| CBS 124745 | Paeonia suffruticosa | Paeoniaceae | USA | KM116272 | KM199360 | KM199430 | KM199524 | |
| N. samarangensis | CBS 115451; HKUCC 9095 | Unidentified tree | — | Hong Kong | — | KM199365 | KM199447 | KM199556 |
| MFLUCC 12-0233* | Syzygium samarangense | Myrtaceae | Thailand | — | JQ968609 | JQ968610 | JQ968611 | |
| N. saprophytica | CBS 115452; HKUCC 8684 | Litsea rotundifolia | Lauraceae | Hong Kong | KM116251 | KM199345 | KM199433 | KM199538 |
| MFLUCC 12-0282; NN047136* | Magnolia sp. | Magnoliaceae | China | — | JX398982 | JX399017 | JX399048 | |
| Neopestalotiopsis sp. Clade 4 | CBS 233.79 | Crotalaria juncea | Fabaceae | India | KM116249 | KM199373 | KM199464 | KM199528 |
| Neopestalotiopsis sp. Clade 10 | CBS 110.20 | — | — | — | KM116250 | KM199342 | KM199442 | KM199540 |
| Neopestalotiopsis sp. Clade 15 | CBS 177.25 | Dalbergia sp. | Fabaceae | — | KM116246 | KM199370 | KM199445 | KM199533 |
| CBS 274.29 | Cocos nucifera | Arecaceae | Indonesia: Java | KM116261 | KM199375 | KM199448 | KM199534 | |
| CBS 322.76 | Camellia sp. | Theaceae | France | KM116259 | KM199366 | KM199446 | KM199536 | |
| CBS 664.94 | Cocos nucifera | Arecaceae | Netherlands | KM116254 | KM199354 | KM199449 | KM199525 | |
| Neopestalotiopsis sp. Clade 20 | CBS 164.42 | Dune sand | — | France | KM116268 | KM199367 | KM199434 | KM199520 |
| CBS 360.61 | Cinchona sp. | Rubiaceae | Guinea | KM116260 | KM199346 | KM199440 | KM199522 | |
| Neopestalotiopsis sp. Clade 22 | CBS 119.75 | Achras sapota | Sapotaceae | India | KM116265 | KM199356 | KM199439 | KM199531 |
| CBS 266.80 | Vitis vinifera | Vitaceae | India | KM116264 | KM199352 | — | KM199532 | |
| Neopestalotiopsis sp. Clade 26 | CBS 266.37; BBA 5087; IMI 083708 | Erica sp. | Ericaceae | Germany | KM116273 | KM199349 | KM199459 | KM199547 |
| CBS 323.76 | Erica gracilis | Ericaceae | France | KM116262 | KM199350 | KM199458 | KM199550 | |
| CBS 361.61 | Cissus sp. | Vitaceae | Netherlands | KM116274 | KM199355 | KM199460 | KM199549 | |
| N. steyaertii | IMI 192475* | Eucalyptus viminalis | Myrtaceae | Australia | KM116285 | KF582796 | KF582794 | KF582792 |
| N. surinamensis | CBS 111494; STE-U 1779 | Protea eximia | Proteaceae | Zimbabwe | JX556250 | JX556232 | KM199462 | KM199530 |
| CBS 450.74* | Soil under Elaeis guineensis | Arecaceae | Suriname | KM116258 | KM199351 | KM199465 | KM199518 | |
| N. umbrinospora | MFLUCC 12-0285; NN042986* | Unidentified plant | — | China | — | JX398984 | JX399019 | JX399050 |
| N. zimbabwana | CBS 111495; STE-U 1777* | Leucospermum cunciforme cv. ‘Sunbird’ | Proteaceae | Zimbabwe | JX556249 | JX556231 | KM199456 | KM199545 |
| Pestalotiopsis adusta | ICMP 6088* | On refrigerator door PVC gasket | — | Fiji | — | JX399006 | JX399037 | JX399070 |
| MFLUCC 10-146 | Syzygium sp. | Myrtaceae | Thailand | — | JX399007 | JX399038 | JX399071 | |
| P. anacardiacearum | IFRDCC 2397* | Mangifera indica | Anacardiaceae | China | — | KC247154 | KC247155 | KC247156 |
| P. arceuthobii | CBS 434.65* | Arceuthobium campylopodum | Santalaceae | USA | KM116243 | KM199341 | KM199427 | KM199516 |
| P. arengae | CBS 331.92* | Arenga undulatifolia | Arecaceae | Singapore | KM116207 | KM199340 | KM199426 | KM199515 |
| P. australasiae | CBS 114126; STE-U 2896* | Knightia sp. | Proteaceae | New Zealand | KM116218 | KM199297 | KM199409 | KM199499 |
| CBS 114141; STE-U 2949 | Protea sp. | Proteaceae | Australia: New South Wales | KM116203 | KM199298 | KM199410 | KM199501 | |
| P. australis | CBS 111503; STE-U 1770 | Protea neriifolia × susannae cv. ‘Pink Ice’ | Proteaceae | South Africa | KM116200 | KM199331 | KM199382 | KM199557 |
| CBS 114193; STE-U 3011* | Grevillea sp. | Proteaceae | Australia: New South Wales | KM116197 | KM199332 | KM199383 | KM199475 | |
| CBS 114474; STE-U 1769 | Protea neriifolia × susannae cv. ‘Pink Ice’ | Proteaceae | South Africa | KM116220 | KM199334 | KM199385 | KM199477 | |
| CBS 119350; CMW 20013 | Brabejum stellatifolium | Proteaceae | South Africa | KM116209 | KM199333 | KM199384 | KM199476 | |
| P. biciliata | CBS 124463* | Platanus × hispanica | Platanaceae | Slovakia | KM116224 | KM199308 | KM199399 | KM199505 |
| CBS 236.38 | Paeonia sp. | Proteaceae | Italy | KM116214 | KM199309 | KM199401 | KM199506 | |
| CBS 790.68 | Taxus baccata | Taxaceae | Netherlands | KM116235 | KM199305 | KM199400 | KM199507 | |
| P. brassicae | CBS 170.26* | Brassica napus | Brassicaceae | New Zealand | — | KM199379 | — | KM199558 |
| P. camelliae | CBS 443.62 | Camellia sinensis | Theaceae | Turkey | KM116225 | KM199336 | KM199424 | KM199512 |
| MFLUCC 12-0277* | Camellia japonica | Theaceae | China | — | JX399010 | JX399041 | JX399074 | |
| MFLUCC 12-0278 | Camellia japonica | Theaceae | China | KM116284 | JX399011 | JX399042 | JX399075 | |
| P. chamaeropis | CBS 113604; STE-U 3078 | — | — | — | KM116201 | KM199323 | KM199389 | KM199471 |
| CBS 113607; STE-U 3080 | — | — | — | KM116211 | KM199325 | KM199390 | KM199472 | |
| CBS 186.71* | Chamaerops humilis | Arecaceae | Italy | KM116210 | KM199326 | KM199391 | KM199473 | |
| CBS 237.38 | — | — | Italy | KM116217 | KM199324 | KM199392 | KM199474 | |
| P. clavata | MFLUCC 12-0268; NN0471340* | Buxus sp. | Buxaceae | China | — | JX398990 | JX399025 | JX399056 |
| P. colombiensis | CBS 118553; CPC 10969* | Eucalyptus eurograndis | Myrtaceae | Colombia | KM116222 | KM199307 | KM199421 | KM199488 |
| P. diploclisiae | CBS 115449; HKUCC 9103 | Psychotria tutcheri | Rubiaceae | Hong Kong | KM116215 | KM199314 | KM199416 | KM199485 |
| CBS 115585; HKUCC 8394 | Diploclisia glaucescens | Menispermaceae | Hong Kong | KM116213 | KM199315 | KM199417 | KM199483 | |
| CBS 115587; HKUCC 10130* | Diploclisia glaucescens | Menispermaceae | Hong Kong | KM116242 | KM199320 | KM199419 | KM199486 | |
| P. diversiseta | MFLUCC 12-0287; NN0472610* | Rhododendron sp. | Ericaceae | China | — | JX399009 | JX399040 | JX399073 |
| P. ericacearum | IFRDCC 2439* | Rhododendron delavayi | Ericaceae | China | — | KC537807 | KC537821 | KC537814 |
| P. furcata | MFLUCC 12-0054; CPC 20280* | Camellia sinensis | Theaceae | Thailand | KM116283 | JQ683724 | JQ683708 | JQ683740 |
| P. gaultheria | IFRD 411-014* | Gaultheria forrestii | Ericaceae | China | — | KC537805 | KC537819 | KC537812 |
| P. grevilleae | CBS 114127; STE-U 2919* | Grevillea sp. | Proteaceae | Australia | KM116212 | KM199300 | KM199407 | KM199504 |
| P. hawaiiensis | CBS 114491; STE-U 2215* | Leucospermum sp. cv. ‘Coral’ | Myrtaceae | USA: Hawaii | KM116239 | KM199339 | KM199428 | KM199514 |
| P. hollandica | CBS 265.33* | Sciadopitys verticillata | Sciadopityaceae | Netherlands | KM116228 | KM199328 | KM199388 | KM199481 |
| P. humus | CBS 115450; HKUCC 9100 | Ilex cinerea | Aquifoliaceae | Hong Kong | KM116208 | KM199319 | KM199418 | KM199487 |
| CBS 336.97* | Soil | — | Papua New Guinea | KM116230 | KM199317 | KM199420 | KM199484 | |
| P. inflexa | MFLUCC 12-0270; NN0470980* | Unidentified tree | — | China | — | JX399008 | JX399039 | JX399072 |
| P. intermedia | MFLUCC 12-0259; NN0476420* | Unidentified tree | — | China | — | JX398993 | JX399028 | JX399059 |
| P. jesteri | CBS 109350 = MONT 6M-B-3* | Fragraea bodenii | Gentianaceae | Papua New Guinea | KM116281 | KM199380 | KM199468 | KM199554 |
| P. kenyana | CBS 442.67* | Coffea sp. | Rubiaceae | Kenya | KM116234 | KM199302 | KM199395 | KM199502 |
| CBS 911.96 | Raw material from agar-agar | — | — | KM116204 | KM199303 | KM199396 | KM199503 | |
| P. knightiae | CBS 111963; STE-U 2905 | Knightia sp. | Proteaceae | New Zealand | KM116241 | KM199311 | KM199406 | KM199495 |
| CBS 114138; STE-U 2906* | Knightia sp. | Proteaceae | New Zealand | KM116227 | KM199310 | KM199408 | KM199497 | |
| P. linearis | MFLUCC 12-0271; NN0471900* | Trachelospermum sp. | Apocynaceae | China | — | JX398992 | JX399027 | JX399058 |
| P. malayana | CBS 102220* | Macaranga triloba | Euphorbiaceae | Malaysia | KM116238 | KM199306 | KM199411 | KM199482 |
| P. monochaeta | CBS 144.97* | Quercus robur | Fagaceae | Netherlands | KM116229 | KM199327 | KM199386 | KM199479 |
| CBS 440.83; IFO 32686 | Taxus baccata | Taxaceae | Netherlands | KM116196 | KM199329 | KM199387 | KM199480 | |
| P. novae-hollandiae | CBS 130973* | Banksia grandis | Proteaceae | Australia | KM116232 | KM199337 | KM199425 | KM199511 |
| P. oryzae | CBS 111522; STE-U 2083 | Telopea sp. | Proteaceae | USA: Hawaii | — | KM199294 | KM199394 | KM199493 |
| CBS 171.26 | — | — | Italy | KM116206 | KM199304 | KM199397 | KM199494 | |
| CBS 353.69* | Oryza sativa | Poaceae | Denmark | KM116221 | KM199299 | KM199398 | KM199496 | |
| P. papuana | CBS 331.96* | Coastal soil | — | Papua New Guinea | KM116240 | KM199321 | KM199413 | KM199491 |
| CBS 887.96 | Cocos nucifera | Arecaceae | Papua New Guinea | KM116231 | KM199318 | KM199415 | KM199492 | |
| P. parva | CBS 265.37; BBA 2820* | Delonix regia | Fabaceae | — | KM116226 | KM199312 | KM199404 | KM199508 |
| CBS 278.35 | Leucothoe fontanesiana | Ericaceae | — | KM116205 | KM199313 | KM199405 | KM199509 | |
| P. portugalica | CBS 393.48* | — | — | Portugal | KM116233 | KM199335 | KM199422 | KM199510 |
| P. rhododendri | IFRDCC 2399* | Rhododendron sinogrande | Ericaceae | China | — | KC537804 | KC537818 | KC537811 |
| P. rosea | MFLUCC 12-0258; NN0471350* | Pinus sp. | Pinaceae | China | — | JX399005 | JX399036 | JX399069 |
| P. scoparia | CBS 176.25* | Chamaecyparis sp. | Cupressaceae | — | KM116216 | KM199330 | KM199393 | KM199478 |
| Pestalotiopsis sp. Clade 33 | CBS 263.33 | Rhododendron ponticum | Ericaceae | Netherlands | KM116198 | KM199316 | KM199414 | KM199489 |
| CBS 264.33 | Cocos sp. | Arecaceae | Indonesia: Sulawesi | KM116199 | KM199322 | KM199412 | KM199490 | |
| P. spathulata | CBS 356.86* | Gevuina avellana | Proteaceae | Chile | KM116236 | KM199338 | KM199423 | KM199513 |
| P. telopeae | CBS 113606; STE-U 3082 | Telopea sp. | Proteaceae | Australia | KM116202 | KM199295 | KM199402 | KM199498 |
| CBS 114137; STE-U 2952 | Protea neriifolia × susannae cv. ‘Pink Ice’ | Proteaceae | Australia | KM116219 | KM199301 | KM199469 | KM199559 | |
| CBS 114161; STE-U 3083* | Telopea sp. | Proteaceae | Australia | — | KM199296 | KM199403 | KM199500 | |
| P. trachicarpicola | IFRDCC 2403 | Podocarpus macrophyllus | Podocarpaceae | China | — | KC537809 | KC537823 | KC537816 |
| MFLUCC 12-0263; NN0470720 | Unidentified tree | — | China | — | JX399000 | JX399031 | JX399064 | |
| MFLUCC 12-0264; NN0471960 | Chrysophyllum sp. | Sapotaceae | China | — | JX399004 | JX399035 | JX399068 | |
| MFLUCC 12-0265; NN0469830 | Schima sp. | Theaceae | China | — | JX399003 | JX399034 | JX399067 | |
| MFLUCC 12-0266; NN0469780 | Sympolocos sp. | Symplocaceae | China | — | JX399002 | JX399033 | JX399066 | |
| MFLUCC 12-0267; NN0470990 | Unidentified tree | — | China | — | JX399001 | JX399032 | JX399065 | |
| OP068; IFRDCC 2440* | Trachycarpus fortunei | Arecaceae | China | — | JQ845947 | JQ845945 | JQ845946 | |
| P. unicolor | MFLUCC 12-0275; NN0473080 | Unidentified tree | — | China | — | JX398998 | JX399029 | JX399063 |
| MFLUCC 12-0276; NN0469740* | Rhododendron sp. | Ericaceae | China | — | JX398999 | JX399030 | — | |
| P. verruculosa | MFLUCC 12-0274; NN0473090* | Rhododendron sp. | Ericaceae | China | — | JX398996 | — | JX399061 |
| Pseudopestalotiopsis cocos | CBS 272.29* | Cocos nucifera | Arecaceae | Indonesia: Java | KM116276 | KM199378 | KM199467 | KM199553 |
| Ps. indica | CBS 459.78* | Hibiscus rosa-sinensis | Malvaceae | India | — | KM199381 | KM199470 | KM199560 |
| Ps. theae | MFLUCC 12-0055; CPC 20281* | Camellia sinensis | Theaceae | Thailand | KM116282 | JQ683727 | JQ683711 | JQ683743 |
| SC011 | Camellia sinensis | Theaceae | Thailand | — | JQ683726 | JQ683710 | JQ683742 | |
ATCC: American Type Culture Collection, Virginia, USA; BBA: Institute for Plant Virology, Microbiology and Biosafety, Federal Biological Research Centre for Agriculture and Forestry (BBA), Germany; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands; CGMCC: China General Microbiological Culture Collection Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; CMW: Tree Pathology Co-operative Program, Forestry and Agricultural Biotechnology Institute, University of Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; HKUCC: The University of Hong Kong Culture Collection, Hong Kong, China; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; IFO: Institute for Fermentation Culture Collection, Osaka, Japan; IFRDCC: International Fungal Research & Development Centre Culture Collection, China; IMI: Culture collection of CABI Europe UK Centre, Egham, UK; INIFAT: Alexander Humboldt Institute for Basic Research in Tropical Agriculture, Ciudad de La Habana, Cuba; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; NN: Novozymes, Beijing, China; QM: Quarter Master Culture Collection, Amherst, MA, USA; STE-U: Culture collection of the Department of Plant Pathology, University of Stellenbosch, South Africa. * = ex-holotype or ex-epitype culture.
LSU: large subunit (28S) of the nrRNA gene operon; ITS: internal transcribed spacers and intervening 5.8S nrDNA; TUB: partial beta-tubulin gene; TEF: partial translation elongation factor 1-alpha gene.
Phylogenetic analyses
The sequences generated in this study were supplemented with additional sequences obtained from GenBank (Table 1) based on blast searches and literature. Multiple sequence alignments were generated with MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html); the alignment was visually improved with Mesquite v. 2.75 (Maddison & Maddison 2011) and MEGA v. 5.2.2 (Kumar et al. 2012) or BioEdit v. 7.0.5.2 (Hall 1999). Three different datasets were used to estimate three phylogenies: an Amphisphaeriaceae family tree, a combined Neopestalotiopsis and Pseudopestalotiopsis species tree, and a Pestalotiopsis species tree. The first tree focuses on the placement and further division of Pestalotiopsis into two new genera in Amphisphaeriaceae by using the LSU region. The second and third phylogenetic analyses were produced to show species relationships in Pestalotiopsis, Neopestalotiopsis and Pseudopestalotiopsis based on the combined datasets (ITS, TUB and TEF). The combined alignments were split between the genera to improve the robustness of the alignment across the three loci. Phylogenetic analyses of the sequence data consisted of Bayesian Inference (BI), Maximum Likelihood (ML) and Maximum Parsimony (MP) analyses of both the individual data partitions as well as the combined aligned dataset. Ambiguously aligned regions were excluded from all analyses and gaps were treated as “fifth character state” in the parsimony analysis. Suitable models for the Bayesian analysis were first selected using models of nucleotide substitution for each gene, as determined using MrModeltest v. 2.2 (Nylander 2004), and included for each gene partition. The Bayesian analyses (MrBayes v. 3.2.1; Ronquist et al. 2012) of four simultaneous Markov Chain Monte Carlo (MCMC) chains were run from random trees for 10 000 000 generations and sampled every 1 000 generations. The temperature value was lowered to 0.15, burn-in was set to 0.25, and the run was automatically stopped as soon as the average standard deviation of split frequencies reached below 0.01. A maximum likelihood analysis was performed using raxmlGUI v. 1.3 (Silvestro & Michalak 2011). The optimal ML tree search was conducted with 100 separate runs, using the default algorithm of the program from a random starting tree for each run. The final tree was selected among suboptimal trees from each run by comparing likelihood scores under the GTR+GAMMA substitution model. The MP analysis was performed with PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003). Trees were inferred by using the heuristic search option with TBR branch swapping and 1 000 random sequence additions. The maximum number of retained trees were limited to 5 000, branches of zero length were collapsed and all multiple equally most parsimonious trees were saved. Tree length [TL], consistency index [CI], retention index [RI], rescaled consistency index [RC], homoplasy index [HI], and log likelihood [-ln L] (HKY model) values were calculated. The robustness of the equally most parsimonious trees was evaluated by 1 000 bootstrap replications (Felsenstein 1985) resulting from a maximum parsimony analysis, each with 10 replicates of random stepwise addition of taxa. The Kishino-Hasegawa tests (Kishino & Hasegawa 1989) were performed to determine whether the trees inferred under different optimality criteria were significantly different. The resulting trees were printed with FigTree v. 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) and the layout was done with Adobe Illustrator CS v. 6. The alignments and trees were deposited in TreeBASE (www.treebase.org/treebase/index.html).
Results
Phylogeny
The LSU alignment was used to resolve the generic placement of Pestalotiopsis strains in the Amphisphaeriaceae (Fig. 3). The alignment comprised 74 strains (including the outgroup taxon Xylaria hypoxylon) and the manually adjusted dataset comprised 807 characters including gaps; the data partition contained 173 unique site patterns. Dirichlet base frequencies and the GTR+I+G model with inverse gamma-distributed rate were recommended by the MrModeltest analysis and used in the Bayesian analysis. The Bayesian analysis lasted 1 435 000 generations and the 50 % consensus trees and posterior probabilities were calculated from the 2 154 trees left after discarding 718 trees (the first 25 % of generations) for burn-in (Fig. 3). The parsimony analysis indicated that 617 characters were constant, 73 variable characters parsimony-uninformative and 117 characters parsimony-informative. After a heuristic search using PAUP, 125 equally most parsimonious trees were obtained (tree length = 408 steps, CI = 0.591, RI = 0.871, RC = 0.514, HI = 0.409). The Bayesian analysis resulted in a tree with the same topology and clades as the ML and MP trees. The BI, ML and MP analyses of LSU indicated that Pestalotiopsis comprises three major monophyletic clades, each supported with high bootstrap confidence or posterior probability. Species possessing morphology similar to the type species of Pestalotiopsis (P. maculans) clustered in one clade designated as Pestalotiopsis s. str. Two well-supported clades clustered outside Pestalotiopsis s. str., for which two new genera, Neopestalotiopsis and Pseudopestalotiopsis are introduced. In all analyses, Pseudopestalotiopsis was always sister to Pestalotiopsis and clustered as a basal sister clade to Neopestalotiopsis. The species containing versicolourous median cells form a monophyletic clade named Neopestalotiopsis and appear to have evolved from the Pseudopestalotiopsis lineage, whose members have concolourous median cells.
Fig. 3.
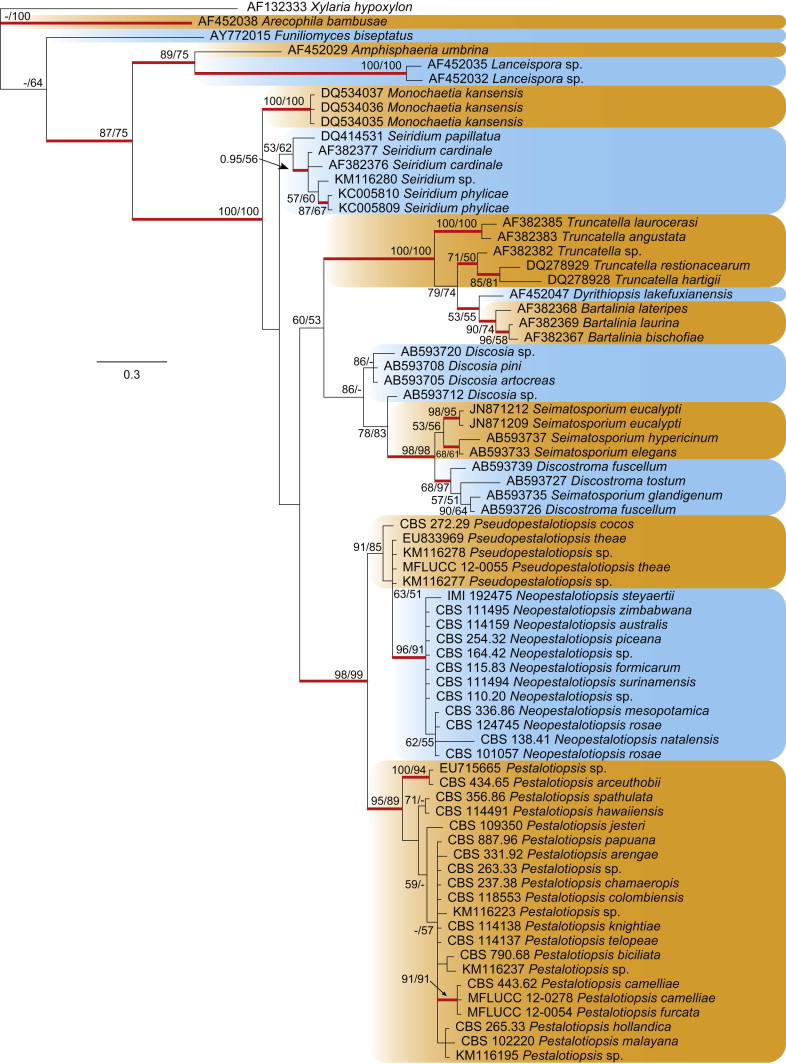
Consensus phylogramme (50 % majority rule) of 2 154 trees resulting from a Bayesian analysis of the LSU sequence alignment of Neopestalotiopsis, Pestalotiopsis, Pseudopestalotiopsis and other genera in family Amphisphaeriaceae. Genera are indicated in coloured blocks and red-thickened lines indicate Bayesian posterior probabilities (PP) above 95 %. RAxML bootstrap support values (MLB) and maximum parsimony bootstrap support values (MPB) are given at the nodes (MLB/MPB). The scale bar represents the expected number of changes per site. The tree was rooted to Xylaria hypoxylon (GenBank AF132333).
Species relationships in Neopestalotiopsis and Pseudopestalotiopsis are shown in Fig. 4. For the combined genes, BI, ML, and MP consensus trees revealed the same phylogenetic relationships between the significantly supported clades. The combined ITS, TUB and TEF alignment comprises 59 strains (including 24 ex-type / ex-epitype strains for species of Neopestalotiopsis, three ex-type / ex-epitype strains for species of Pseudopestalotiopsis, and Pestalotiopsis trachicarpicola as the outgroup taxon) and 1 418 characters including gaps with 66, 145 and 180 unique site patterns for ITS, TUB and TEF, respectively. Suitable models were selected using models of nucleotide substitution for each gene, as determined using MrModeltest. The GTR+I model with a proportion of invariable sites for ITS and the HKY+G model with gamma-distributed rate model for TUB and the GTR+I+G model with inverse gamma rate were selected for TEF and included for each gene partition. The Bayesian analysis lasted 2 585 000 generations and the 50 % consensus trees and posterior probabilities were calculated from the 3 880 trees left after discarding 1 293 trees (the first 25 % of generations) for burn-in (Fig. 4). Among these 1 418 characters (ITS = 491, TUB = 442 and TEF = 485), 990 were constant, 172 variable characters parsimony uninformative and 256 characters parsimony-informative. The parsimony analysis resulted in 108 equally most parsimonious trees (tree length = 805 steps, CI = 0.688, RI = 0.810, RC = 0.557, HI = 0.312). Neopestalotiopsis and Pseudopestalotiopsis isolates clustered into two well-supported clades (BI = 1, ML = 100 and MP = 100). Furthermore, thirty clades are recognised in Neopestalotiopsis and discussed here (Fig. 4).
Fig. 4.
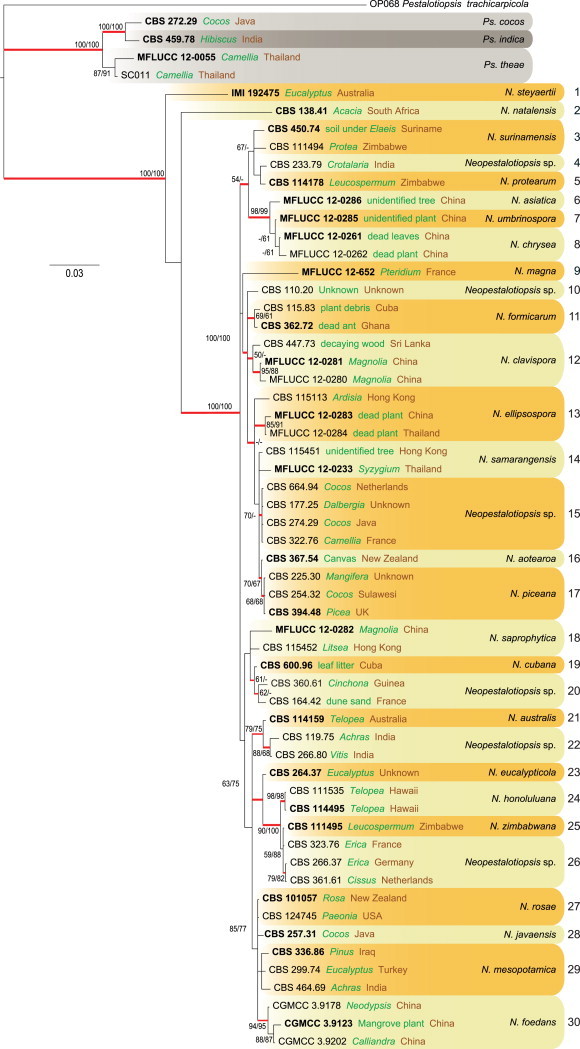
Consensus phylogramme (50 % majority rule) of 3 880 trees resulting from a Bayesian analysis of the combined (ITS+TUB+TEF) alignment of the analysed Neopestalotiopsis and Pseudopestalotiopsis sequences. Pseudopestalotiopsis is indicated in grey shades and Neopestalotiopsis clades are indicated in yellow and orange coloured blocks. Clades are numbered to the right of the blocks (1–30). Red-thickened lines indicate Bayesian posterior probabilities (PP) above 95 %. RAxML bootstrap support values (MLB) and maximum parsimony bootstrap supports (MPB) are given at the nodes (MLB/MPB). Strain accession numbers (sequences derived from ex-type are printed in bold) are followed by the isolation source (green) and country of origin (brown). The correct species name is indicated to the right of the clade. The scale bar represents the expected number of changes per site. The tree was rooted to Pestalotiopsis trachicarpicola (OP068).
To clarify species boundaries within Pestalotiopsis, a combined alignment of ITS, TUB and TEF contained 96 sequences (including the outgroup Neopestalotiopsis saprophytica; MFLUCC 12-0282), and 1 519 characters including alignment gaps with 101, 213 and 268 unique site patterns for ITS, TUB and TEF, respectively (Fig. 5). Dirichlet base frequencies and the GTR+I+G model with inverse gamma-distributed rate for ITS and HKY+I+G model with inverse gamma-distributed rate were selected for TUB and TEF and set in MrBayes. The Bayesian analysis lasted 745 000 generations and the 50 % consensus trees and posterior probabilities were calculated from the 1 120 trees left after discarding 373 trees (the first 25 % of generations) for burn-in (Fig. 5). Of the 1 519 characters (ITS = 552, TUB = 463 and TEF = 504), 890 were constant, 250 variable characters parsimony uninformative and 379 characters parsimony-informative. A MP analysis yielded 96 equally most parsimonious trees (tree length = 1 628 steps, CI = 0.596, RI = 0.808, RC = 0.482, HI = 0.404). The Bayesian analysis resulted in a tree with the same topology and terminal clades as the ML and MP trees. Fourty-three clades are recognised and discussed here (Fig. 5).
Fig. 5.
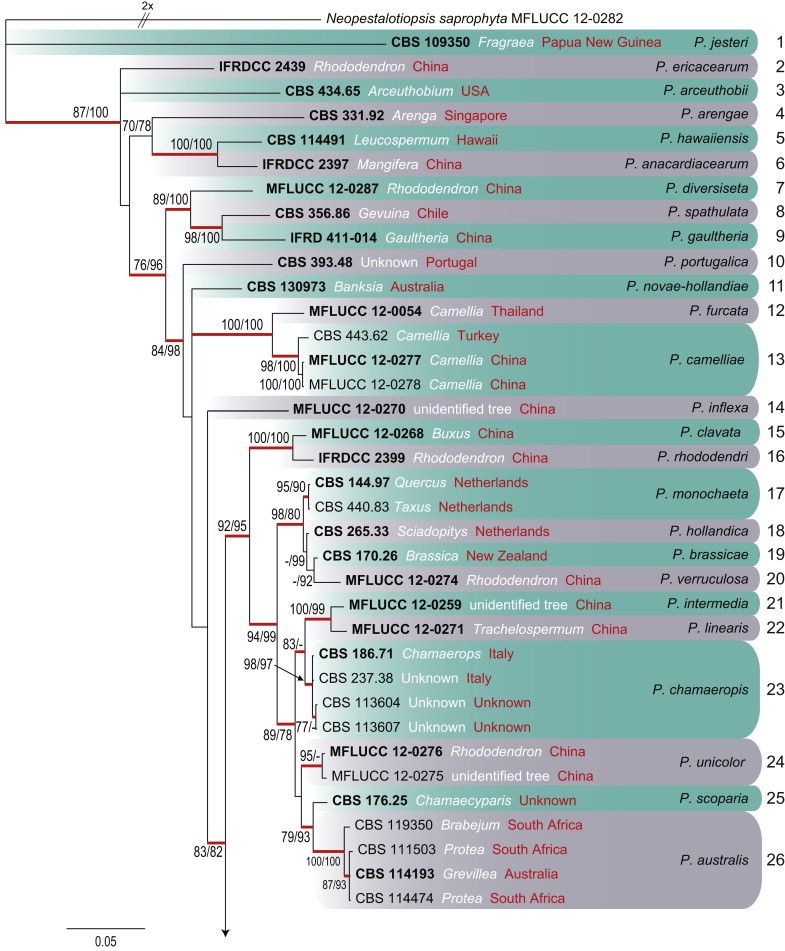
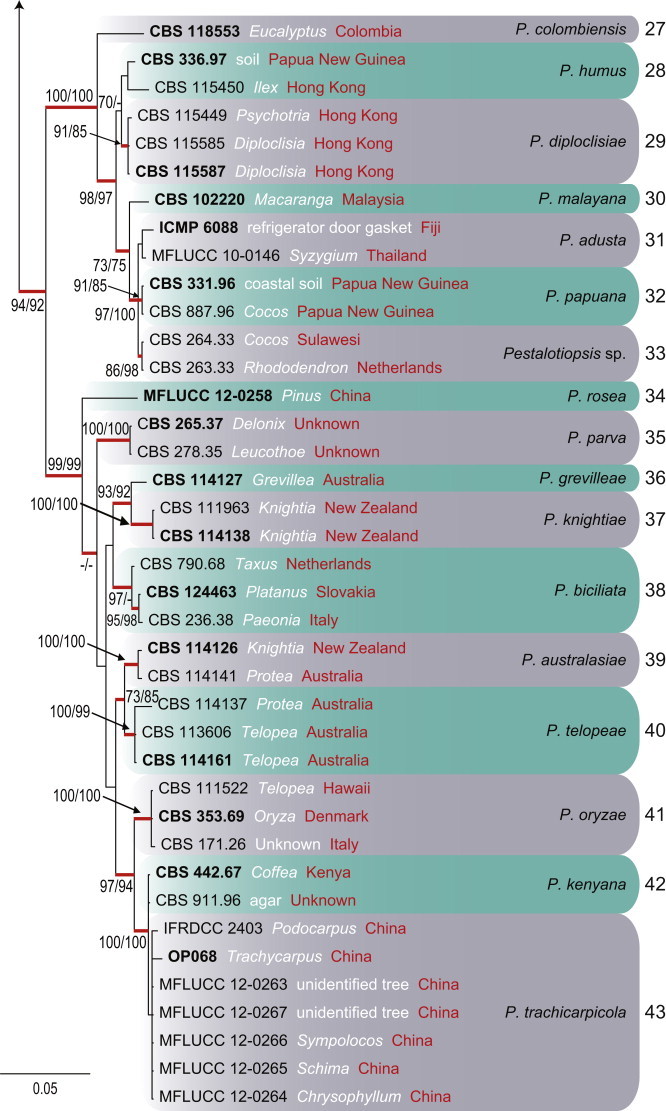
Consensus phylogramme (50 % majority rule) of 1 120 trees resulting from a Bayesian analysis of the combined (ITS+TUB+TEF) alignment of the analysed Pestalotiopsis isolates. Clades are indicated in coloured blocks. Clades are numbered to the right of the boxes (1–43). Red-thickened lines indicate Bayesian posterior probabilities (PP) above 95 %. RAxML bootstrap support values (MLB) and maximum parsimony bootstrap supports (MPB) are given at the nodes (MLB/MPB). Strain accession numbers (sequences derived from ex-type are printed in bold) are followed by the isolation source (white) and country of origin (red). The correct species name is indicated to the right of the clade. The scale bar represents the expected number of changes per site. The tree is rooted to Neopestalotiopsis saprophytica (MFLUCC 12-0282).
Taxonomy
Phylogenetic analyses based on the LSU alignment, together with an appraisal of the literature and morphology, resulted in the proposal of two novel genera in Amphisphaeriaceae. The new genera Neopestalotiopsis and Pseudopestalotiopsis, which segregate off Pestalotiopsis, are proposed based on the types Neopestalotiopsis protearum and Pseudopestalotiopsis theae, respectively. Descriptions of the new genera Neopestalotiopsis and Pseudopestalotiopsis are provided. Based on the results of ITS, TUB and TEF sequence analyses, 30 internal clades (clades 1–30; Fig. 4) can be distinguished in Neopestalotiopsis; three clades in Pseudopestalotiopsis (Fig. 4) and 43 clades in Pestalotiopsis (clades 1–43; Fig. 5). Several Pestalotiopsis species are transferred to Neopestalotiopsis and Pseudopestalotiopsis. Eleven new species of Neopestalotiopsis are described and one ex-type re-examined. Two novel species are introduced in Pseudopestalotiopsis. Twenty-four new species of Pestalotiopsis are described and illustrated here and two ex-types are re-examined. Based on the molecular phylogeny, several remaining isolates represent unnamed species; these are not treated further as most of these isolates did not sporulate, or due to lack of ecological diversity.
Neopestalotiopsis Maharachch., K.D. Hyde & Crous, gen. nov. MycoBank MB809759.
Etymology: Named after its morphological similarity to Pestalotiopsis.
Conidiomata acervular or pycnidial, subglobose, globose, clavate, solitary or aggregated, dark brown to black, immersed to erumpent, unilocular or irregularly plurilocular; exuding dark brown to black conidia in a slimy, globose mass. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical, ampulliform to lageniform, hyaline, smooth, thin-walled; conidiogenesis initially holoblastic, becoming percurrent to produce additional conidia at slightly higher levels. Conidia fusoid, ellipsoid to subcylindrical, straight to slightly curved, 4-septate; basal cell conic to subcylindrical, with a truncate base, hyaline or pale brown to olivaceous, thin and rugose to smooth-walled; three median cells doliiform, wall rugose to verruculose, versicoloured, septa darker than the rest of the cell; apical cell hyaline, conic to cylindrical, thin- and smooth-walled; with tubular apical appendages, one to many, filiform or attenuated, flexuous, branched or unbranched; basal appendage single, tubular, unbranched, centric.
Type species: Neopestalotiopsis protearum (Crous & L. Swart) Maharachch., K.D. Hyde & Crous (see below).
Notes: Based on LSU sequence data (Fig. 3), Neopestalotiopsis clusters in Amphisphaeriaceae and is distinct from Pseudopestalotiopsis and Pestalotiopsis, and is best treated as a separate genus. Liu et al. (2010), based on the length of the ITS alignment, also revealed that species of Pestalotiopsis cluster in three groups. The ITS sequence lengths in groups A, B, and C (i.e. Neopestalotiopsis, Pestalotiopsis and Pseudopestalotiopsis) were 480–484 bp, 489–495 bp and 536–540 bp, respectively. Morphologically Neopestalotiopsis can also be easily distinguished from Pseudopestalotiopsis and Pestalotiopsis by its versicolourous median cells. Furthermore, in Neopestalotiopsis conidiophores are indistinct and often reduced to conidiogenous cells. In the key provided by Guba (1961) and Steyaert (1949) the species in the versicolourous group divided into two subgroups: umber-olivaceous (two upper median cells umber and lowest median cell yellow-brown) and fuliginous-olivaceous (two upper median cells fuliginous, usually opaque, and lowest median cell pale brown). In his monograph Guba (1961) treated the versicolourous umber-olivaceous group, which comprised 40 species and the versicolourous fuliginous-olivaceous group, which comprised 56 species. The two groups were differentiated depending on the intensities of the median cells, while most species have similar conidial measurements. Jeewon et al. (2003), Liu et al. (2010) and Maharachchikumbura et al. (2011) concluded that the division of the versicolourous group based on colour intensities of the median conidial cell is not a taxonomically good character. Instead of using two groups, we propose Neopestalotiopsis as a new genus for the versicolourous group.
Neopestalotiopsis aotearoa Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809760. Fig. 6.
Fig. 6.
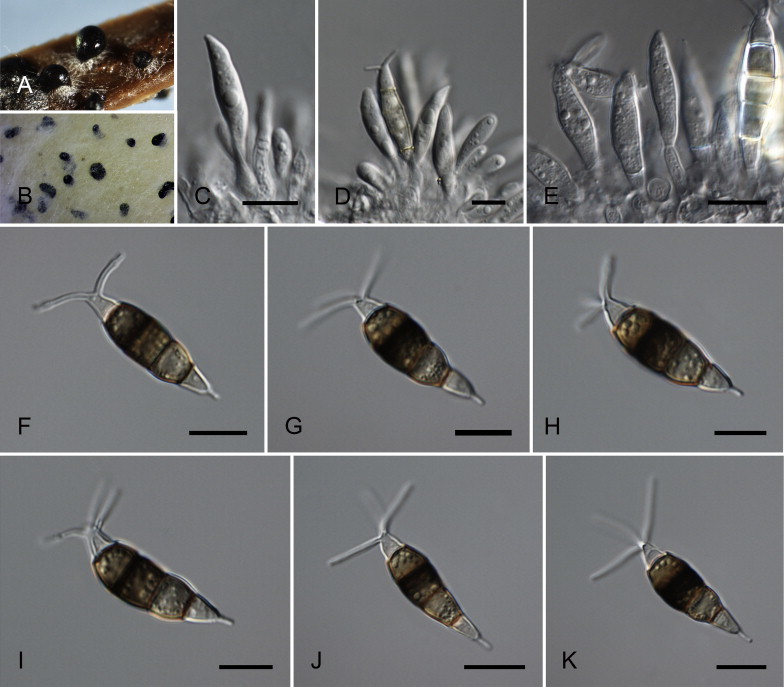
Neopestalotiopsis aotearoa CBS 367.54T. A. Conidiomata sporulating on PNA (pine needle agar). B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the Maori name (= Aotearoa) for the country where it was collected, New Zealand.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary or confluent, embedded or semi-immersed to erumpent, dark brown, 200–450 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, subcylindrical to ampulliform, hyaline, proliferating 2–4 times percurrently, 5–20 × 2–10 μm, apex 2–5 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (19.5–)21–28(–29) × (6–)6.5–8.5(–9) μm, ± SD = 24.8 ± 1.6 × 7.7 ± 0.5 μm; basal cell conic with a truncate base, hyaline, rugose and thin-walled, 4–6.5 μm long; three median cells doliiform, (13–)14–18(–18.5) μm long, ± SD = 15.9 ± 1.1 μm, wall verruculose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 4–6 μm long; third cell honey-brown, 3.5–7 μm long; fourth cell brown, 4–6.5 μm long); apical cell 3.5–5.5 μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (3–)5–12(–13) μm long, ± SD = 8.1 ± 1.2 μm; basal appendage single, tubular, unbranched, centric, 1.5–4 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with undulate edge, pale honey-coloured, sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: Saprobe on canvas.
Known distribution: New Zealand.
Material examined: New Zealand, from canvas, Sep. 1954, G.C. Wade (CBS H-15765, holotype, ex-type culture CBS 367.54 = ATCC 11763 = QM 381).
Notes: Neopestalotiopsis aotearoa (clade 16; Fig. 4) is described from a canvas in New Zealand. In the phylogenetic analyses, N. aotearoa proved to be sister to N. piceana (clade 17; Fig. 4), but the two species are morphologically easily distinguishable. Neopestalotiopsis piceana is distinct from N. aotearoa by its clavate conidia, longer basal, and apical appendages.
Neopestalotiopsis asiatica (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809761.
Basionym: Pestalotiopsis asiatica Maharachch. & K.D. Hyde, Fungal Divers. 56: 104. 2012.
Material examined: China, Hunan Province, Yizhang County, Mangshan, from living leaves of unidentified tree, 12 Apr. 2002, W.P. Wu (HMAS047638, holotype; MFLU 12-0422, isotype, ex-type culture NN0476380 = MFLUCC 12-0286).
Note: This species (clade 6; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis australis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809762. Fig. 7.
Fig. 7.
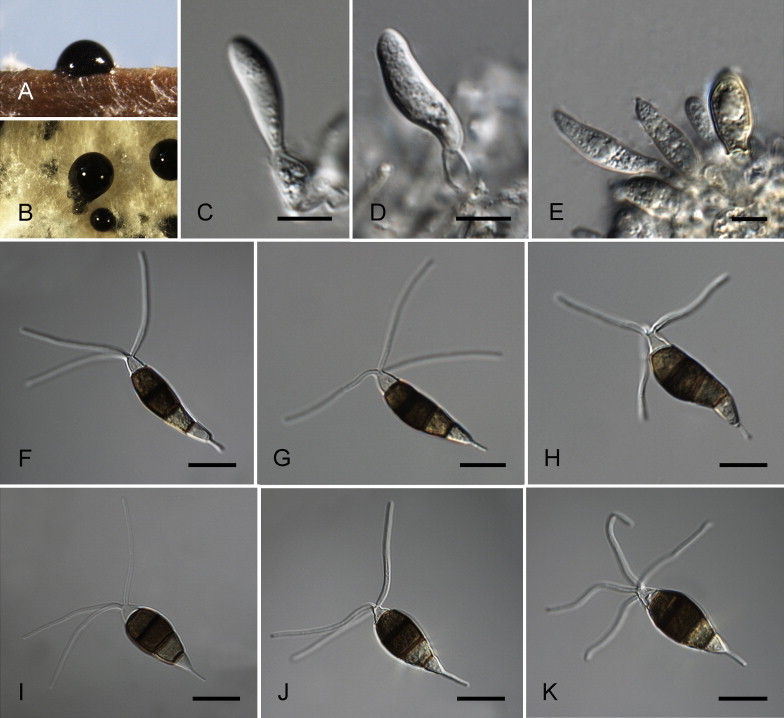
Neopestalotiopsis australis CBS 114159T. A. Conidioma sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Australia.
Conidiomata pycnidial in culture on PDA, globose to clavate, solitary or aggregated in clusters, semi-immersed, brown to black, 100–500 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, rugose-walled, simple, proliferating 1–3 times percurrently, 5–12 × 2–7 μm, apex 1–2 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (19–)21–27(–28) × (7–)7.5–9(–9.5) μm, ± SD = 24.6 ± 1.8 × 8 ± 0.4 μm; basal cell conic with a truncate base, hyaline, rugose and thin-walled, 3.5–5.5 μm long; three median cells doliiform, (13–)14–18(–18.5) μm long, ± SD = 16.1 ± 1 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 3.5–6.5 μm long; third cell darker brown, 4–7 μm long; fourth cell brown, 5–6.5 μm long); apical cell 3–6 μm long, hyaline, subcylindrical to obconic, rugose and thin-walled; with 3–4 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous, (19–)21–32(–34) μm long, ± SD = 26.6 ± 3 μm; basal appendage single, tubular, unbranched, centric, 3–7 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale honey-coloured, with dense aerial mycelium on the surface with black, concentric conidiomata; reverse similar in colour.
Habitat: On Telopea sp.
Known distribution: Australia.
Material examined: Australia, New South Wales, from Telopea sp., 12 Oct. 1999, P.W. Crous (CBS H-21773, holotype, ex-type culture CBS 114159 = STE-U 3017).
Notes: Neopestalotiopsis australis (clade 21; Fig. 4) was isolated from Telopea sp. in New South Wales, Australia. The conidiogenous cells and conidia of N. australis resemble those of the two Indian isolates, CBS 266.80 and CBS 119.75 (clade 22; Fig. 4), which were isolated from Vitis vinifera and Eucalyptus globulus, respectively. Since there is geographical variation of the two Indian isolates and a slight distinction in phylogeny, they are tentatively maintained as Neopestalotiopsis sp. Clade 22 until additional collections and cultures become available. There are various fungal pathogens recorded from Proteaceae, which is an important plant family in world floriculture markets (Crous et al. 2011). Neopestalotiopsis and Pestalotiopsis have subsequently been isolated from several Protea and Leucospermum hosts (Swart et al. 1999), and intercepted at quarantine inspection points (Taylor 2001). Neopestalotiopsis australis, N. honoluluana, N. protearum and N. zimbabwana are recorded from Proteaceae plants. Most of these species cause leaf spots and tip dieback, and can be easily identified based on diagnostic morphology and phylogeny.
Neopestalotiopsis chrysea (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809763.
Basionym: Pestalotiopsis chrysea Maharachch. & K.D. Hyde, Fungal Divers. 56: 107. 2012.
Materials examined: China, Guangxi Province, Shangsi, Shiwandashan, Wangle, dead leaves of unidentified plant, 2 Jan. 1997, W.P. Wu (HMAS042855, holotype; MFLU 12-0411, isotype, ex-type culture NN042855 = MFLUCC 12-0261); Hunan Province, Yizhang County, Mangshanon, dead plant material, 12 Apr. 2002, W.P. Wu, culture NN047037 = MFLUCC 12-0262.
Note: This species (clade 8; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis clavispora (G.F. Atk.) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809764.
Basionym: Pestalotia clavispora G.F. Atk., Bull. Cornell Univ. 3: 37. 1897.
≡ Pestalotiopsis clavispora (G.F. Atk.) Steyaert, Bull. Jard. bot. État Brux. 19: 335. 1949.
Materials examined: China, Guangxi Province, Shiwandashan, on dead leaves of Magnolia sp., 28 Dec. 1997, W.P. Wu (HMAS043133 = MFLU 12-0418, epitype, ex-epitype culture NN043133 = MFLUCC 12-0281); Guangxi Province, Yunnan, Shiwandashan, on dead leaves of Magnolia sp., 28 Dec. 1997, W.P. Wu, culture NN043011 = MFLUCC 12-0280. Sri Lanka, decaying wood, 23 Jan. 1973, W. Gams, culture CBS 447.73. USA, Auburn, Alabama, on fallen leaves of Quercus rubra, 10 Mar. 1891, F. Atkinson (CUP-A-032389, holotype).
Note: This species (clade 12; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis cubana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809765. Fig. 8.
Fig. 8.
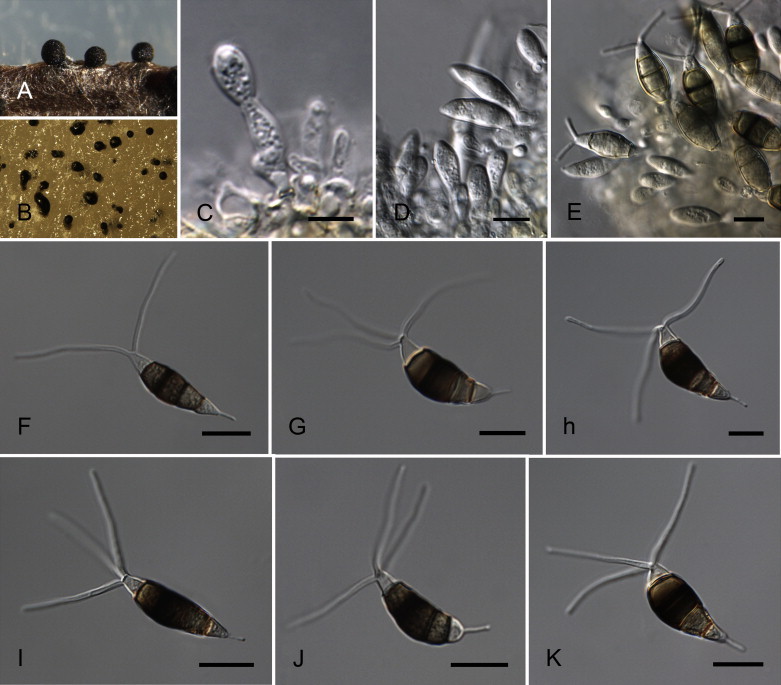
Neopestalotiopsis cubana CBS 600.96T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Cuba.
Conidiomata pycnidial in culture on PDA, globose, solitary or aggregated, embedded or semi-immersed, dark brown to black, up to 250 μm diam; exuding globose, brown to black conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, 5–12 × 2–4 μm, or ampulliform to lageniform, 3–8 × 1–4 μm, hyaline, smooth-walled, proliferating 2–4 times percurrently, 5–15 × 2–5 μm, collarette present and not flared. Conidia fusoid, ellipsoid, straight to slightly curved, somewhat constricted at septa, 4-septate, (19–)20–25(–27) × (7.5–)8–9.5(–10) μm, ± SD = 23.4 ± 1.4 × 8.8 ± 0.4 μm; basal cell obconic to conic with a truncate base, hyaline, rogose and thin-walled, 3–5 μm long; three median cells doliiform, (13.5–)14–16.5(–17.5) μm long, ± SD = 15.5 ± 0.9 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 4.5–6 μm long; third cell honey-brown, 4.5–6.5 μm long; fourth cell brown, 4–5.5 μm long); apical cell 4–5 μm long, hyaline, subcylindrical, thin- and smooth-walled; with 2–4 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous, (19–)21–27(–28) μm long, ± SD = 24 ± 2 μm; basal appendage single, tubular, unbranched, centric, 4–7 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale honey coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On leaf litter.
Known distribution: Cuba.
Material examined: Cuba, from leaf litter, Jun. 1996, R.F. Castañeda (CBS H-21772, holotype, ex-type culture CBS 600.96 = INIFAT C96/44-4).
Notes: Neopestalotiopsis cubana (clade 19; Fig. 4) is from leaf litter isolated in Cuba, and forms a sister clade to CBS 164.42 and CBS 360.61, which were isolated from sand dunes in France and Cinchona sp. in Guinea, respectively. The latter isolates are morphologically somewhat similar to N. cubana, even though, due to clear ecological differences we prefer to maintain them as Neopestalotiopsis sp. Clade 20 until we have obtained more cultures and collections. Neopestalotiopsis cubana is distinguished from the sister N. saprophytica (clade 18; Fig. 4) (22–30 × 5–6 μm) by its wider conidia.
Neopestalotiopsis ellipsospora (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809766.
Basionym: Pestalotiopsis ellipsospora Maharachch. & K.D. Hyde, Fungal Divers. 56: 112. 2012.
Materials examined: China, Yunnan Province, on dead plant materials, L.D. Guo (MFLU 12-0420, holotype, ex-type culture MFLUCC 12-0283); Hong Kong, on fruits of Ardisia crenata, 1 Jan. 2002, unknown collector, culture CBS 115113 = HKUCC 9136. Thailand, Chiang Rai, Tool Kwan, Huay Mesak waterfall, on dead plant material, 12 Jan. 2010, S.S.N. Maharachchikumbura, culture MFLUCC 12-0284.
Note: This species (clade 13; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis eucalypticola Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809767. Fig. 9.
Fig. 9.

Neopestalotiopsis eucalypticola CBS 264.37T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Eucalyptus.
Conidiomata (on PDA) pycnidial, globose, solitary or aggregated in clusters, semi-immersed, brown to black, 100–400 μm diam; exuding globose, dark brown conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, smooth, thin-walled, simple, proliferating up to several times percurrently, 3–10 × 2–8 μm, opening 2–6 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (22–)23–30(–31) × (9–)7.5–9(–9.5) μm, ± SD = 26.7 ± 1.3 × 8.3 ± 0.4 μm; basal cell conic to obconic with a truncate base, hyaline, rugose and thin-walled, 5–7 μm long; three median cells doliiform, (15.5–)16–19.5(–20) μm long, ± SD = 17.6 ± 1.1 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 5–7 μm long; third cell darker brown, 4.5–7.5 μm long; fourth cell darker brown, 5–7 μm long); apical cell 4.5–7.5 μm long, hyaline, cylindrical to subcylindrical, rugose and thin-walled; with 1–2 tubular apical appendages, arising as an extension of the apical cell, unbranched, attenuated, flexuous, (20–)32–55(–66) μm long, ± SD = 43 ± 6 μm; basal appendage single, tubular, unbranched, centric, 6–11 μm long.
Culture characteristics: Colonies on PDA attaining 30–50 mm diam after 7 d at 25 °C, with smooth edge, white to pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Eucalyptus globulus.
Known distribution: Unknown.
Material examined: Unknown country, from Eucalyptus globulus, Jun. 1937, H.W. Wollenweber (CBS H-15658, holotype, ex-type culture CBS 264.37 = BBA 5300).
Notes: Neopestalotiopsis eucalypticola (clade 23; Fig. 4), which was isolated from Eucalyptus globulus, is phylogenetically and morphologically well distinguished from all other species in the genus. The 1–2, long tubular apical appendages, which are sometimes branched, attenuated, arising as an extension of the apical cell, notably distinguish N. eucalypticola from other species.
Neopestalotiopsis foedans (Sacc. & Ellis) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809768.
Basionym: Pestalotia foedans Sacc. & Ellis, Michelia 2: 575. 1882.
≡ Pestalotiopsis foedans (Sacc. & Ellis) Steyaert, Bull. Jard. bot. État Brux. 14: 329. 1949.
Materials examined: China, Xinglong, Hainan, on mangrove plant leaves, Apr. 2005, A.R. Liu (MFLU 12-0424, epitype, ex-epitype culture CGMCC 3.9123); Xinglong, Hainan, on leaves of Calliandra haematocephala, May 2004, A.R. Liu, culture CGMCC 3.9202; Xinglong, Hainan, on leaves of Neodypsis decaryi, May 2004, A.R. Liu, culture CGMCC 3.9178. USA, Newfield, New Jersey, on decaying bark of white cedar, Thuja occidentalis, Oct. 1880, Ellis & Harkness (BPI 0405695, holotype).
Note: This species (clade 30; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis formicarum Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809769. Fig. 10.
Fig. 10.
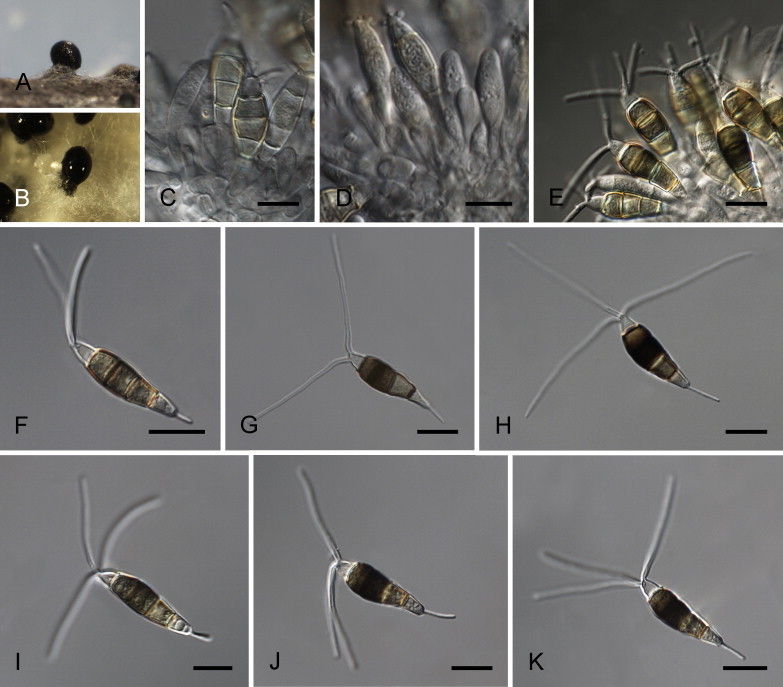
Neopestalotiopsis formicarum CBS 362.72T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the insect host family from which it was isolated, Formicidae.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary or aggregated in clusters, semi-immersed, brown to black, 200–500 μm diam; exuding globose, dark brown conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, smooth, thin-walled, simple, proliferating several times percurrently, 3–10 × 2–5 μm, apex 1–3 μm diam. Conidia ellipsoid, straight to slightly curved, 4-septate, (20–)21–28(–29) × 7.5–9.5 μm, ± SD = 24.6 ± 1.4 × 8.6 ± 0.4 μm; somewhat constricted at septa; basal cell conic to acute with truncate base, rugose and thin-walled, 4.5–6 μm long; three median cells (14–)15–16.5(–17) μm long, ± SD = 15.1 ± 1 μm, doliiform, verruculose, versicoloured, brown, septa darker than the rest of the cell (second cell from base pale brown, 4–6.5 μm long; third cell dark brown, 4–6 μm long; fourth cell brown, 4.5–6.5 μm long); apical cell subcylindrical, hyaline, thin- and smooth-walled, 4–5.5 μm long; with 2–3 tubular apical appendages, arising from the apical crest, flexuous, unbranched, (20–)23–33(–36) μm long, ± SD = 27 ± 4 μm; basal appendage single, tubular, unbranched, centric, 4–8 μm long.
Culture characteristics: Colonies on PDA reaching 30–40 mm diam after 7 d at 25 °C, edge undulate, whitish to pale honey-coloured, with moderate aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On dead ants and plant debris.
Known distribution: Cuba and Ghana.
Materials examined: Cuba, from plant debris, 1982, sent to CBS for ident. by G. Arnold (via W. Gams), CBS H-15752, culture CBS 115.83. Ghana, from dead ant (Formicidae), Nov. 1971, H.C. Evans (CBS H-15661, holotype, ex-type culture CBS 362.72).
Notes: Neopestalotiopsis formicarum (clade 11; Fig. 4) is a saprobic species collected from dead ants in Ghana and plant debris from Cuba. This species is a sister taxon to N. clavispora and Neopestalotiopsis sp. Clade 10 (clades 12 and clade 10, respectively; Fig. 4). It differs from N. clavispora in having larger conidia and longer apical appendages.
Neopestalotiopsis honoluluana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809770. Fig. 11.
Fig. 11.
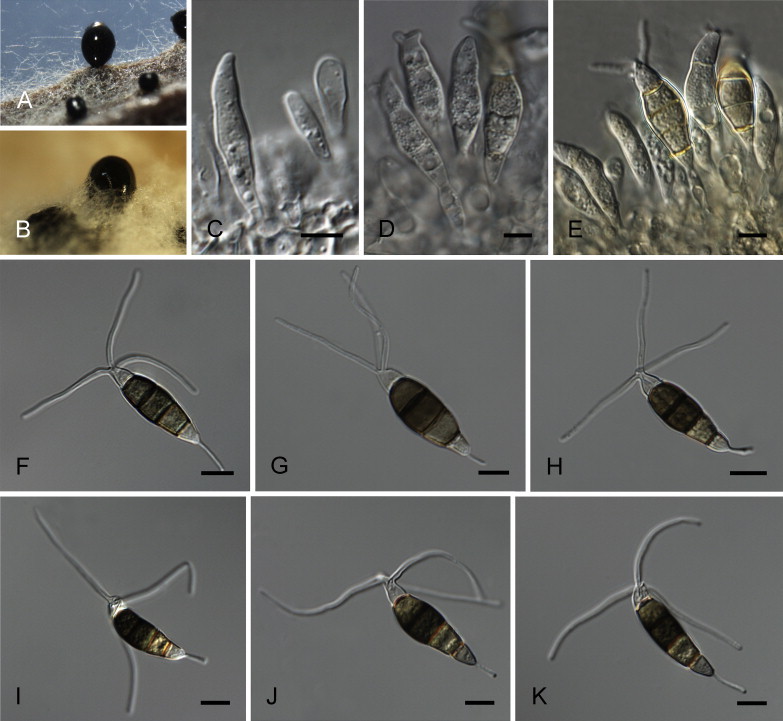
Neopestalotiopsis honoluluana CBS 114495T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the city where it was collected, Honolulu in Hawaii.
Conidiomata pycnidial in culture on PDA, globose to clavate, solitary or aggregated in clusters, semi-immersed, brown to black, 100–400 μm diam; exuding globose, dark brown conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, subcylindrical to ampulliform, hyaline, smooth, thin-walled, simple, proliferating up to 3 times percurrently, 5–20 × 2–6 μm, opening 1–3 μm diam. Conidia ellipsoid, straight to slightly curved, somewhat constricted at septa, 4-septate, (21–)24–34(–35) × (7–)7.5–9.5(–10) μm, ± SD = 28 ± 2.3 × 8.3 ± 0.6 μm, basal cell obconic with truncate base, rugose and thin-walled, 4.5–7 μm long; three median cells (14.5–)15–20(–21) μm long, ± SD = 17.3 ± 1.6 μm, doliiform, rugose, versicoloured, brown to olivaceous (second cell from base pale brown, 4.5–7 μm long; third cell darker brown, 4–6.5 μm long; fourth cell brown, 5.5–7.5 μm long); apical cell subcylindrical, hyaline, thin- and smooth-walled, 4–7.5 μm long; with 3 tubular apical appendages, arising from the apical crest, flexuous, unbranched, (22–)23–40(–47) μm long, ± SD = 32 ± 6.0 μm; basal appendage single, unbranched, centric, 2.5–10 μm long.
Culture characteristics: Colonies on PDA reaching 30–50 mm diam after 7 d at 25 °C, edge entire, whitish to pale honey-coloured, with moderate aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Telopea sp.
Known distribution: USA (Hawaii).
Materials examined: USA, Hawaii, Honolulu, from Telopea sp., 8 Dec. 1998, P.W. Crous & M.E. Palm (CBS H-21771, holotype, ex-type culture CBS 114495 = STE-U 2076); Waimea, Telopea sp., 8 Dec. 1998, P.W. Crous & M.E. Palm, culture CBS 111535 = STE-U 2078.
Notes: Neopestalotiopsis honoluluana (clade 24; Fig. 4) is confined to Telopea sp. in Hawaii, and is a sister taxon to N. eucalypticola and N. zimbabwana. Neopestalotiopsis eucalypticola differs from N. honoluluana in its longer and fewer apical appendages. The conidia of N. zimbabwana are smaller and apical appendages are shorter than those in N. honoluluana. Neopestalotiopsis australis was isolated from the same host genus Telopea, in Australia. Morphologically, however, conidia of N. australis are smaller and apical appendages are somewhat shorter.
Neopestalotiopsis javaensis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809771. Fig. 12.
Fig. 12.

Neopestalotiopsis javaensis CBS 257.31T. A. Conidiomata sporulating on PNA. B. Conidioma on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the island where it was collected, Java.
Conidiomata pycnidial in culture on PDA, globose to clavate, solitary, semi-immersed, dark brown to black, up to 250 μm diam; exuding dark brown to black conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, rugose-walled, proliferating 2–3 times percurrently, 5–25 × 3–10 μm, apex 2–4 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (24–)25–30(–31) × (6.5–)7–8.5(–9) μm, ± SD = 27.3 ± 1.6 × 7.6 ± 0.3 μm; basal cell conic to obconic with a truncate base, hyaline, rugose and thin-walled, 4.5–6.5 μm long; three median cells doliiform, (14.5–)15–18.5(–19) μm long, ± SD = 17.1 ± 1.2 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 5–7 μm long; third cell brown, 5–7 μm long; fourth cell brown, 5.5–7.5 μm long); apical cell subcylindrical, hyaline, thin- and smooth-walled, 3.5–5.5 μm long; with 1–3 tubular apical appendages, arising from the apical crest, unbranched, filiform, 2–10(–18) μm long, ± SD = 5.7 ± 3 μm; basal appendage single, tubular, unbranched, centric, 2–4 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale honey-coloured, sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On leaves of Cocos nucifera.
Known distribution: Java.
Material examined: Indonesia, Java, Manado, from leaf of Cocos nucifera, collection date unknown, R.L. Steyaert (CBS H-15764, holotype, ex-type culture CBS 257.31).
Notes: Neopestalotiopsis javaensis (clade 28; Fig. 4) was isolated from leaves of coconut in Java. It forms a separate cluster in the DNA phylogeny, as sister to a species assemblage including N. foedans, N. mesopotamica and N. rosae. Nestalotiopsis javaensis has relatively larger conidial dimensions when compared with N. foedans (19–23.5 × 5.5–7 μm) (Maharachchikumbura et al. 2012). Nestalotiopsis javaensis differs from N. mesopotamica and N. rosae in having notably shorter apical appendages (see notes under N. rosae).
Neopestalotiopsis magna (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809772.
Basionym: Pestalotiopsis magna Maharachch. & K.D. Hyde, Mycol. Prog. 13: 618. 2013.
Material examined: France, Ariège, Rimont, on decaying leaves of Pteridium sp., Aug. 2011, K.D. Hyde (MFLU 13-0594, holotype, ex-type culture MFLUCC 12-0652 = ICMP 20011).
Note: This species (clade 9; Fig. 4) was treated in detail by Maharachchikumbura et al. (2013d).
Neopestalotiopsis mesopotamica Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809773. Fig. 13.
Fig. 13.
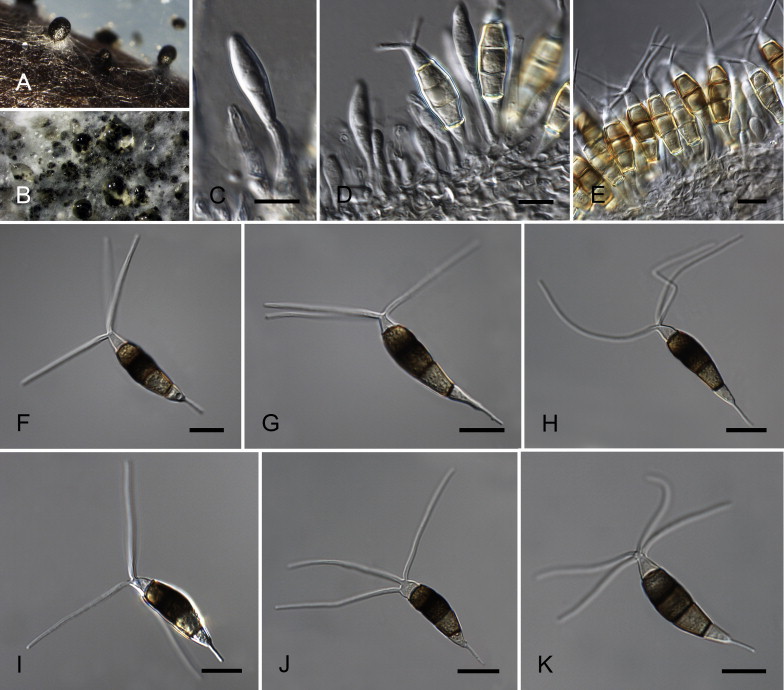
Neopestalotiopsis mesopotamica CBS 336.86T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where the type specimen was collected, Iraq, hence Mesopotamia.
Conidiomata (on PDA) pycnidial, globose or clavate, aggregated or confluent, embedded or semi-immersed, black, up to 250 μm diam; exuding brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, 8–20 × 2–7 μm, hyaline, smooth-walled, proliferating 2–3 times percurrently, 5–18 × 2–4 μm, collarette present and not flared, with prominent periclinal thickening. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (25–)26–32(–34) × (7–)7.5–9(–9.5) μm, ± SD = 29.6 ± 1.1 × 8 ± 0.4 μm; basal cell conic with a truncate base, hyaline, rugose and thin-walled, 6–7.5 μm long; three median cells doliiform, (17–)17.5–20(–21) μm long, ± SD = 18.5 ± 1.2 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 5–7.5 μm long; third cell honey brown, 5.5–7.5 μm long; fourth cell honey brown, 6.5–7.5 μm long); apical cell 4.5–6 μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 3–4 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (25–)28–38(–41) μm long, ± SD = 33.3 ± 3.2 μm; basal appendage single, tubular, unbranched, centric, 4–6.5 μm long.
Culture characteristics: Colonies on PDA attaining 30–50 mm diam after 7 d at 25 °C, with lobate edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, concentric conidiomata; reverse similar in colour.
Habitat: On Achras sapota, Eucalyptus sp. and Pinus brutia.
Known distribution: India, Iraq and Turkey.
Materials examined: India, New Delhi, from Achras sapota, May 1969, unknown collector, culture CBS 464.69. Iraq, from Pinus brutia, 23 Jun. 1986, sent to CBS for ident. by A.I. Al-Kinany, Mosul University, Mosul, Iraq (CBS H-15782, holotype, ex-type culture CBS 336.86). Turkey, from Eucalyptus sp., 2 Apr. 1974, G. Turhan, CBS H-15739 = CBS H-15741, culture CBS 299.74.
Notes: Neopestalotiopsis mesopotamica (clade 29; Fig. 4) forms a sister group to N. javaensis and N. rosae, and deviates in having larger conidia and longer apical appendages (see notes under N. rosae).
Neopestalotiopsis natalensis (J.F.H. Beyma) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809774. Fig. 14.
Fig. 14.
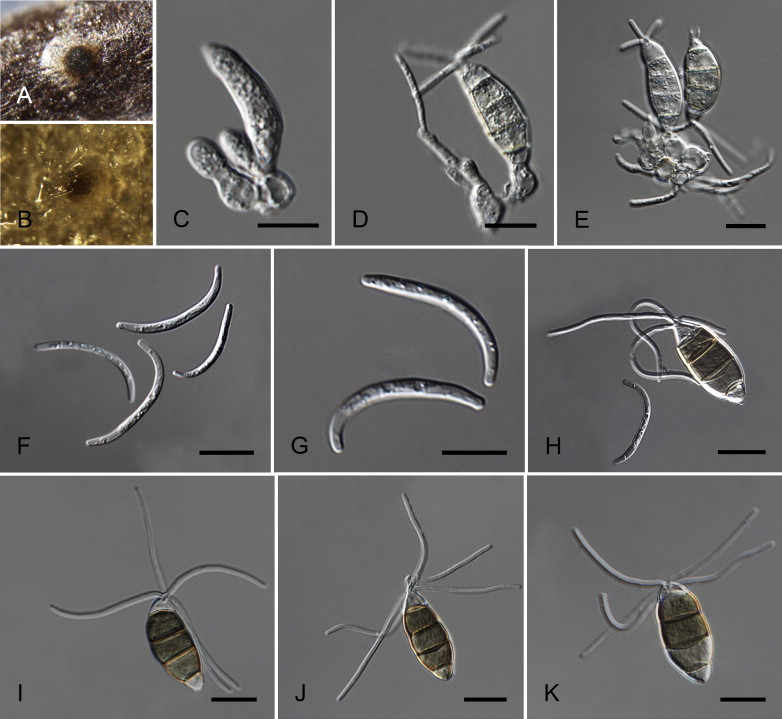
Neoestalotiopsis natalensis CBS 138.41T. A. Conidioma sporulating on PNA. B. Conidioma on PDA. C–E. Conidiogenous cells. F–G. β-conidia. H. Beta and alpha conidia. I–K. α-conidia. Scale bars = 10 μm.
Basionym: Pestalotia natalensis J.F.H. Beyma, Antonie van Leeuwenhoek 6: 288. 1940.
=Pestalotiopsis natalensis (J.F.H. Beyma) Steyaert, Bull. Jard. bot. État Brux. 19: 344. 1949.
Conidiomata (on PDA) pycnidial, globose, solitary or aggregated, immersed or semi-immersed, dark brown, 50–150 μm diam. α-conidiophores indistinct, often reduced to conidiogenous cells. α-conidiogenous cells discrete, hyaline, rugose, simple, ampulliform, sometimes slightly wide at the base, truncate at apex, proliferating once or twice, 4–10 × 3–9 μm. α-conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (21–)23–28(–29) × (7.5–)8–10(–10.5) μm, ± SD = 25.0 ± 1.6 × 9 ± 0.4 μm; basal cell hemispherical, hyaline or slightly brown, thin- and smooth-walled, 4–7 μm long; three median cells (15.5–)16–19(–19.5) μm long, ± SD = 17.5 ± 0.8 μm, concolourous or two upper median cells slightly darker than the lower median cell, brown, septa darker than the rest of the cell, and conidium constricted at septum (second cell from the base 5.5–8 μm long; third cell 5.5–8 μm long; fourth cell 5–7 μm long); apical cell 4–6.5 μm long, hyaline, conic; with 3–5 tubular apical appendages, arising from the apical crest, unbranched, (15–)18–32(–35) μm long, ± SD = 25 ± 4 μm; lacking basal appendages, when present unbranched, centric, 2–8 μm long. β-conidiophores 1–2 septate, subcylindrical, hyaline, smooth, up to 12 μm long or often reduced to conidiogenous cells. β-conidiogenous cells discrete, hyaline, smooth, cylindrical, terminated in an apex with 1–2 loci which gave rise to β-conidia in a sympodial arrangement. 5–15 × 2–6 μm. β-conidia (20–)22–29(–31) × 1–3 μm, ± SD = 25.6 ± 2 × 1.9 ± 0.2 μm, widest in the middle, curved, hyaline, apex subobtuse, base truncate.
Culture characteristics: Colonies on PDA attaining 25–35 mm diam after 7 d at 25 °C, with smooth edge, whitish, with sparse aerial mycelium on the surface; reverse similar in colour. Cultures sporulate poorly on PDA, only few conidiomata can be seen upon 4 mo of incubation.
Habitat: On Acacia mollissima.
Known distribution: South Africa.
Material examined: South Africa, KwaZulu-Natal, from Acacia mollissima (black wattle), Jan. 1941, M.S.J. Ledeboer, ex-type culture CBS 138.41.
Notes: An unusual feature of N. natalensis (clade 2; Fig. 4) is the presence of a synanamorph in culture. Most species form β-conidia on the host tissue. Crous et al. (2006) observed α- and β-conidia in Pestalotiopsis disseminata isolated from Eucalyptus eurograndis in Colombia. However, α- and β-conidia were only observed on the original host substrate and not in culture. According to the original description of Van Beyma (1940), the conidia of N. natalensis are narrower (25–33 × 6–9 μm) and apical appendages are longer (30–40 μm) than observed here.
Neopestalotiopsis piceana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809775. Fig. 15.
Fig. 15.
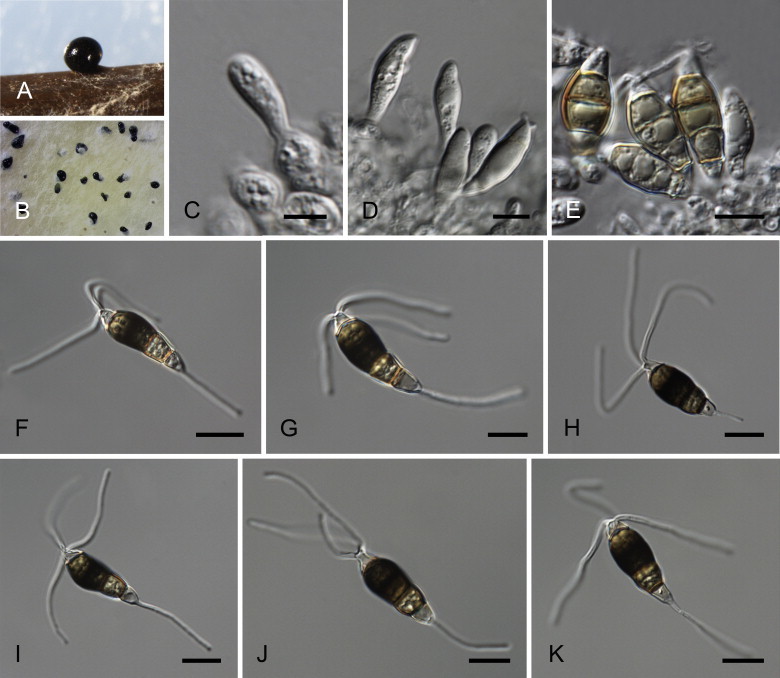
Neopestalotiopsis piceana CBS 394.48T. A. Conidioma sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Picea.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary, semi-immersed, brown to black, 100–300 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, smooth- and thin-walled, simple, (4–12 × 2–10 μm), apex 2–5 μm diam. Conidia ellipsoid to clavate, straight to slightly curved, 4-septate, (19–)19.5–25(–26) × (7–)7.5–9(–9.5) μm, ± SD = 22.1 ± 0.8 × 8.1 ± 0.6 μm; somewhat constricted at septa; basal cell obconic with truncate base, rugose and thin-walled, 3.5–5.5 μm long; three median cells (13–)13.5–16(–16.5) μm long, ± SD = 15 ± 0.9 μm, doliiform, verruculose, versicoloured, septa darker than the rest of the cell (second cell from base pale brown, 4–6 μm long; third cell dark brown, 4.5–6.5 μm long; fourth cell brown, 5–7 μm long); apical cell obconic, hyaline, thin- and smooth-walled, 3–6 μm long; with 3 tubular apical appendages, arising from the apical crest, flexuous, unbranched, (19–)21–31(–33) μm long, ± SD = 24.8 ± 3 μm; basal appendage single, tubular, unbranched, centric, 6–23 μm long.
Culture characteristics: Colonies on PDA reaching 40–50 mm diam after 7 d at 25 °C, edge entire, whitish to pale honey-coloured, with sparse aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On wood of Picea sp., Cocos nucifera and fruit of Mangifera indica.
Known distribution: Indonesia (Sulawesi) and UK.
Materials examined: Indonesia, Sulawesi, from Cocos nucifera, unknown collection date and collector, CBS H-15645, culture CBS 254.32. UK, from wood of Picea sp., Aug. 1948, S.M. Hasan (CBS H-15705, holotype, ex-type culture CBS 394.48). Unknown country, from fruit of Mangifera indica, Apr. 1930, Levie, CBS H-15688, culture CBS 225.30.
Notes: Neopestalotiopsis piceana (clade 17; Fig. 4) is characterised by clavate conidia with a long basal appendage. Neopestalotiopsis piceana is sister to N. aotearoa (clade 16; Fig. 4), which has been described from a canvas in New Zealand. The two species are distinguishable by TEF (3 bp) sequence data and not by their ITS and TUB sequences. The species differ by shape of their conidia and length of their apical appendages (see notes under N. aotearoa).
Neopestalotiopsis protearum (Crous & L. Swart) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809776.
Basionym: Pestalotiopsis protearum Crous & L. Swart, Persoonia 27: 34. 2011.
Material examined: Zimbabwe, Harare, Aveley Farm, on living leaves of Leucospermum cuneiforme cv. ‘Sunbird’, 6 Mar. 1998, L. Swart (PREM 56186, holotype, ex-type culture CBS 114178 = STE-U 1765).
Note: This species (clade 5; Fig. 4) was treated in detail by Crous et al. (2011).
Neopestalotiopsis rosae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809777. Fig. 16.
Fig. 16.
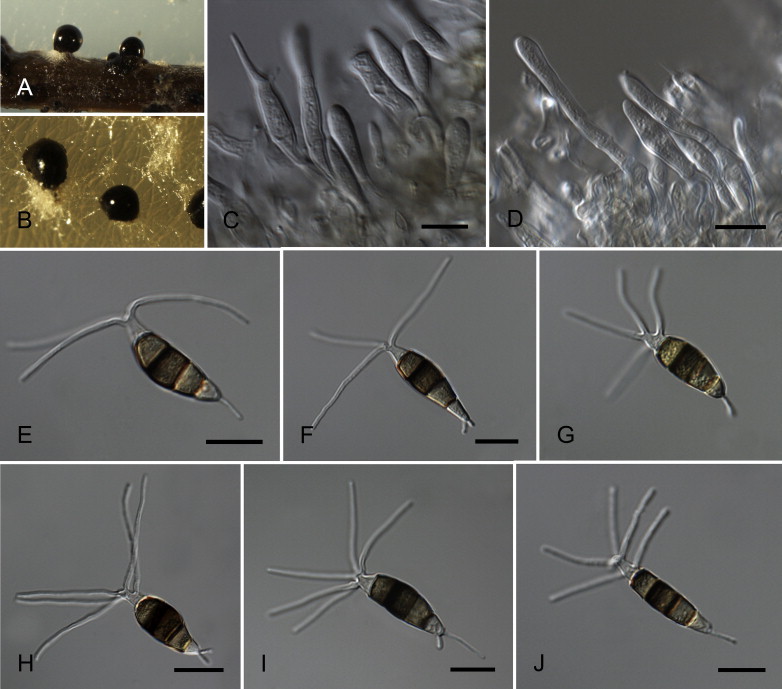
Neopestalotiopsis rosae CBS 101057T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Rosa.
Conidiomata (on PDA) pycnidial, globose, solitary, semi-immersed, dark brown to black, 100–300 μm diam; exuding globose, dark brown, glistening conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical, hyaline, smooth-walled, simple, proliferating 2–4 times percurrently, tapering towards a truncate apex with visible periclinal thickening, 5–20 × 2–8 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (20–)22–37(–29) × (7–)7.5–9.5(–10.5) μm, ± SD = 24.8 ± 1.5 × 8.5 ± 0.6 μm; basal cell conic to obconic with a truncate base, hyaline, rugose and thin-walled, 3.5–6 μm long, often with a short oblique appendage projecting from the base adjoining the point of attachment of the basal appendage; three median cells doliiform, (14–)14.5–18(–18.5) μm long, ± SD = 16 ± 1.1 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 4.5–6.5 μm long; third cell honey brown, 5–7 μm long; fourth cell brown, 5–7 μm long); apical cell 3.5–5.5 μm long, hyaline, cylindrical, thin- and smooth-walled; with 3–5 tubular apical appendages, not arising from the apical crest, but each inserted at a different locus in the upper half of the apical cell, unbranched, filiform, (22–)24–31(–33) μm long, ± SD = 27 ± 2.1 μm; basal appendage single, tubular, unbranched, centric, 5–8 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale yellow-coloured, with moderate aerial mycelium on the surface with black, concentric conidiomata; reverse similar in colour.
Habitat: On stem of Paeonia suffruticosa and stem lesion in Rosa sp.
Known distribution: New Zealand and USA.
Materials examined: New Zealand, from stem lesion in Rosa sp., Jul. 1998, J. Reeve (CBS H-21770, holotype, ex-type culture CBS 101057). USA, Connecticut, Torrington, from stem of Paeonia suffruticosa, 17 May 2007, R.E. Marra, culture CBS 124745.
Notes: Neopestalotiopsis rosae (clade 27; Fig. 4) was isolated from a stem lesion in Rosa sp. in New Zealand and stem of Paeonia suffruticosa in USA, and is morphologically quite distinct from other taxa in the genus. It has 3–5 tubular apical appendages, which do not arise from the apical crest; instead they arise at different regions in the upper half of the apical cell. Sequences of N. rosae form a sister group to N. javaensis (clade 28; Fig. 4) and N. mesopotamica (clade 29; Fig. 4), but N. rosae could be separated from N. javaensis by Bayesian analysis. However the two clades were supported in the ML and MP analyses. The two species are separable by TEF (5 bp) sequence data. There is only a 2-bp difference in ITS sequence between N. javaensis and N. rosae. Neopestalotiopsis javaensis can be differentiated morphologically from N. rosae by its long and thin conidia, and shorter apical appendages. The conidia of N. rosae are wider than those of N. mesopotamica, and the conidia and apical appendages are shorter.
Neopestalotiopsis samarangensis (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809778.
Basionym: Pestalotiopsis samarangensis Maharachch. & K.D. Hyde, Trop. Plant Pathol. 38: 229. 2013.
Materials examined: China, Hong Kong, leaf of unidentified tree, 6 Mar. 2002, unknown collector, culture CBS 115451 = HKUCC 9095. Thailand, Chiang Mai Province, Chiang Mai, on fruits of Syzygium samarangense, 20 Jan. 2010, S.S.N. Maharachchikumbura (MFLU 12-0133, holotype, ex-type culture MFLUCC 12-0233); ibid., 15 May 2011, S.S.N. Maharachchikumbura, MFLU 12-0134; Chiang Rai Province, Chiang Rai, 15 Sep. 2011, S.S.N. Maharachchikumbura, MFLU 12-0135.
Note: This species (clade 14; Fig. 4) was treated in detail by Maharachchikumbura et al. (2013b).
Neopestalotiopsis saprophytica (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809780.
Basionym: Pestalotiopsis saprophyta Maharachch. & K.D. Hyde, Fungal Divers. 56: 119. 2012.
Materials examined: China, Hong Kong, on fruits of Litsea rotundifolia, 19 Nov. 2001, unknown collector, culture CBS 115452 = HKUCC 8684; Yunnan Province, Kunming, Kunming Botanical Garden, on leaves of Magnolia sp., 19 Mar. 2002, W.P. Wu (HMAS047136, holotype; MFLU 12-0419, isotype, ex-type culture NN047136 = MFLUCC 12-0282).
Note: This species (clade 18; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis sp. Clade 4
Material examined: India, from leaf Crotalaria juncea, Feb. 1979, M. Mathur, culture CBS 233.79.
Notes: Culture CBS 233.79 (clade 4; Fig. 4) represents a Neopestalotiopsis sp. that was isolated from a leaf of Crotalaria juncea in India. Sequences of this taxon form a sister group to N. protearum (clade 5; Fig. 4). However, due to clear ecological differences, we retain this isolate as Neopestalotiopsis sp. until we obtain more collections and cultures for further study.
Neopestalotiopsis sp. Clade 10
Material examined: Unknown country, unknown host, Dec. 1920, N.A. Brown, culture CBS 110.20.
Note: Although phylogenetically slightly distinct (clade 10; Fig. 4), this culture proved to be sterile, and thus is not treated further.
Neopestalotiopsis sp. Clade 15
Materials examined: France, from twig of Camellia sp., Apr. 1976, J. Vegh, culture CBS 322.76. Indonesia, Java, Cocos nucifera, C.M. Doyer, culture CBS 274.29. Netherlands, from commercial Cocos nucifera imported from Africa, Jan. 1995, A. Aptroot, culture CBS 664.94. Unknown country, from Dalbergia sp., unknown collector and collection date, culture CBS 177.25.
Notes: Although these isolates (clade 15; Fig. 4) appear to represent an undescribed species based on phylogenetic data, due to clear ecological differences of the isolates, we maintain this clade as Neopestalotiopsis sp. until more cultures and collections are obtained.
Neopestalotiopsis sp. Clade 20
Materials examined: France, on dune sand, Mar. 1942, F. Moreau, culture CBS 164.42. Guinea, from young shoot of Cinchona sp. (attacked by Phytophthora canker), Nov. 1961, J. Chevaugeon, culture CBS 360.61.
Note: Although phylogenetically distinct (clade 20; Fig. 4), both cultures of this species proved to be sterile, and thus are not treated further.
Neopestalotiopsis sp. Clade 22
Materials examined: India, from Achras sapota, Feb. 1975, H.S. Sohi, culture CBS 119.75; from berries, leaves and canes of Vitis vinifera, Apr. 1980, H.R. Reddy, culture CBS 266.80.
Notes: Although phylogenetically and ecologically distinct, these two isolates (clade 22; Fig. 4) are morphologically similar to N. australis (clade 21; Fig. 4). Therefore, until more cultures and collections become available, we prefer to maintain this as Neopestalotiopsis sp. Clade 22.
Neopestalotiopsis sp. Clade 26
Materials examined: France, from Erica gracilis, Aug. 1975, sent to CBS for ident. by J. Vegh, culture CBS 323.76. Germany, from Erica sp., unknown date, H.W. Wollenweber, culture CBS 266.37 = BBA 5087 = IMI 083708. Netherlands, from Cissus sp., unknown collector and collection date, culture CBS 361.61.
Note: See notes under N. zimbabwana.
Neopestalotiopsis steyaertii (Mordue) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809779.
Basionym: Pestalotiopsis steyaertii Mordue, Trans Brit. Mycol. Soc. 85: 379. 1985.
Material examined: Australia, Australian Capital Territory, Brindabella mountains, from roots of Eucalyptus viminalis grown in soil, 24 Mar. 1975, G.C. Johnson (ex-type culture IMI 192475).
Note: This species (clade 1; Fig. 4) was treated in detail by Maharachchikumbura et al. (2013d).
Neopestalotiopsis surinamensis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809781. Fig. 17.
Fig. 17.
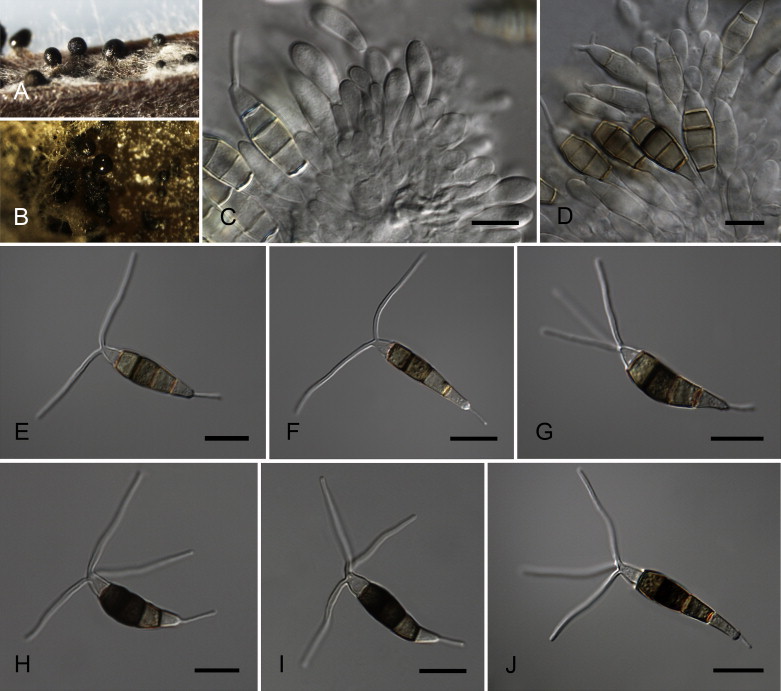
Neopestalotiopsis surinamensis CBS 450.74T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Suriname.
Conidiomata (on PDA) pycnidial, globose, mostly aggregated in clusters, semi-immersed or erumpent, black, up to 350 μm diam; exuding globose, brown conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, 4–10 × 2–6 μm, hyaline, smooth-walled, simple, proliferating 2–3 times percurrently, wide at the base, opening 1–2 μm diam. Conidia fusoid, ellipsoid to subcylindrical, straight to slightly curved, 4-septate, (23–)24–28(–29) × (7–)7.5–9(–9.5) μm, ± SD = 27.7 ± 1 × 8.1 ± 0.4 μm; basal cell obconic to subcylindrical with a truncate base, hyaline, rugose and thin-walled, 5–7.5 μm long; three median cells doliiform, (14.5–)15–17(–17.5) μm long, ± SD = 16.5 ± 0.6 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown, 5.5–6.5 μm long; third cell honey brown, 5–6.5 μm long; fourth cell brown, 4.5–6 μm long); apical cell 4–5.5 μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (15–)18–27(–28) μm long, ± SD = 21.6 ± 3 μm; basal appendage single, tubular, unbranched, centric, 5–7 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale honey-coloured, with dense aerial mycelium on the surface with black, concentric conidiomata; reverse similar in colour.
Habitat: On soil under Elaeis guineensis and leaves of Protea eximia.
Known distribution: Suriname and Zimbabwe.
Materials examined: Suriname, Brokobaka, from soil under Elaeis guineensis, Mar. 1974, J.H. van Emden (CBS H-15730, holotype, ex-type culture CBS 450.74). Zimbabwe, Karoi, Glenellen Farm, on living leaves of Protea eximia, 10 Mar. 1998, L. Swart, PREM 56190, culture CBS 111494 = STE-U 1779.
Notes: Neopestalotiopsis surinamensis (clade 3; Fig. 4) was isolated from soil under Elaeis guineensis (African oil palm) in Suriname, which is the principal source of palm oil and leaves of Protea eximia in Zimbabwe. Although phylogenetically closely related to N. protearum (clade 5; Fig. 4) (Crous et al. 2011), the two species can be distinguished by their ITS (4 bp) and TEF (9 bp) sequences, and less easily by their TUB (1 bp) sequences. In morphology, N. surinamensis differs from N. protearum in having wider conidia, as well as longer and fewer apical appendages.
Neopestalotiopsis umbrinospora (Maharachch. & K.D. Hyde) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809782.
Basionym: Pestalotiopsis umberspora Maharachch. & K.D. Hyde, Fungal Divers. 56: 121. 2012.
Material examined: China, Guangxi Province, Shiwandashan, on dead leaves of unidentified plant, 30 Dec. 1997, W.P. Wu (HMAS042986, holotype; MFLU 12-0421, isotype, ex-type culture NN042986 = MFLUCC 12-0285).
Note: This species (clade 7; Fig. 4) was treated in detail by Maharachchikumbura et al. (2012).
Neopestalotiopsis zimbabwana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809783. Fig. 18.
Fig. 18.
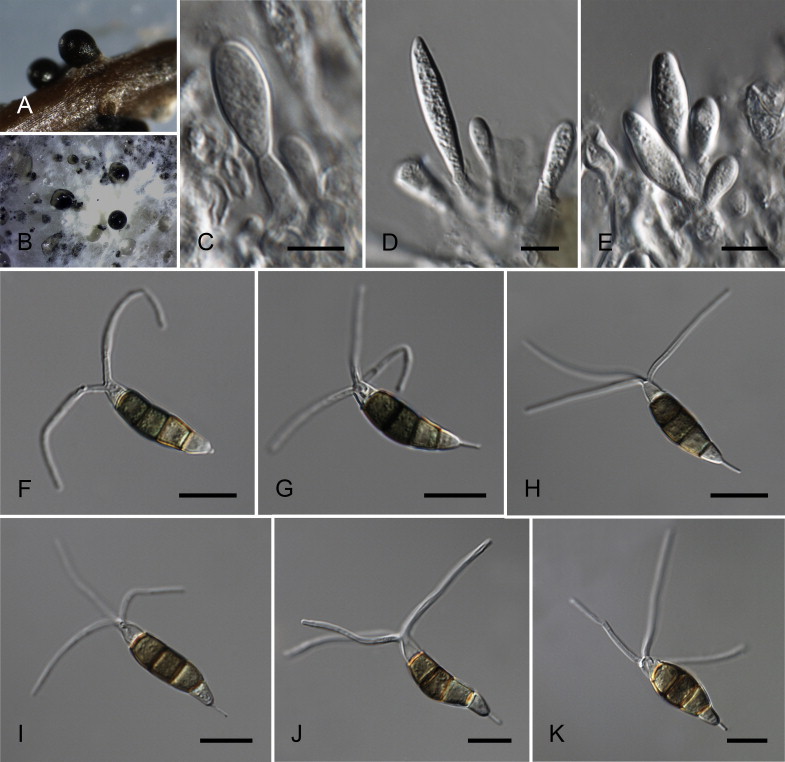
Neopestalotiopsis zimbabwana CBS 111495T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Zimbabwe.
Conidiomata (on PDA) pycnidial, globose, aggregated or scattered, semi-immersed, black, 150–400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform, hyaline, smooth-walled, simple, proliferating several times percurrently, 5–15 × 3–8 μm, apex 2–5 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (22–)23–29(–30) × (6.5–)7–8.5(–9) μm, ± SD = 25.3 ± 1.2 × 7.7 ± 0.3 μm; basal cell conic to obconic with a truncate base, hyaline, rugose and thin-walled, 3.5–5.5 μm long; three median cells doliiform, (15–)15.5–17.5(–18) μm long, ± SD = 16.5 ± 0.6 μm, wall rugose, versicoloured, septa darker than the rest of the cell (second cell from the base pale brown to pale olivaceous, 4.5–6.5 μm long; third cell brown to olivaceous, 4.5–6.5 μm long; fourth cell brown to olivaceous, 5–7 μm long); apical cell 4–6.5 μm long, hyaline, cylindrical to subcylindrical, rugose and thin-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (18–)23–35(–41) μm long, ± SD = 28.6 ± 4 μm; basal appendage single, tubular, unbranched, centric, 3–9.5 μm long.
Culture characteristics: Colonies on PDA attaining 30–45 mm diam after 7 d at 25 °C, with smooth edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Leucospermum cuneiforme.
Known distribution: Zimbabwe.
Material examined: Zimbabwe, Banket, Mariondale Farm, on living leaves of Leucospermum cuneiforme cv. ‘Sunbird’, 9 Mar. 1998, L. Swart (CBS H-21769, holotype; PREM 56188, isotype, ex-type culture CBS 111495 = STE-U 1777).
Notes: Neopestalotiopsis zimbabwana (clade 25; Fig. 4) occurs on leaf spots of Leucospermum cuneiforme in Zimbabwe. In our phylogenetic analyses, N. zimbabwana proved to be allied to CBS 266.37, CBS 361.61 and CBS 323.76 (clade 26; Fig. 4), which were isolated from Erica sp. in Germany, Cissus sp. in Netherlands and Erica gracilis in France, respectively. Even though the latter isolates have overlapping morphological characters with N. zimbabwana, due to clear geographical differences, we maintain these isolates as Neopestalotiopsis sp. Clade 26 until we have obtained more collections and cultures. Neopestalotiopsis protearum (clade 5; Fig. 4) was also identified as a pathogen on Leucospermum cuneiforme in Zimbabwe. However, N. protearum and N. zimbabwana are phylogenetically distinct.
Pestalotiopsis adusta (Ellis & Everh.) Steyaert
Materials examined: Fiji, on refrigerator door PVC gasket, 1 Jun. 1978, E.H.C. McKenzie (MFLU 12-0425, epitype, ex-epitype culture ICMP 6088 = PDDCC 6088). Thailand, Chiang Rai, on living leaves of Syzygium sp., 6 Feb. 2010, S.S.N. Maharachchikumbura, culture MFLUCC 10-0146.
Note: This species (clade 31; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis anacardiacearum Y. M. Zhang, Maharachchikumbura & K. D. Hyde
Material examined: China, Yunnan Province, Mangshi, Dehong, on living leaves of Mangifera indica, Sep. 2011, Y.M. Zhang (IFRD 411-015, holotype, ex-type culture IFRDCC 2397).
Note: This species (clade 6; Fig. 5) was treated in detail by Maharachchikumbura et al. (2013c).
Pestalotiopsis arceuthobii Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809728. Fig. 19.
Fig. 19.
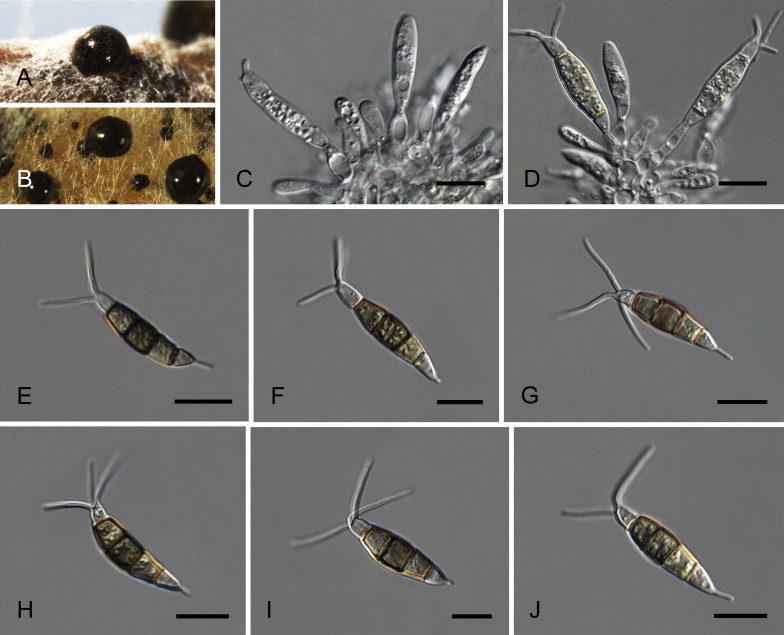
Pestalotiopsis arceuthobii CBS 434.65T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Arceuthobium.
Conidiomata pycnidial in culture on PDA, globose to clavate, solitary or aggregated in clusters, brown to black, semi-immersed, 100–500 μm diam; exuding dark brown conidia in a slimy, globose mass. Conidiophores mostly reduced to conidiogenous cells, branched or unbranched, 0–2-septate, hyaline and smooth, up to 10 μm long. Conidiogenous cells discrete, subcylindrical (3–12 × 1–3 μm) or ampulliform to lageniform (3–10 × 2–6 μm), hyaline, smooth, thin-walled, proliferating up to 4 times percurrently, collarette present and not flared. Conidia ellipsoid, straight to slightly curved, somewhat constricted at septa, 4-septate, (21–)22–25.5(–26) × 6.5–8(–8.5) μm, ± SD = 24.4 ± 1.3 × 7.2 ± 0.5 μm, basal cell obconic with truncate base, rugose and thin-walled, 5–6 μm long; three median cells (14–)15–16.5 μm long, ± SD = 15.6 ± 0.9 μm, doliiform, verruculose, concolourous, brown (second cell from base 5–6 μm long; third cell 5.5–6.5 μm long; fourth cell 4.5–6 μm long); apical cell cylindrical, hyaline, thin- and smooth-walled, 4–5 μm long; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, flexuous, unbranched, (10–)11–14.5(–16) μm long, ± SD = 12.8 ± 1.0 μm; basal appendage single, tubular, unbranched, centric, 3–6 μm long.
Culture characteristics: Colonies on PDA reaching 60–70 mm diam after 7 d at 25 °C, edge entire, whitish to pale honey-coloured, with aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Arceuthobium campylopodum.
Known distribution: USA.
Material examined: USA, Washington, King County, North Bend, from Arceuthobium campylopodum, Aug. 1965, E.F. Wicker (CBS H-15695, holotype, ex-type culture CBS 434.65).
Notes: Pestalotiopsis arceuthobii is a distinct species represented by a single isolate (clade 3; Fig. 5), sister to P. ericacearum (clade 2; Fig. 5). Pestalotiopsis arceuthobii can be distinguished from P. ericacearum (conidia size = 15–21 × 5–9 μm) by its narrow conidia (size = 21–26 × 6.5–8.5 μm) as well as short apical appendages (10–16 μm). In P. ericacearum the apical appendages are longer (19–45 μm), and knobbed.
Pestalotiopsis arengae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809729. Fig. 20.
Fig. 20.

Pestalotiopsis arengae CBS 331.92T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Arenga.
Conidiomata (on PDA) pycnidial, globose or clavate, solitary or aggregated, semi-immersed, dark brown to black, 200–400 μm diam; exuding dark brown conidial masses. Conidiophores most often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, smooth, thin-walled, 3–15 × 3–10 μm, proliferating several times percurrently, with minute periclinal thickenings. Conidia ellipsoid, straight to slightly curved, slightly constricted at septa, 4-septate, (24–)25–32(–33) × 7–9.5(–10) μm, ± SD = 27.6 ± 2 × 8 ± 0.4 μm; basal cell conic with a truncate base, rugose and thin-walled, 4–7 μm long; three median cells (17–)17.5–21.5(–22) μm long, ± SD = 19 ± 1.3 μm, doliiform, verruculose, concolourous, brown, septa darker than the rest of the cell (second cell from base 5.5–7 μm long; third cell 5.5–8 μm long; fourth cell 6–7.5 μm long); apical cell subcylindrical, hyaline, thin- and smooth-walled, 2.5–4.5 μm long; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (4–)4.5–11(–12) μm long, ± SD = 7.3 ± 1.3 μm; basal appendage single, tubular, unbranched, centric, 1.5–3 μm long.
Culture characteristics: Colonies on PDA reaching 70–80 mm diam after 7 d at 25 °C, undulate at the margin, white to pale luteous-coloured, with moderate aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On dead leaves of Arenga undulatifolia.
Known distribution: Singapore.
Material examined: Singapore, Botanical Gardens, from dead leaves of Arenga undulatifolia, Nov. 1991, W. Gams (CBS H-21768, holotype, ex-type culture CBS 331.92).
Notes: Pestalotiopsis arengae (clade 4; Fig. 5) forms a separate cluster in the combined phylogeny as basal sister to P. anacardiacearum (clade 6; Fig. 5) and P. hawaiiensis (clade 5; Fig. 5), which were isolated from mango from China and Leucospermum sp. from Hawaii, respectively. In morphology, P. arengae differs from P. anacardiacearum and P. hawaiiensis by its smaller conidia and shorter apical appendages.
Pestalotiopsis australasiae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809730. Fig. 21.
Fig. 21.
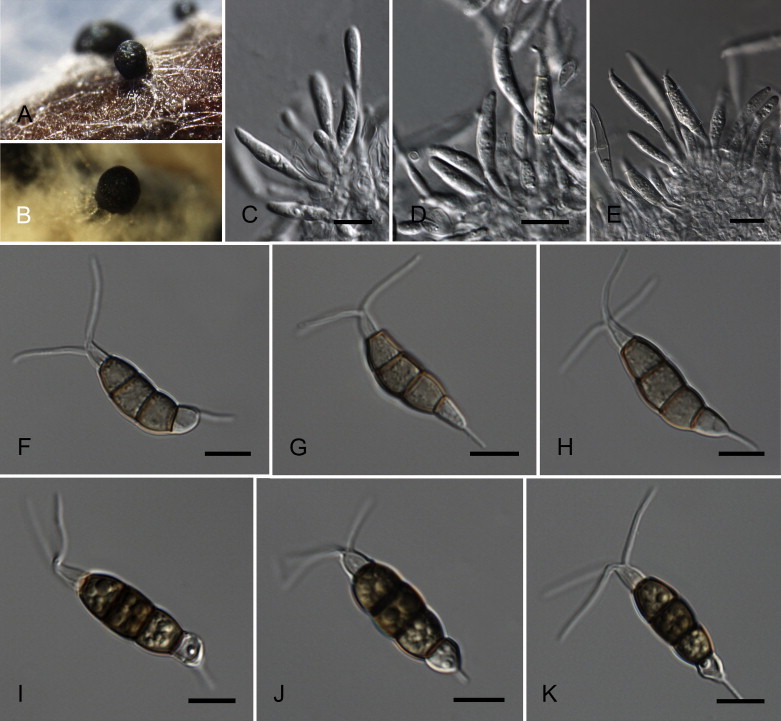
Pestalotiopsis australasiae CBS 114126T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Refers to the broader geographical region (Australia and New Zealand) where the fungus was isolated.
Conidiomata pycnidial in culture on PDA, globose, scattered, semi-immersed, up to 200 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete or integrated, ampulliform or cylindrical, hyaline, minutely verruculose, proliferating 2–4 times percurrently, tapering to a long, thin neck, 15–50 × 3–9 μm, with flaring collarettes. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (23–)24.5–29(–31) × (6–)6.5–8(–8.5) μm, ± SD = 26 ± 1.4 × 7.5 ± 0.2 μm; basal cell obconic to hemispherical, hyaline, verruculose and thin-walled, 5–6.5 μm long; three median cells doliiform, (15–)15.5–18(–18.5) μm long, ± SD = 16.7 ± 0.7 μm, wall verruculose, concolourous, brown, septa darker than the rest of the cell (second cell from the base 5–6.5 μm long; third cell 5.5–7 μm long; fourth cell 5.5–7 μm long); apical cell 3.5–5 μm long, hyaline, cylindrical to subcylindrical; with 2–3 tubular apical appendages, arising from an apical crest, unbranched, filiform, flexuous, (9–)10–15(–16) μm long, ± SD = 12.6 ± 1.7 μm; basal appendage single, tubular, unbranched, centric, 2.5–4.5 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, flat with entire edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Knightia sp. and Protea sp.
Known distribution: Australia and New Zealand.
Materials examined: Australia, New South Wales, from Protea neriifolia × susannae cv. ‘Pink Ice’, 12 Oct. 1999, P.W. Crous, culture CBS 114141 = STE-U 2949. New Zealand, from Knightia sp., 2002, P.W. Crous (CBS H-21767, holotype, ex-type culture CBS 114126 = STE-U 2896).
Notes: Morphologically P. australasiae (clade 39; Fig. 5) is comparable to P. knightiae (clade 37; Fig. 5), P. parva (clade 35; Fig. 5) and P. grevilleae (clade 36; Fig. 5), but differs in having larger conidia when compared to P. parva, and shorter apical appendages when compared to P. knightiae and P. grevilleae. It has an overlapping conidial size with P. telopeae (clade 40; Fig. 5), which causes a leaf spot disease on Telopea spp. Since the two species are genetically distinct, we maintain them as two separate species (see notes under P. telopea).
Pestalotiopsis australis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809731. Fig. 22.
Fig. 22.

Pestalotiopsis australis CBS 114193T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Australia.
Conidiomata pycnidial in culture on PDA, globose or clavate, aggregated or scattered, semi-immersed or partly erumpent, dark brown to black, up to 400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores 1–3-septate, sparsely branched at the base, subcylindrical, hyaline, verruculose, up to 25 μm long. Conidiogenous cells discrete or integrated, ampulliform or cylindrical, hyaline, smooth, proliferating 2–4 times percurrently, 20–60 × 2–6 μm, collarette present and slightly flared. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (26–)27–34(–36) × 7–8.5 μm, ± SD = 30.8 ± 2.1 × 7.7 ± 0.3 μm; basal cell conic to obconic with a truncate base, hyaline, minutely verruculose and thin-walled, 6–10 × μm long; three median cells doliiform, (16–)17–21(–21.5) μm long, ± SD = 19.1 ± 1.2 μm, wall minutely verruculose, concolourous, brown, septa darker than the rest of the cell (second cell from the base 5.5–7.5 μm long; third cell 5.5–7.5 μm long; fourth cell 6–8 μm long); apical cell 4–6.5 × μm long, hyaline, cylindrical to subcylindrical, thin- and smooth walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (11–)12–20(–22) μm long, ± SD = 15.5 ± 2.7 μm; basal appendage single, tubular, unbranched, centric, 3–7 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with smooth edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Brabejum stellatifolium, Grevillea sp. and Protea neriifolia × susannae.
Known distribution: Australia and South Africa.
Materials examined: Australia, New South Wales, from Grevillea sp. 12 Oct. 1999, P.W. Crous (CBS H-21766, holotype, ex-type culture CBS 114193 = STE-U 3011). South Africa, KwaZulu-Natal, from Protea neriifolia × susannae cv. ‘Pink Ice’, 15 May 1998, L. Swart, culture CBS 114474 = STE-U 1769; ibid., 15 May 1998, L. Swart, culture CBS 111503 = STE-U 1770; on dead leaves of Brabejum stellatifolium, 3 Nov. 2000, S. Lee, PREM 59519, culture CBS 119350 = CMW 20013.
Notes: Pestalotiopsis australis (clade 26; Fig. 5) is a distinct species, which can be isolated from members of Proteaceae. Pestalotiopsis australis is closely related to P. scoparia (clade 25; Fig. 5), and is distinguished morphologically from related species by its large conidia.
Pestalotiopsis biciliata Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809732. Fig. 23.
Fig. 23.
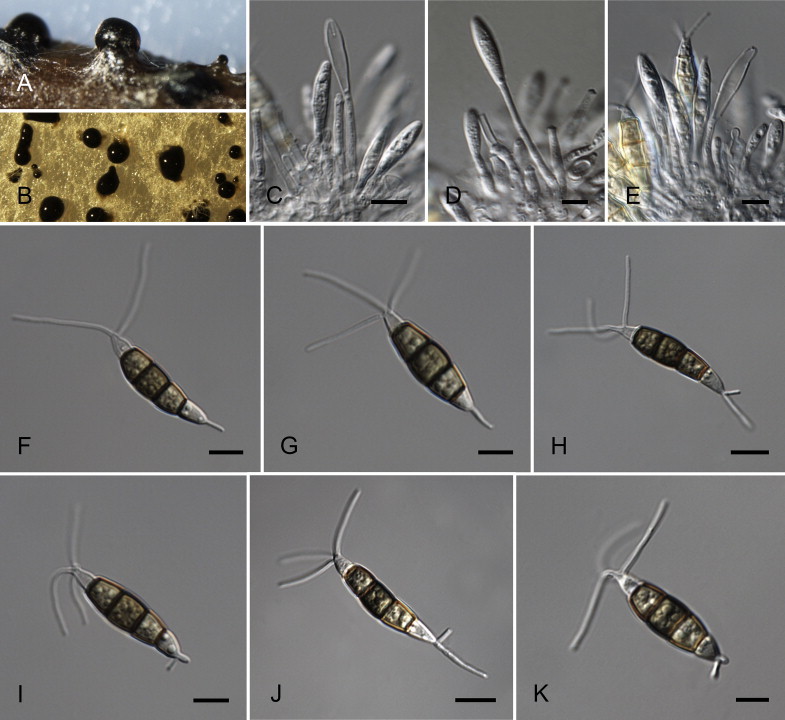
Pestalotiopsis biciliata CBS 124463T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Name refers to its two basal appendages.
Conidiomata pycnidial in culture on PDA, globose to clavate, aggregated or scattered, semi-immersed, dark brown to black, up to 300 μm diam; exuding globose, slimy, dark brown conidial droplets. Conidiophores sparsely septate and unbranched or irregularly branched at the base, up to 40 μm long, or reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, hyaline, smooth, tapering to a long, thin neck, 10–45 × 2–5 μm, proliferating several times percurrently near apex, with flaring collarettes. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (21–)22–28.5(–30) × (5.5–)6–7.5(–8) μm, ± SD = 25.3 ± 2 × 6.7 ± 0.3 μm; basal cell obconic to hemispherical with a truncate base, hyaline, verruculose and thin-walled, 4–7 μm long; three median cells doliiform, (13.5–)14.5–17.5(–18.5) μm long, ± SD = 16 ± 1.1 μm, wall verruculose, concolourous, olivaceous, septa darker than the rest of the cell (second cell from the base 4–6.5 μm long; third cell 4–7 μm long; fourth cell 4–6.5 μm long); apical cell 3–4.5 μm long, hyaline, subcylindrical, rugose and thin-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (6–)8–18(–20) μm long, ± SD = 13.3 ± 3.2 μm; two basal appendages; centric appendage tubular, 3–8 μm long and excentric appendage tubular, 1–3 μm long.
Culture characteristics: Colonies on PDA attaining 40–50 mm diam after 7 d at 25 °C, with lobate edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse pale honey-coloured.
Habitat: On Paeonia sp., bark of Platanus × hispanica and Taxus baccata dry needles.
Known distribution: Italy, Netherlands and Slovakia.
Materials examined: Italy, from Paeonia sp., Jun. 1938, O. Servazzi, culture CBS 236.38. Netherlands, from Taxus baccata dry needles attached to the tree, 23 Oct. 1968, H.A. van der Aa, culture CBS 790.68. Slovakia, Giraltovce, from bark of Platanus × hispanica, unknown collection date, M. Pastircak (CBS H-21765, holotype, ex-type culture CBS 124463).
Notes: Pestalotiopsis biciliata (clade 38; Fig. 5) is a species often having two basal appendages. Pestalotiopsis biciliata overlaps morphologically with P. trachicarpicola (clade 43; Fig. 5). However, in the phylogenetic analyses it formed a distinct lineage apart from Pestalotiopsis kenyana (which has wider conidia; clade 42; Fig. 5) and P. trachicarpicola (clade 43; Fig. 5).
Pestalotiopsis brassicae (Guba) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809734. Fig. 24.
Fig. 24.

Pestalotiopsis brassicae CBS 170.26isoT. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Basionym: Pestalotia brassicae Guba, Monograph of Monochaetia and Pestalotia: 245. 1961.
Conidiomata acervular to pycnidial in culture on PDA, globose, scattered or gregarious and confluent, semi-immersed or erumpent, dark brown to black, up to 500 μm diam; exuding globose, black conidial masses. Conidiophores septate near base, branched, subcylindrical, hyaline, up to 10 μm long. Conidiogenous cells discrete, cylindrical 20–70 × 2–10 μm or ampulliform to lageniform 4–10 × 3–8 μm, hyaline, smooth-walled, proliferating 2–4 times percurrently, wide at base, collarette present and not flared, with prominent periclinal thickening. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (29–)30–37(–40) × (8–)8.5–11(–11.5) μm, ± SD = 34 ± 2.1 × 9.7 ± 0.7 μm; basal cell obconic with a truncate base, hyaline, minutely verruculose and thin-walled, 5–8.5 × μm long; three median cells doliiform to subcylindrical, (20–)20.5–24.5(–25) μm long, ± SD = 22.6 ± 1.5 μm, wall verruculose, concolourous, but occasionally the two upper median cells slightly darker than the lower median cell, brown to olivaceous, septa darker than the rest of the cell (second cell from the base 5.5–9 μm long; third cell 7–9.5 μm; fourth cell 6–9 μm); apical cell 3.5–7 × μm long, hyaline, cylindrical to subcylindrical, thin- and smooth walled; with 3–5 tubular apical appendages (mostly 4), arising from the apical crest, unbranched, filiform, flexuous, (27–)28.5–48(–50) μm long, ± SD = 37 ± 5 μm; basal appendage single, tubular, unbranched, centric, 10–25 μm long.
Culture characteristics: Colonies on PDA attaining 25–40 mm diam after 7 d at 25 °C, with smooth edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On seeds of Brassica napus.
Known distribution: New Zealand.
Material examined: New Zealand, from seeds of Brassica napus, May 1926, G.H. Cunningham (CBS H-7542, isotype, ex-isotype culture CBS 170.26).
Notes: According to the original description of Guba (1961), conidia of P. brassicae are somewhat smaller (25–32 × 8.5–9.5 μm) and the apical appendages are shorter (20–35 μm) than in the present observation. In his monograph Guba placed this species in a group with species having versicoloured median cells. However, our phylogenetic analyses (Fig. 5) do not support placing P. brassicae (clade 19; Fig. 5) within the versicoloured group (genus Neopestalotiopsis; Fig. 4). Pestalotiopsis brassicae formed a sister group to P. hollandica (clade 18; Fig. 5), which was isolated from Sciadopitys verticillata in the Netherlands. The latter species is clearly distinguished from P. brassicae by having wider conidia, and branched, sub-apically attached apical appendages. Furthermore, P. brassicae is distinguished from its other closest phylogenetic neighbour, P. verruculosa (clade 20; Fig. 5) (28–35 × 9–11 μm) by its larger conidia.
Pestalotiopsis camelliae Y.M. Zhang, Maharachch. & K.D. Hyde
Materials examined: China, Yunnan Province, Chuxiong, Shuangbai, on living leaves of Camellia japonica, Jul. 2011, Y.M. Zhang (IFRD OP111, holotype, ex-type culture MFLUCC 12-0277); ibid., Aug. 2011, IFRD OP131, culture MFLUCC 12-0278. Turkey, Samsun, on leaf of Camellia sinensis, collection date unknown, O. Orbas, culture CBS 443.62.
Note: This species (clade 13; Fig. 5) was treated in detail by Zhang et al. (2012b).
Pestalotiopsis chamaeropis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809735. Fig. 25.
Fig. 25.
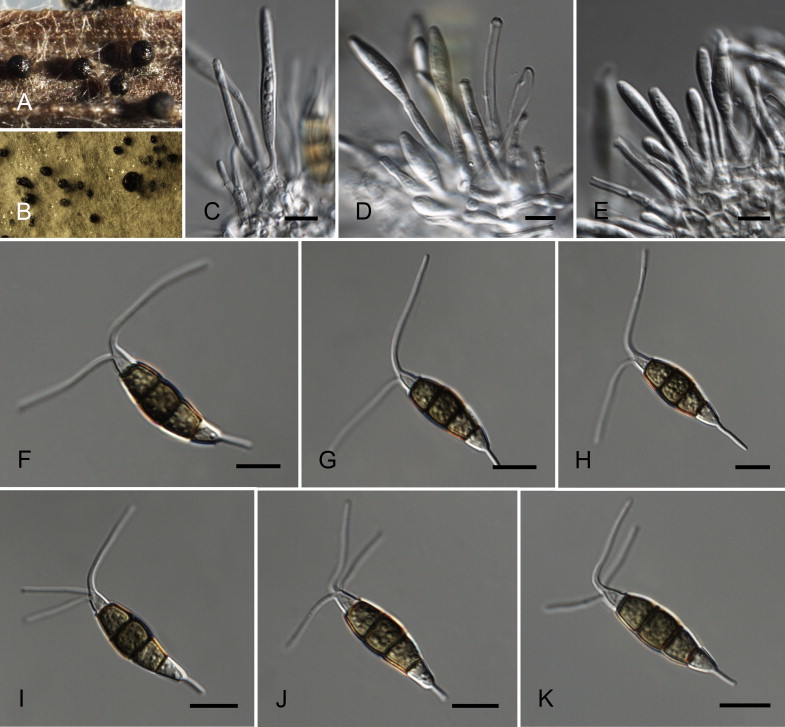
Pestalotiopsis chamaeropis CBS 186.71T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus, Chamaeropis.
Conidiomata pycnidial in culture on PDA, globose, semi-immersed or partly erumpent, aggregated or scattered, up to 250 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores 1–3-septate, branched, subcylindrical, hyaline, verruculose, up to 25 μm long. Conidiogenous cells discrete, cylindrical, hyaline, smooth-walled, proliferating 2–4 times percurrently, 20–50 × 2–5 μm, collarette present and not flared, with prominent periclinal thickening. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (21–)22.5–27(–28) × (6–)7–9(–9.5) μm, ± SD = 25.2 ± 1.3 × 8 ± 0.4 μm; basal cell obconic with a truncate base, hyaline, minutely verruculose and thin-walled, 5–6.5 μm long; three median cells doliiform to subcylindrical, (15–)16–17.5(–18.5) μm long, ± SD = 16.7 ± 0.8 μm, wall verruculose, concolourous, but occasionally the two upper median cells are slightly darker than the lower median cell, brown, septa darker than the rest of the cell (second cell from the base 4.5–6.5 μm long; third cell 4.5–6.5 μm long; fourth cell 4.5–6 μm long); apical cell 4–6 μm long, hyaline, subcylindrical, thin- and smooth walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (13–)14.5–23(–24) μm long, ± SD = 18 ± 3.1 μm; basal appendage single, tubular, unbranched, centric, 4–8.5 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with smooth edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On leaves of Chamaerops humilis.
Known distribution: Italy.
Materials examined: Italy, Sardinia, Dorgali, from leaf of Chamaerops humilis, Feb. 1971, H.A. van der Aa (CBS H-15702, holotype, ex-type culture CBS 186.71); unknown collection details (June 1938 deposited in CBS collection), O. Servazzi, culture CBS 237.38. Unknown locality, unknown collection details, culture CBS 113604 = STE-U 3078, CBS 113607 = STE-U 3080.
Notes: Clade 23 (Fig. 5) is represented by four isolates of P. chamaeropis. It differs from related species in having distinctly wider conidia. Pestalotiopsis chamaeropis forms a separate cluster in the combined phylogeny, as sister to a group including P. intermedia (clade 21; Fig. 5) and P. linearis (clade 22; Fig. 5), which were isolated from dead leaves of an unidentified tree, and as an endophyte of Trachelospermum sp. respectively, both collected in China. In 1938, O. Servazzi deposited two isolates (CBS 237.38 and CBS 236.38) in CBS as authentic strains of Pestalotia paeoniae. Even though these two isolates had overlapping conidial dimensions, the deposited isolates cluster in distinct clades (CBS 237.38 in clade 23 and CBS 236.38 in clade 38; Fig. 5) with species having concolourous median cells. According to the description of Guba (1961), P. paeoniae belongs to the species with versicoloured median cells (presently Neopestalotiopsis; Fig. 4). The reliability of these two “authentic” strains is thus doubtful, and CBS 237.78 is placed in P. chamaeropis, and CBS 236.38 in P. biciliata (clade 38; Fig. 5).
Pestalotiopsis clavata Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on living leaves of Buxus sp., 19 Mar. 2002, W.P. Wu (HMAS047134, holotype, MFLU 12-0412, isotype, ex-type culture NN0471340 = MFLUCC 12-0268).
Note: This species (clade 15; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis colombiensis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809736. Fig. 26.
Fig. 26.
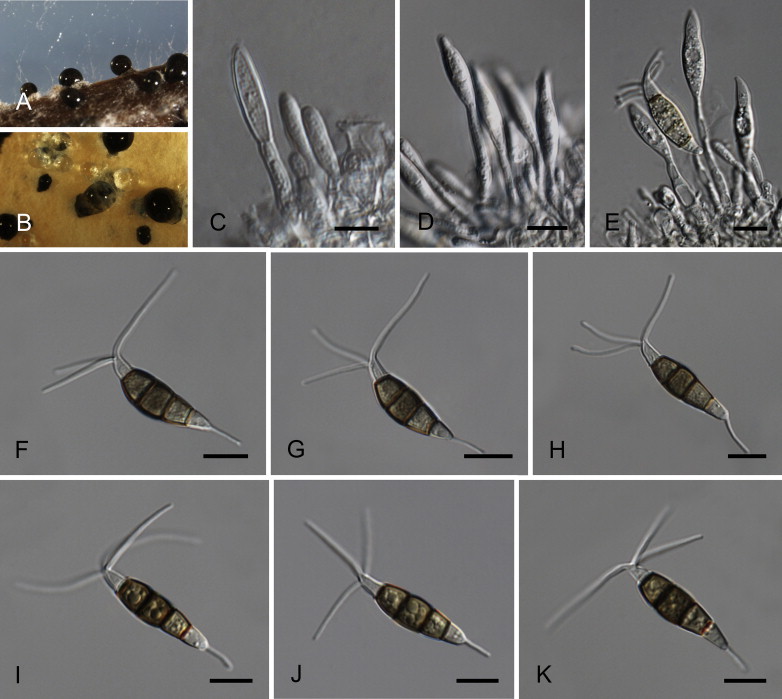
Pestalotiopsis colombiensis CBS 118553T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country from where it was collected, Colombia.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary or aggregated, semi-immersed, dark brown, 200–400 μm diam; exuding globose, dark brown, glistening conidial masses. Conidiophores reduced to conidiogenous cells; when present, septate, unbranched, or irregularly branched, hyaline, thin-walled, 5–12 × 2–6 μm. Conidiogenous cells discrete, cylindrical, proliferating 2–5 times percurrently, tapering to a long, thin neck, 10–50 × 2–8 μm, with prominent periclinal thickening, collarette present and not flared. Conidia ellipsoid, straight to slightly curved, 4-septate, slightly constricted at septa, (19–)21–27(–28.5) × 5.5–7.5(–8) μm, ± SD = 24 ± 1.5 × 6.3 ± 0.5 μm; basal cell conic to acute with truncate base, minutely verruculose and thin-walled, 5–7.5 μm long; three median cells, (13–)13.5–16.5(–17) μm long, ± SD = 15.2 ± 0.8 μm, doliiform, thick-walled, verruculose, concolourous, brown (second cell from base 5–6.5 μm long; third cell 4.5–6 μm long; fourth cell 5–6.5 μm long); apical cell cylindrical to subcylindrical, hyaline, thin- and smooth-walled, 3.5–5 μm long; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (11–)13–25(–28) μm, ± SD = 17.5 ± 3 μm; basal appendage single, tubular, unbranched, centric, 2–5 μm long.
Culture characteristics: Colonies on PDA reaching 70–80 mm diam after 7 d at 25 °C, entire at the edge, whitish to pale grey-coloured, with dense aerial mycelium on the surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On living leaves of Eucalyptus eurograndis.
Known distribution: Colombia.
Material examined: Colombia, from living leaves of Eucalyptus eurograndis, 2004, M.J. Wingfield (CBS H-21764, holotype, ex-type culture CBS 118553 = CPC 10969).
Notes: Pestalotiopsis colombiensis (clade 27; Fig. 5) is a distinct species represented by a Colombian isolate from Eucalyptus. It differs from its closest phylogenetic neighbours, P. diploclisiae (clade 29; Fig. 5) and P. humus (clade 28; Fig. 5) by its longer apical appendages. Furthermore P. colombiensis is geographically distinct from P. diploclisiae and P. humus, which were isolated from Hong Kong and Papua New Guinea, respectively.
Pestalotiopsis diploclisiae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809737. Fig. 27.
Fig. 27.
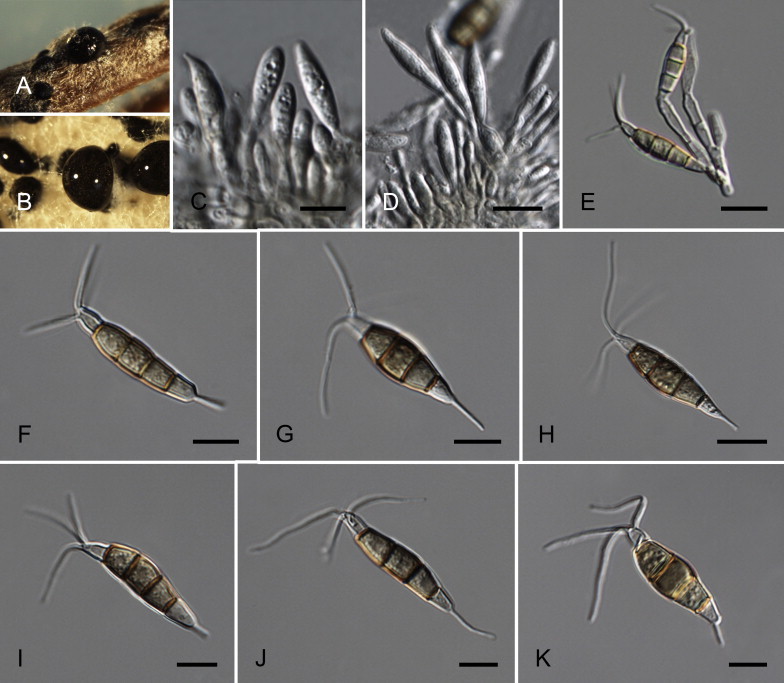
Pestalotiopsis diploclisiae CBS 115587T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Diploclisia.
Conidiomata pycnidial in culture on PDA, globose, solitary or aggregated, semi-immersed, black, up to 500 μm diam; exuding globose, slimy, dark brown, conidial droplets. Conidiophores often reduced to conidiogenous cells, sparsely septate at the base and unbranched or branched, up to 20 μm long. Conidiogenous cells discrete, cylindrical to subcylindrical, hyaline, smooth, simple, proliferating 2–3 times percurrently, 6–20 × 2–5 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (20–)22–26.5(–28) × 5–6.5(–7) μm, ± SD = 24 ± 1.3 × 5.7 ± 0.4 μm; basal cell obconic to subcylindrical with a truncate base, hyaline, rugose and thin-walled, 4–6.5 μm long; three median cells doliiform, (13.5–)14–16(–17) μm long, ± SD = 15.4 ± 0.9 μm, wall minutely verruculose, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 4.5–6 μm; third cell 4.5–7 μm; fourth cell 4.5–6.5 μm); apical cell 3.5–6 μm long, hyaline, subcylindrical, thin- and smooth-walled; with 2–4 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (10–)13–19(–22) μm long, ± SD = 16.6 ± 2.1 μm; basal appendage single, tubular, unbranched, centric, 3–8 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with smooth edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On fruit of Diploclisia glaucescens and Psychotria tutcheri.
Known distribution: China (Hong Kong).
Materials examined: China, Hong Kong, Lamma Island, from fruit of Diploclisia glaucescens, 5 Jul. 2001, K.D. Hyde (CBS H-21763, holotype, ex-type culture CBS 115587 = HKUCC 10130); ibid., culture CBS 115585 = HKUCC 8394; Mount Nicholson, from fruit of Psychotria tutcheri, 15 Feb. 2002, K.D. Hyde, culture CBS 115449 = HKUCC 9103.
Notes: Pestalotiopsis diploclisiae (clade 29; Fig. 5) comprises three isolates originating from Hong Kong. Pestalotiopsis diploclisiae is morphologically very similar to P. colombiensis (clade 27; Fig. 5), but genetically clearly distinct, forming a well-separated clade. Pestalotiopsis diploclisiae is genetically close to P. humus (clade 28; Fig. 5), which was isolated from soil in Papua New Guinea, but can be distinguished by its narrow conidia and longer apical appendages.
Pestalotiopsis diversiseta Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on living leaves of Rhododendron sp., 19 Mar. 2002, W.P. Wu (HMAS047261, holotype, MFLU 12-0423, isotype, ex-type culture NN0472610 = MFLUCC 12-0287).
Note: This species (clade 7; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis ericacearum Y. M. Zhang, Maharachch. & K. D. Hyde
Material examined: China, Yunnan Province, Chuxiong, Zixishan, leaf spots on Rhododendron delavayi, Feb. 2011, Y.M. Zhang (IFRD 410-008, holotype, ex-type culture IFRDCC 2439).
Note: This species (clade 2: Fig. 5) was treated in detail by Zhang et al. (2013).
Pestalotiopsis furcata Maharachch. & K.D. Hyde
Material examined: Thailand, Chiang Mai Province, Mae Taeng District, Ban Pha Deng, Mushroom Research Centre, 19°17.123′N 98°44.009′E, on living leaves of Camellia sinensis, 20 Jan. 2010, S.S.N. Maharachchikumbura (MFLU 12-0112, holotype, ex-type culture MFLUCC 12-0054 = CPC 20280).
Note: This species (clade 12; Fig. 5) was treated in detail by Maharachchikumbura et al. (2013a).
Pestalotiopsis gaultheria Y. M. Zhang, Maharachch. & K. D. Hyde
Material examined: China, Yunnan Province, Dehong, Mangshi, leaf spots on Gaultheria forrestii, Sep. 2011, Y.M. Zhang (IFRD 411-014, holotype).
Note: This species (clade 9; Fig. 5) was treated in detail by Zhang et al. (2013).
Pestalotiopsis grevilleae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809738. Fig. 28.
Fig. 28.
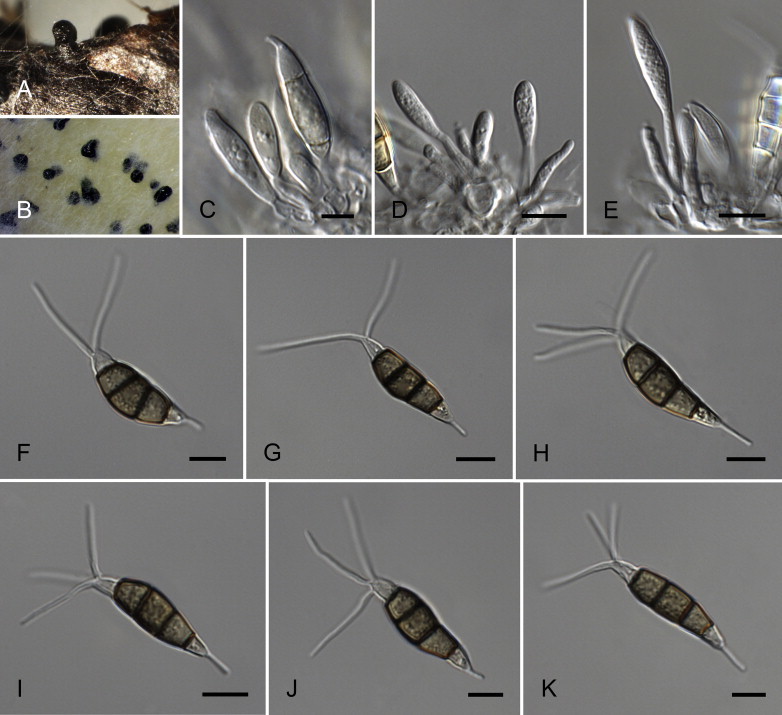
Pestalotiopsis grevilleae CBS 114127T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Grevillea.
Conidiomata pycnidial in culture on PDA, globose, aggregated or scattered, semi-immersed, dark brown to black, up to 200 μm diam; releasing globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, hyaline, smooth, proliferating 2–3 times percurrently, flared collarette, with prominent periclinal thickening, 5–25 × 2–8 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (21–)22.5–28(–29) × (7–)7.5–9(–9.5) μm, ± SD = 25.2 ± 1.2 × 8.2 ± 0.5 μm; basal cell conic with a truncate base, hyaline, rugose and thin-walled, 3.5–5.5 μm long; three median cells doliiform, (12.5–)13–17(–17.5) μm long, ± SD = 15 ± 1.2 μm, wall verruculose, concolourous, olivaceous, septa darker than the rest of the cell (second cell from the base 4.5–6.5 μm; third cell 4.5–6.5 μm; fourth cell 4–6.5 μm); apical cell 3.5–5.5 μm long, hyaline, cylindrical to subcylindrical, rugose and thin-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (12–)14–26.5(–29) μm long, ± SD = 19 ± 3 μm; basal appendage single, tubular, unbranched, centric, 3–8 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with smooth edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Grevillea sp.
Known distribution: Australia.
Material examined: Australia, New South Wales, Sydney, Grevillea sp., 1999, P.W. Crous (CBS H-21762, holotype, ex-type culture CBS 114127 = STE-U 2919).
Notes: Pestalotiopsis grevilleae (clade 36; Fig. 5) forms a sister clade to P. knightiae (clade 37; Fig. 5), being distinct from the latter species in having narrower conidia. Pestalotiopsis grevilleae has overlapping conidial dimensions with P. australasiae (clade 39; Fig. 5), although their basal cells are distinct. In P. grevilleae the basal cells are conic, while in P. australasiae they are obconic to hemispherical. Furthermore, phylogenetic analyses (Fig. 5) indicate that the two species are genetically distinct.
Pestalotiopsis hawaiiensis Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809739. Fig. 29.
Fig. 29.
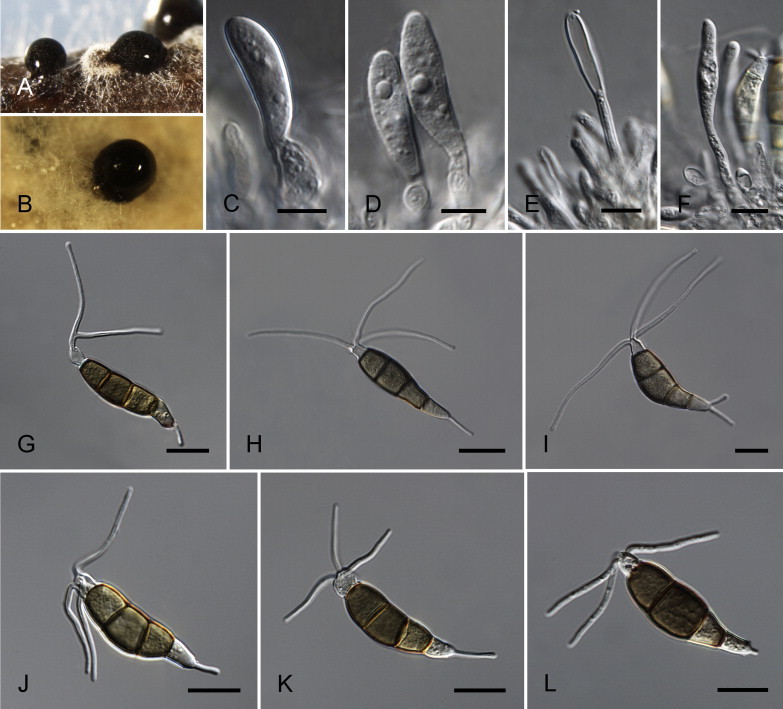
Pestalotiopsis hawaiiensis CBS 114491T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–F. Conidiogenous cells. G–L. Conidia. Scale bars = 10 μm.
Etymology: Named after the island from where it was collected, Hawaii.
Conidiomata (on PDA) pycnidial, globose, solitary, semi-immersed, dark brown to black, 200–600 μm diam; exuding globose, brown to black conidial masses. Conidiophores simple or branched, hyaline, subcylindrical, smooth-walled, 5–15 × 3–8 μm. Conidiogenous cells discrete, cylindrical, hyaline, smooth-walled, proliferating 2–4 times percurrently near apex, 20–50 × 3–6 μm, collarette present and not flared, with prominent periclinal thickening. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (26–)27–34.5(–37) × (7–)7.5–10(–10.5) μm, ± SD = 31.6 ± 2 × 8.7 ± 0.6 μm; basal cell conic to obconic with a truncate base, hyaline, minutely verruculose and thin-walled, 4–8 μm long; three median cells doliiform to subcylindrical, (19–)19.5–23(–25) μm long, ± SD = 21.4 ± 1.2 μm, wall verruculose, concolourous brown, septa darker than the rest of the cell (second cell from the base 5–8.5 μm; third cell 6.5–9.5 μm; fourth cell 6–9 μm); apical cell 4–7 × μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (14–)19–33(–36) μm long, ± SD = 25.3 ± 4.1 μm; basal appendage single, tubular, unbranched, centric, 5–11 μm long.
Culture characteristics: Colonies on PDA attaining 30–45 mm diam after 7 d at 25 °C, with undulate edge, whitish, sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Leucospermum sp.
Known distribution: USA (Hawaii).
Material examined: USA, Hawaii, from Leucospermum sp. cv. ‘Coral’, 9 Dec. 1999, P.W. Crous (CBS H-21761, holotype, ex-type culture CBS 114491 = STE-U 2215).
Notes: Pestalotiopsis hawaiiensis (clade 5; Fig. 5), known from Hawaii on Leucospermum sp., has overlapping conidial dimensions with P. anacardiacearum (27–39 × 7–10 μm; clade 6; Fig. 5), which was isolated from leaves of Mangifera indica in China (Maharachchikumbra et al. 2013c). However, P. anacardiacearum differs from P. hawaiiensis by having longer apical appendages (20–45 μm). Furthermore, the two species are genetically, geographically and ecologically distinct, and thus we maintain them as two separate species.
Pestalotiopsis hollandica Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809740. Fig. 30.
Fig. 30.

Pestalotiopsis hollandica CBS 265.33T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the pars pro toto name “Holland” for the country where it was collected, the Netherlands.
Conidiomata (on PDA) pycnidial, 200–350 μm diam, globose or clavate, solitary or aggregated, semi-immersed, dark brown to black; exuding dark brown conidial masses. Conidiophores septate, branched at base, sometimes reduced to conidiogenous cells, hyaline, smooth-walled, up to 30 μm long. Conidiogenous cells discrete, cylindrical, proliferating 2–5 times percurrently near apex, tapering to a long, thin neck, collarette present and not flared. Conidia ellipsoid, straight to slightly curved, 4-septate, slightly constricted at septa, (25–)25.5–33(–34) × 8.5–10(–10.5) μm, ± SD = 28 ± 2 × 9.4 ± 0.3 μm; basal cell conic to obconic with truncate base, thin-walled, 5–7.5 μm long; three median cells (16.5–)17–23(–24) μm long; ± SD = 28 ± 2 × 9.4 ± 0.3 μm, doliiform, thick-walled, verruculose, concolourous, but occasionally the two upper median cells slightly darker than the lower median cell, wall rugose (second cell from base 5–8.5 μm; third cell 6–9 μm; fourth cell 6–8 μm); apical cell conic, hyaline, thin- and smooth-walled, 3.5–5 μm long; with 1–4 tubular apical appendages, with some branched appendages, arising from the apex of the apical cell and sometimes from just above the septum separating the apical and subapical cell, 20–40 μm long, ± SD = 27 ± 1.5 μm; basal appendage single, tubular, unbranched, centric, 3–9 μm long.
Culture characteristics: Colonies on PDA reaching 60–70 mm diam. after 7 d at 25 °C, with an undulate edge, whitish to pale grey-coloured, with dense aerial mycelium on surface, and black, gregarious conidiomata; reverse similar in colour.
Habitat: On Sciadopitys verticillata.
Known distribution: Netherlands.
Material examined: Netherlands, Baarn, from Sciadopitys verticillata, Jul. 1933, A. Punt (CBS H-15703, holotype, ex-type culture CBS 265.33).
Notes: Pestalotiopsis hollandica (clade 18; Fig. 5) differs from all other related species (clades 17, 19 and 20; Fig. 5) in having some appendages that arise from different parts of the apical cell. Pestalotiopsis hollandica differs from P. monochaetioides (22–30 × 5–10 μm; no culture available for molecular study), which was isolated from a dead twig of Chamaecyparis lawsoniana in the Netherlands (Guba 1961), by its branched, subapically attached apical appendages.
Pestalotiopsis humus Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809727. Fig. 31.
Fig. 31.
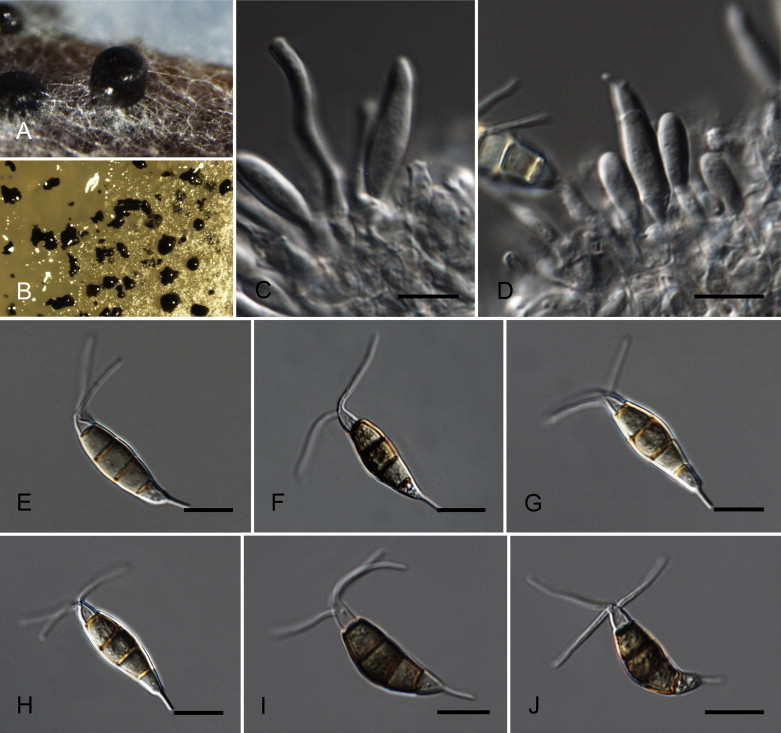
Pestalotiopsis humus CBS 336.97T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the substrate from which it was isolated, soil.
Conidiomata pycnidial in culture on PDA, globose, semi-immersed, aggregated or scattered, up to 400 μm diam; exuding dark brown to black, globose conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical, hyaline, smooth-walled, simple, proliferating up to 3 times percurrently, 8–28 × 2–5 μm, apex 1–2 μm diam. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, constricted at septum, (17–)18.5–22(–23) × 5–7(–7.5) μm, ± SD = 20 ± 1.4 × 6 ± 0.4 μm; basal cell obconic to conic with a truncate base, hyaline, minutely verruculose and thin-walled, 3.5–5.5 μm long; three median cells subcylindrical, (11.5–)12–14(–14.5) μm long, ± SD = 12.8 ± 0.8 μm, wall rugose, concolourous, brown, septa darker than the rest of the cell (second cell from the base 3.5–5.5 μm long; third cell 3.5–6 μm long; fourth cell 3.5–5.5 μm long); apical cell 3.5–4.5 × μm long, hyaline, subcylindrical; with 2–3 tubular apical appendages, arising from an apical crest, unbranched, filiform, flexuous, (6–)6.5–12(–13) μm long, ± SD = 9.0 ± 1.5 μm; basal appendage single, tubular, unbranched, centric, 2–5 μm long.
Culture characteristics: Colonies on PDA attaining 45–50 mm diam after 7 d at 25 °C, with smooth edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On fruits of Ilex cinerea and soil.
Known distribution: China (Hong Kong) and Papua New Guinea.
Materials examined: China, Hong Kong, from fruit of Ilex cinerea, 20 Jan. 2002, K.D. Hyde, culture CBS 115450 = HKUCC 9100. Papua New Guinea, from soil in tropical rain forest, Nov. 1995, A. Aptroot (CBS H-21760, holotype, ex-type culture CBS 336.97).
Notes: Clade 28 (Fig. 5) comprises P. humus, isolated from rain forest soil in Papua New Guinea and fruit of Ilex cinerea in Hong Kong. Sequences of Pestalotiopsis humus form a sister clade to P. diploclisiae (clade 29; Fig. 5). Pestalotiopsis diploclisiae differs from P. humus in conidial morphology, in having narrower conidia (20–28 × 5–7 μm), and longer apical appendages (10–22 μm).
Pestalotiopsis inflexa Maharachch. & K.D. Hyde
Material examined: China, Hunan Province, Yizhang County, Mangshan, on living leaves of unidentified tree, 12 Apr. 2002, W.P. Wu (HMAS047098, holotype, MFLU 12-0413, isotype, ex-type culture NN0470980 = MFLUCC 12-0270).
Note: This species (clade 14; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis intermedia Maharachch. & K.D. Hyde
Material examined: China, Hubei Province, Shengnongjia, on dead leaves of unidentified tree, 24 Mar. 2003, W.P. Wu (HMAS047642, holotype, MFLU 12-0410, isotype, ex-type culture NN0476420 = MFLUCC 12-0259).
Note: This species (clade 21; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis jesteri Strobel, J. Yi Li, E.J. Ford & W.M. Hess, Mycotaxon 76: 260. 2000. Fig. 32.
Fig. 32.

Pestalotiopsis jesteri CBS 109350. A. Conidioma sporulating on PNA. B. Conidioma on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Conidiomata (on PDA) pycnidial, globose, solitary or aggregated, immersed, medium to dark brown, 100–450 μm diam; releasing globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, lageniform to subcylindrical, hyaline, smooth, proliferating once or twice, 5–20 × 3–7 μm; collarette flared, apex 2–5 μm diam. Conidia fusoid, ellipsoid to subcylindrical, straight to slightly curved, 4-septate, (21–)22.5–31(–34.5) × 7–9 μm, ± SD = 26.8 ± 3 × 8.2 ± 0.2 μm; basal cell narrowly obconic with a truncate base, hyaline, thin- and smooth-walled, 4.5–6.5 μm long; three median cells doliiform to subcylindrical, (15.5–)16–20(–21) μm long, ± SD = 17.5 ± 1.4 μm, wall rugose, concolourous, golden brown, septa darker than the rest of the cell (second cell from the base 4.5–7 μm long; third cell 5.5–7.5 μm long; fourth cell 5.5–7.5 μm long); apical cell 3.5–7.5 μm long, hyaline, obconic with an acute apex, thin- and smooth-walled; appendages tubular, attenuated; apical appendage single, 14–25 long; lateral appendages 2–4, arising just above the septum separating the apical cell and upper median cell, unbranched, 14–25 long; basal appendage single, tubular, unbranched, centric, 4–14 μm long.
Culture characteristics: Colonies on PDA attaining 20–30 mm diam after 7 d at 25 °C, with undulate edge, whitish, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On bark of Fragraea bodenii.
Known distribution: Papua New Guinea.
Material examined: Papua New Guinea, Southern Highlands, Aluak ambe village, from bark of Fragraea bodenii, E. Eroli & G. Strobel (deposited in CBS collection Mar. 2001 by G. Strobel) (MONT Strobel 6T-L-3, holotype, MONT Strobel 6M-B-3 and MONT Strobel 6B-S-4, isotypes, ex-type culture CBS 109350 = MONT 6M-B-3).
Notes: Pestalotiopsis jesteri (clade 1; Fig. 5) is described from bark of Fragraea bodenii in Papua New Guinea and is well-characterised and easily recognisable by the unique appendages attached to the apical cell. The arrangement of apical appendages in P. jesteri is comparable with Pestalotia montellica (Guba 1961). However, P. jesteri differs from Pestalotia montellica by the presence of knobbed apical appendages. Furthermore, P. jesteri is a basal species in the species phylogeny (Fig. 5), and forms a lineage distinct from all other species.
Pestalotiopsis kenyana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809741. Fig. 33.
Fig. 33.
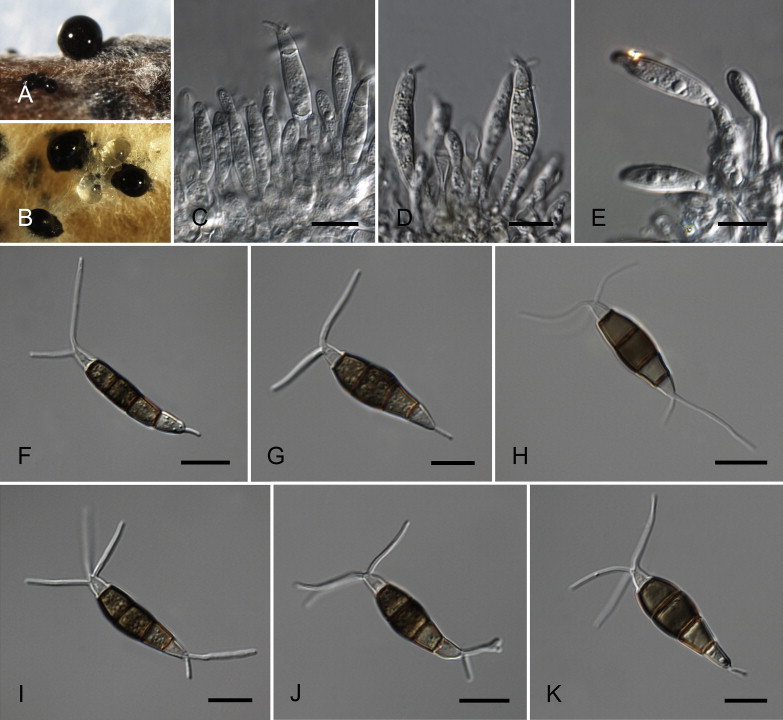
Pestalotiopsis kenyana CBS 442.67T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Kenya.
Conidiomata pycnidial in culture on PDA, globose, scattered, semi-immersed, black, up to 400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores sparsely septate at base, branched or unbranched, subcylindrical, hyaline, smooth, up to 15 μm or reduced to conidiogenous cells. Conidiogenous cells discrete, lageniform to subcylindrical, hyaline, smooth, proliferating 1–3 times percurrently, 10–25 × 2–5 μm, apex with minute periclinal thickening and collarette. Conidia fusoid, ellipsoid to subcylindrical, straight to slightly curved, 4-septate, (22–)23–28(–29) × 7–9 μm, ± SD = 25.5 ± 1.2 × 8 ± 0.4 μm; basal cell conic to obconic with a truncate base, hyaline, minutely verruculose and thin-walled, 4–6 μm long; three median cells doliiform, (15–)15.5–18.5(–19) μm long, ± SD = 17 ± 0.7 μm, wall verruculose concolourous, brown, septa darker than the rest of the cell (second cell from the base 4.5–6 μm long; third cell 5.5–7.5 μm long; fourth cell 3.5–4.5 μm long); apical cell 3.5–5.5 μm long, hyaline, subcylindrical, rugose and thin-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, (8–)9–18(–20) μm long, ± SD = 14 ± 3 μm; two basal appendages; centric appendage tubular, flexuous, 3–20 μm long and eccentric appendage tubular, 1–4 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with undulate edge, whitish, with medium dense aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On branches of Coffea sp., and raw material from agar-agar, kobe 1.
Known distribution: Kenya.
Materials examined: Kenya, from Coffea sp. branch, Oct. 1967, H. Vermeulen (CBS H-15657, holotype, ex-type culture CBS 442.67). Unknown country, from raw material from agar-agar, kobe 1 (stips), Sep. 1996, A.K. Johansen, culture CBS 911.96.
Notes: Pestalotiopsis kenyana (clade 42; Fig. 5) formed a separate clade in the phylogenetic analyses as sister to P. trachicarpicola (clade 43; Fig. 5). Both P. kenyana and P. trachicarpicola often have two basal appendages. Pestalotiopsis kenyana differs from both P. trachicarpicola and P. biciliata (clade 38; Fig. 5) in having wider conidia (see comparison under P. biciliata).
Pestalotiopsis knightiae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809742. Fig. 34.
Fig. 34.
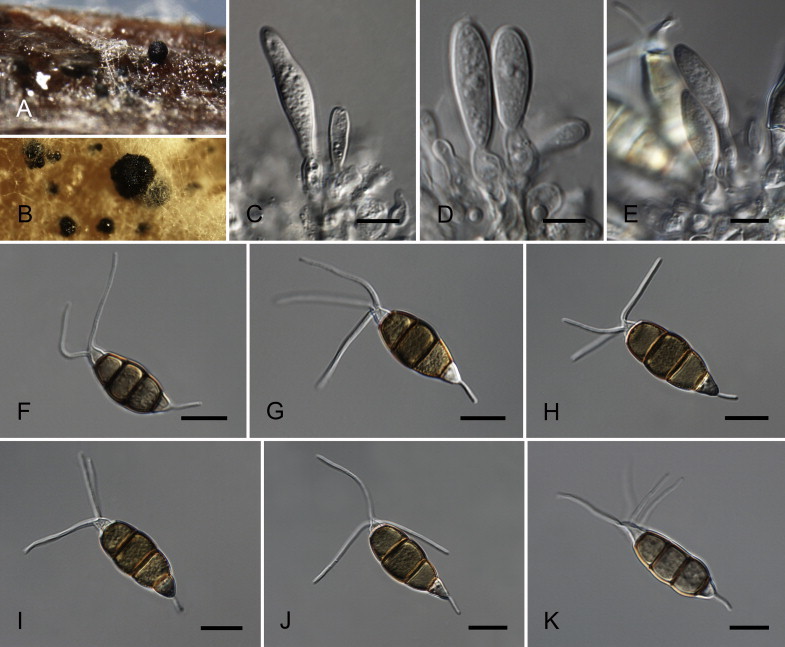
Pestalotiopsis knightiae CBS 114138T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Knightia.
Conidiomata pycnidial, globose, aggregated or scattered, semi-immersed to erumpent on PDA, dark brown to black, 100–200 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform or lageniform, hyaline, smooth, simple, proliferating once or twice, wide at the base, 10–30 × 2–10 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (21–)22–27(–29) × (8–)8.5–10.5(–11) μm, ± SD = 24.8 ± 1.3 × 9.6 ± 0.4 μm; basal cell obconic to conic with a truncate base, hyaline, thin- and smooth-walled, 3–6.5 μm long; three median cells doliiform, (15.5–)16–18.5(–19.5) μm long, ± SD = 17.4 ± 1.2 μm, wall minutely rugose, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 5.5–7 μm long; third cell 6–7.5 μm long; fourth cell 5.5–7 μm long); apical cell 3–4.5(–5) μm long, hyaline, cylindrical to subcylindrical; with 2–4 tubular apical appendages (mostly 3), not arising from the apical crest, but each inserted at a different locus in the upper half of the cell, unbranched, filiform, (8–)12–20(–23) μm long, ± SD = 15 ± 3.9 μm; basal appendage single, tubular, unbranched, centric, 2.5–7.5 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with lobate edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Knightia sp.
Known distribution: New Zealand.
Materials examined: New Zealand, from Knightia sp., 1999, P.W. Crous (CBS H-21759, holotype, ex-type culture CBS 114138 = STE-U 2906); Tamaki, Maori Village, from Knightia sp., 1999, P.W. Crous, culture CBS 111963 = STE-U 2905.
Notes: Pestalotiopsis knightiae (clade 37; Fig. 5) is a species occurring on Knightia sp. in New Zealand, and is distinct from other morphologically closely related species (clades 36 and 38; Fig. 5) based on its DNA phylogeny. It forms a sister clade with P. grevilleae (clade 36; Fig. 5), and is distinguishable from other phylogenetically closely related species by its wider conidia.
Pestalotiopsis linearis Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on living leaves of Trachelospermum sp., 19 Mar. 2002, W.P. Wu (HMAS047190, holotype, MFLU 12-0414, isotype, ex-type culture NN0471900 = MFLUCC 12-0271).
Note: This species (clade 22; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis malayana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809743. Fig. 35.
Fig. 35.

Pestalotiopsis malayana CBS 102220T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Malaysia.
Conidiomata (on PDA) pycnidial, globose, scattered or aggregated, semi-immersed, dark brown to black, up to 400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores 2–5-septate, irregular branched, cylindrical, hyaline, verruculose-walled, up to 50 μm, sometimes reduced to conidiogenous cells. Conidiogenous cells discrete, subcylindrical to ampulliform, hyaline, smooth, tapering to a long, thin neck, 6–18 × 2–4 μm, proliferating several times percurrently near apex, with flaring collarettes. Conidia fusoid, ellipsoid, straight to slightly curved, slightly constricted at septa, 4-septate, (20.5–)22–29.5(–31) × 5–7.5 μm, ± SD = 25.6 ± 2 × 6.3 ± 0.4 μm; basal cell obconic to conic with a truncate base, hyaline, minutely verruculose and thin-walled, 3.5–7.5 μm long; three median cells doliiform, 15–18 μm long, ± SD = 16.5 ± 0.8 μm, wall minutely verruculose, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 4.5–7 μm long; third cell 4.5–6.5 μm long; fourth cell 5–7 μm long); apical cell 3–6 μm long, hyaline, cylindrical to subcylindrical; with 1–3 tubular apical appendages (mostly 2), arising as an extension of the apical cell, unbranched, filiform, (11–)11.5–18.5(–19) μm long, ± SD = 15.1 ± 1.4 μm; basal appendage single, tubular, unbranched, centric, 2–5 μm long.
Culture characteristics: Colonies on PDA reaching 22–30 mm after 7 d at 25 °C, edge rhisoid, white to pale honey-coloured, conidiomata black, gregarious; reverse of culture same colours.
Habitat: On stem of Macaranga triloba colonised by ants.
Known distribution: Malaysia.
Material examined: Malaysia, from stem of Macaranga triloba colonised by ants, Sep. 1999, W. Federle (CBS H-21758, holotype, ex-type culture CBS 102220).
Notes: Clade 30 (Fig. 5) represents Pestalotiopsis malayana (CBS 102220), which is characterised by having two apical appendages. Pestalotiopsis malayana formed a distinct lineage in the phylogenetic analyses from its closely related species P. adusta (clade 31; Fig. 5), P. papuana (clade 32; Fig. 5) and Pestalotiopsis sp. Clade 33 (clade 33; Fig. 5). Furthermore, morphologically P. malayana is well distinguished from allied species by its larger conidia and longer apical appendages.
Pestalotiopsis monochaeta Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809744. Fig. 36.
Fig. 36.
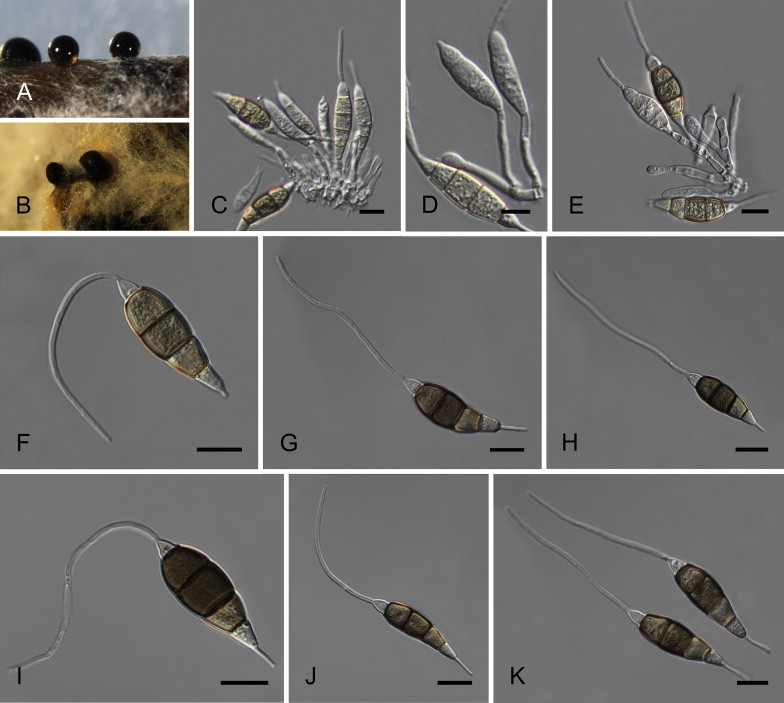
Pestalotiopsis monochaeta CBS 144.97T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the unique single apical appendage.
Conidiomata pycnidial in culture on PDA, globose or clavate, aggregated or scattered, semi-immersed or partly erumpent, 250–500 μm diam; exuding a globose, dark brown to black conidial masses. Conidiophores septate, sparsely branched and sometimes reduced to conidiogenous cells, hyaline, smooth-walled, up to 50 μm long. Conidiogenous cells discrete or integrated, ampulliform to lageniform (4–12 × 2–4 μm) or cylindrical (10–60 × 2–8 μm), proliferating 2–4 times percurrently near apex, tapering to a long, thin neck, collarette present and not flared. Conidia ellipsoid, straight to slightly curved, 4-septate, slightly constricted at septa, (25–)27–40(–42) × 7–11(–11.5) μm, ± SD = 32.8 ± 3.5 × 9.6 ± 0.6 μm; basal cell conic to obconic with a truncate base, rugose and thin-walled, 5.5–9.5 μm long; three median cells (17–)18–25(–26) μm, ± SD = 21 ± 2 μm, doliiform, verruculose, concolourous, but occasionally the two upper median cells slightly darker than the lower median cell (second cell from base 5–8.5 μm long; third cell 7–9 μm long; fourth cell 7–9 μm long); apical cell conic, hyaline, thin- and smooth-walled, 4–6.5 μm long; with single, central, tubular apical appendage, unbranched, filiform, (40–)43–67(–75) μm, ± SD = 51 ± 6 μm; basal appendage single, tubular, unbranched, centric, 6–14 μm long.
Culture characteristics: Colonies on PDA reaching 50–60 mm diam after 7 d at 25 °C, with undulate edge, whitish to pale yellow-coloured, with dense, with aerial mycelium on surface, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Taxus baccata and endophytic in branches of Quercus robur.
Known distribution: Netherlands.
Materials examined: Netherlands, Baarn, Eemnesserweg, endophytes on branches of Quercus robur, Jul. 1996, H.A. van der Aa (CBS H-21757, holotype, ex-type culture CBS 144.97); Baarn, Eemnesserweg 90, from Taxus baccata, 14 Apr. 1983, H.A. van der Aa, CBS H-14560, culture CBS 440.83 = IFO 32686.
Notes: Pestalotiopsis monochaeta (clade 17; Fig. 5) differs from all other species in the genus in having a single apical appendage. Pestalotiopsis brassicae (clade 19; Fig. 5), P. hollandica (clade 18; Fig. 5) and P. verruculosa (clade 20; Fig. 5) are closely related species, but have conidia with more than two apical appendages. This species can easily be misidentified as Monochaetia, since it has borderline morphological features of both genera.
Pestalotiopsis novae-hollandiae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809745. Fig. 37.
Fig. 37.

Pestalotiopsis novae-hollandiae CBS 130973T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the historical European name, New Holland or Hollandia Nova, for the country where it was collected, Australia.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary to aggregated, embedded or semi-immersed, dark brown, 200–450 μm diam, exuding a globose, dark brown, glistening conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, simple, straight to curved, lageniform, smooth, thin-walled, hyaline, 5–20 × 5–10 μm. Conidia fusoid to ellipsoid, straight to slightly curved, 4-septate, (24–)25–31(–32) × (7.5–)8–10(–10.5) μm, ± SD = 28.1 ± 1.6 × 9 ± 0.7 μm; basal cell obconic with truncate base, hyaline or slightly olivaceous, rugose and thin-walled, 4–7 μm long; three median cells (16–)16.5–20.5(–21) μm long, ± SD = 19 ± 1.3 μm, doliiform to subcylindrical, verruculose, concolourous, olivaceous, constricted at the septa (second cell from base 6–8 μm long; third cell 6–7 μm long; fourth cell 5–7 μm long); apical cell hyaline, conic to cylindrical, hyaline, thin- and smooth-walled, 3–5 μm long; with 3–9 tubular apical appendages, arising not in an apical crest, but each inserted at a different locus in the upper half of the cell, unequal in length, some appendages branched, filiform, flexuous, (20–)22–44(–50) μm long, ± SD = 31 ± 9 μm; basal appendage single, tubular, unbranched, centric, 2–5 μm long.
Culture characteristics: Colonies on PDA reaching 50–80 mm diam after 7 d at 25 °C, undulated at the edge, whitish to pale yellow-coloured, with dense aerial mycelium on surface, forming black, gregarious conidiomata; reverse similar in colour.
Habitat: On old inflorescence of Banksia grandis.
Known distribution: Australia.
Material examined: Australia, Perth, Jarrah Forest, from old inflorescence of Banksia grandis, 2010, W. Gams (CBS H-21756, holotype, ex-type culture CBS 130973).
Notes: This species (clade 11; Fig. 5) is characterised by a large number of apical appendages and in having a short basal appendage. Species such as P. camelliae (clade 13; Fig. 5) and P. furcata (clade 12; Fig. 5) have as large a number of apical appendages as P. novae-hollandiae, but they lack a basal appendage. Pestalotiopsis novae-hollandiae is sister to P. portugalica (clade 10; Fig. 5), which has rather smaller conidia (15–21 × 5–7 μm), and few apical appendages (1–3).
Pestalotiopsis oryzae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809746. Fig. 38.
Fig. 38.
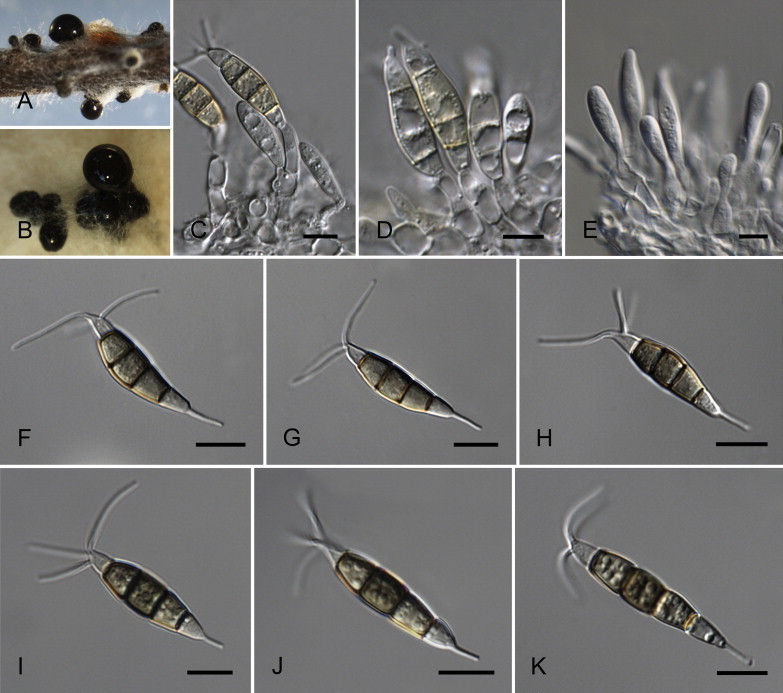
Pestalotiopsis oryzae CBS 353.69T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Oryza.
Conidiomata pycnidial in culture on PDA, globose to clavate, aggregated or scattered, dark brown to black, semi-immersed or partially erumpent, up to 300 μm diam; releasing globose, dark brown to black conidial masses. Conidiophores sparsely septate at base, branched or unbranched, subcylindrical, hyaline, smooth, up to 20 μm. Conidiogenous cells discrete, ampulliform to lageniform, hyaline, smooth, proliferating 2–5 times percurrently, 10–25 × 3–7 μm. Conidia fusoid, ellipsoid to subcylindrical, straight to slightly curved, 4-septate, (23–)24.5–29(–30) × 6–8 μm, ± SD = 26.9 ± 1.4 × 7 ± 0.2 μm; basal cell obconic to conic with a truncate base, hyaline, verruculose and thin-walled, 4.5–6.5 μm long; three median cells doliiform, (14–)16–18.5(–19) μm long, ± SD = 17 ± 1.3 μm, wall minutely verruculose, concolourous or middle median cell is much darker than other cell, olivaceous, septa darker than the rest of the cell (second cell from the base 5–7 μm; third cell 5.5–7 μm; fourth cell 5–6.5 μm); apical cell 3.5–5 μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, filiform, flexuous (9–)18–27(–17) μm long, ± SD = 12.9 ± 1.7 μm; basal appendage single, tubular, unbranched, centric, 3–6 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with undulate edge, convex with papilate surface, hyaline to pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse pale honey-coloured.
Habitat: On Telopea sp. and seeds of Oryza sativa.
Known distribution: Denmark, Italy and USA.
Materials examined: Denmark, from seeds of Oryza sativa, S.B. Mathur (CBS H-15697, holotype, ex-type culture CBS 353.69). Italy, unknown substrate, Dec. 1926, R. Ciferri, culture CBS 171.26. USA, Hawaii, from Telopea sp. (introduced from Australia), 8 Dec. 1998, P.W. Crous & M.E. Palm, CBS H-21753, culture CBS 111522 = STE-U 2083.
Notes: Clade 41 (Fig. 5) consists of three isolates of P. oryzae, including the ex-type strain (CBS 353.69) isolated from seeds of Oryza sativa from Denmark. Pestalotiopsis oryzae has overlapping conidial characters with P. kenyana (clade 42; Fig. 5) and P. trachicarpicola (clade 43; Fig. 5). However, P. oryzae is genetically distinct and has a different geographic distribution from these two species.
Pestalotiopsis papuana Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809747. Fig. 39.
Fig. 39.

Pestalotiopsis papuana CBS 331.96T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Papua New Guinea.
Conidiomata pycnidial, globose to clavate, aggregated or scattered, semi-immersed on PDA, dark brown to black, 100–500 μm diam; exuding globose, dark brown conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, lageniform to subcylindrical, hyaline, smooth, proliferating once or twice, 4–20 × 2–4 μm; apex with minute periclinal thickening and flaring collarettes. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (17–)18–22(–24) × 6–7.5 μm, ± SD = 20.5 ± 1.5 × 6.7 ± 0.3 μm; basal cell obconic with a truncate base, hyaline, verruculose and thin-walled, 3–5 μm long; three median cells doliiform, 12–15 μm long, ± SD = 13.6 ± 0.7 μm, wall verruculose, concolourous, brown, septa darker than the rest of the cell (second cell from the base 3.5–5.5 μm; third cell 4.5–5.5 μm; fourth cell 4.5–6 μm); apical cell 2–4 μm long, hyaline, cylindrical to subcylindrical, rugose and thin-walled; with 1–2 tubular apical appendages, arising from the apical crest, unbranched, filiform, 1.5–7 μm long, ± SD = 4.1 ± 1 μm; basal appendage single, tubular, unbranched, centric, 0.5–2 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with undulate edge, pale honey-coloured, with medium sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On coastal soil and leaves of Cocos nucifera.
Known distribution: Papua New Guinea.
Materials examined: Papua New Guinea, from soil along the coast, Nov. 1995, A. Aptroot (CBS H-21755, holotype, ex-type culture CBS 331.96); from leaves of Cocos nucifera (coastal primary forest), 27 Oct. 1995, A. Aptroot, culture CBS 887.96.
Notes: Pestalotiopsis papuana (clade 32; Fig. 5) is genetically close to P. adusta (clade 31; Fig. 5) and two isolates representing Pestalotiopsis sp. Clade 33 (clade 33; Fig. 5). The latter two isolates are unnamed for the present since both cultures were sterile, making morphological comparisons impossible (see notes under Pestalotiopsis sp. Clade 33). Morphologically, however, P. papuana is quite distinct from P. adusta in having larger conidia and shorter apical appendages.
Pestalotiopsis parva Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809748. Fig. 40.
Fig. 40.
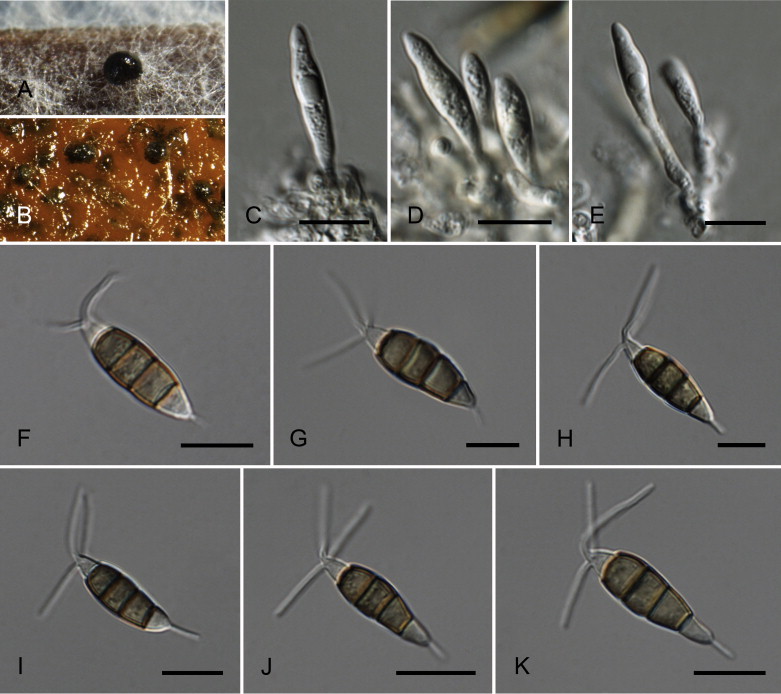
Pestalotiopsis parva CBS 278.35T. A. Conidioma sporulating on PNA. B. Conidiomata on PDA. C–D. Conidiogenous cells. E–I. Conidia. Scale bars = 10 μm.
Etymology: The epithet parva refers to the small conidial size of this species.
Conidiomata pycnidial, globose, aggregated or scattered, dark brown to black, semi-immersed on PDA, 100–200 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, hyaline, smooth-walled, simple, proliferating 2–3 times percurrently, 5–18 × 2–4 μm, apex 1–1.5 μm diam. Conidia fusoid, straight to slightly curved, 4-septate, (16–)16.5–20(–21) × 5–7(–7.5) μm, ± SD = 18.3 ± 1.2 × 6.2 ± 0.5 μm; basal cell obconic to conic with a truncate base, hyaline, thin- and smooth-walled, 3–5 μm long; three median cells doliiform, (10–)10.5–13(–13.5) μm long, ± SD = 12.1 ± 1.0 μm, wall minutely verruculose, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 3.5–5 μm long; third cell 3.5–4.5 μm long; fourth cell 4–5 μm long); apical cell (2–)2.5–4 μm long, hyaline, subcylindrical; with 2–3 tubular apical appendages (mostly 3), arising from the apical crest, unbranched, (6–)6.5–12(–13) μm long, ± SD = 9.0 ± 1.9 μm; basal appendage single, tubular, unbranched, centric, 2–4 μm long.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam after 7 d at 25 °C, with smooth edge, pale honey-coloured, with sparse aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Delonix regia and Leucothoe fontanesiana.
Known distribution: Unknown.
Materials examined: Unknown country, from Leucothoe fontanesiana, 1935, R.P. White (CBS H-15694, holotype, ex-type culture CBS 278.35); from Delonix regia, H.W. Wollenweber, CBS H-15659, culture CBS 265.37 = BBA 2820.
Notes: Pestalotiopsis parva is a distinct species represented by two isolates (clade 35; Fig. 5). Pestalotiopsis rosea (clade 34; Fig. 5), which is an endophyte isolated from living leaves of Pinus sp. in China, is a sister species. Although these two species are morphologically similar, they differ in having distinctly longer apical appendages, which are sometimes branched. Furthermore, the reddish colony is unique to P. rosea and this reddish colour can be seen even in conidiogenous cells and some conidia.
Pestalotiopsis portugalica Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809749. Fig. 41.
Fig. 41.

Pestalotiopsis portugalica CBS 393.48T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C. Conidiogenous cells. D–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, Portugal.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary or aggregated, black, semi-immersed, 200–400 μm diam; releasing brown to black, slimy, globose conidial masses. Conidiophores hyaline, septate, irregularly branched, up to 100 μm in long. Conidiogenous cells cylindrical, hyaline, smooth, proliferating 2–6 times percurrently, 10–60 × 4–12 μm, collarette present and not flared, with prominent periclinal thickening. Conidia fusoid, straight to slightly curved, 4-septate, (14.5–)15.5–20(–21.5) × 5–7 μm, ± SD = 17.9 ± 1.6 × 6.0 ± 0.5 μm; basal cell obconic with a truncate base, hyaline, thin- and smooth-walled, 2.5–4 μm long; three median cells (9–)9.5–13.5(–14) μm long, ± SD = 11.7 ± 1 μm, doliiform to subcylindrical, with thick verruculose walls, constricted at the septa, concolourous, pale brown (second cell from base 3–5 μm long; third cell 3.0–5 μm long; fourth cell 3.5–5 μm long); apical cell conic to cylindrical, hyaline, thin- and smooth-walled, 2–5 μm long; 1–3 tubular apical appendages arising from an apical crest or branched irregular along their length resulting 2–3 branched, filiform, (8–)9–18(–20) μm long, ± SD = 14 ± 3 μm; basal appendage lack or when present single, tubular, unbranched, centric, 1–4 μm long.
Culture characteristics: Colonies on PDA reaching 60–70 mm diam after 7 d at 25 °C, edge entire, whitish to pale honey-coloured, aerial mycelium on surface, conidiomata black, gregarious; reverse similar in colour.
Habitat: Unknown.
Known distribution: Portugal.
Material examined: Portugal, unknown host, Jun. 1948, collector unknown (CBS H-21754, holotype, ex-type culture CBS 393.48).
Notes: Pestalotiopsis portugalica (clade 10; Fig. 5) is a distinct species in terms of morphology and phylogeny. It differs from its phylogenetically related species P. camelliae (clade 13; Fig. 5), P. furcata (clade 12; Fig. 5) and P. novae-hollandiae (clade 11; Fig. 5) by smaller conidia and fewer apical appendages. Its conidial size overlaps with P. rosea (17.5–21.8 × 5.7–7 μm; clade 34; Fig. 5), but those two species are phylogenetically distinct.
Pestalotiopsis rhododendri Y.M. Zhang, Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Chuxiong, Zixishan, leaf spots on Rhododendron sinogrande, May 2011, Y.M. Zhang (IFRD 410-018, holotype, ex-type culture IFRDCC 2399).
Note: This species (clade 16; Fig. 5) was treated in detail by Zhang et al. (2013).
Pestalotiopsis rosea Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on living leaves of Pinus sp., 19 Mar. 2002, W.P. Wu (HMAS047135, holotype, MFLU12 0409, isotype, ex-type culture NN0471350 = MFLUCC 12-0258).
Note: This species (clade 34; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis scoparia Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809750. Fig. 42.
Fig. 42.
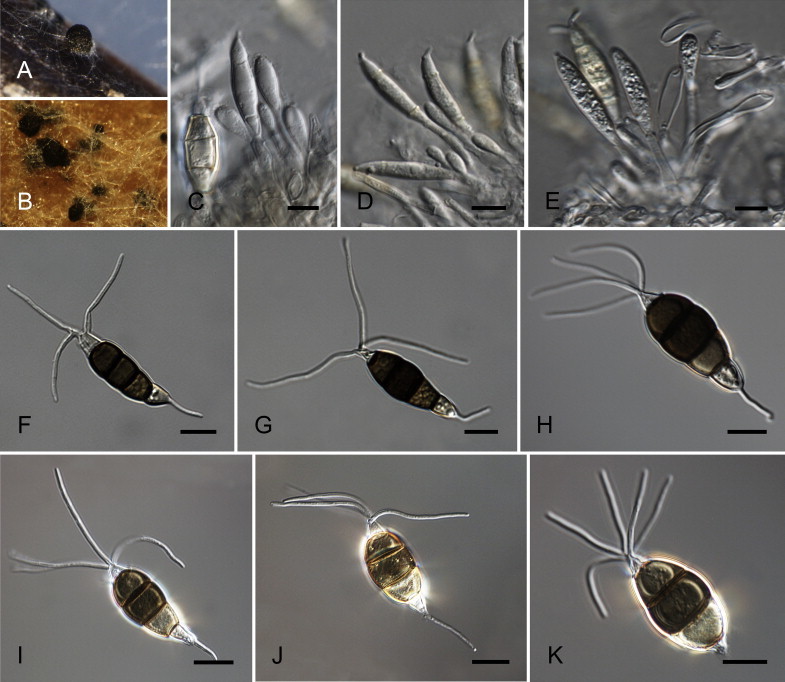
Pestalotiopsis scoparia CBS 176.25T. A. Conidioma sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: The epithet scoparia refers to the broom-shaped apical appendages of this species.
Conidiomata pycnidial, globose, aggregated or scattered, semi-immersed on PDA, dark brown to black, 100–400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical to subcylindrical, hyaline, smooth, proliferating up to 3 times, 10–30 × 2–4 μm, with visible periclinal thickening; collarette slightly flared, up to 3 μm long when present. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (22–)23.5–29(–31) × 6–8.5 μm, ± SD = 26.3 ± 2 × 7.4 ± 0.3 μm; basal cell hemispherical to obconic with a truncate base, hyaline, verruculose and thin-walled, 4–6 μm long; three median cells doliiform, 15.5–19.5 μm long, ± SD = 17 ± 1 μm, wall verruculose, concolourous, but occasionally the two upper median cells darker than the lower median cell, brown, septa darker than the rest of the cell (second cell from the base 5–6.5 μm long; third cell 5–7 μm long; fourth cell 5.5–7.5 μm long); apical cell 4.5–6 μm long, hyaline, subcylindrical, rugose and thin-walled; with 3–5 tubular apical appendages, arising from the apical crest, unbranched, filiform, (20–)23–35(–42) μm long, ± SD = 29.6 ± 4 μm; basal appendage single, tubular, unbranched, centric, 9–25 μm long.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 7 d at 25 °C, with smooth edge, pale honey-coloured, with medium dense aerial mycelium on the surface with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Chamaecyparis sp.
Known distribution: Unknown.
Material examined: Unknown country, from young Chamaecyparis sp. ‘Retinospora’, May 1925, C.M. Doyer (CBS H-21752, holotype, ex-type culture CBS 176.25).
Notes: Pestalotiopsis scoparia (clade 25; Fig. 5) is genetically a clearly distinct species, forming a separate clade in a sister position to P. australis (clade 26; Fig. 5) and P. unicolor (clade 24; Fig. 5). It is well characterised by its rather long broom-shaped, 3–5 apical appendages, long basal appendages and occasionally by having versicoloured median cells.
Pestalotiopsis sp. Clade 33
Materials examined: Indonesia, Sulawesi, from leaf spot in bibit of Cocos sp., unknown collection date, P.M.L. Tammes, culture CBS 264.33. Netherlands, Boskoop, from Rhododendron ponticum, Mar. 1933, W.F. van Hell, culture CBS 263.33.
Note: Although phylogenetically distinct (clade 33; Fig. 5), both cultures of this species proved to be sterile, and thus are not treated further.
Pestalotiopsis spathulata Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809751. Fig. 43.
Fig. 43.
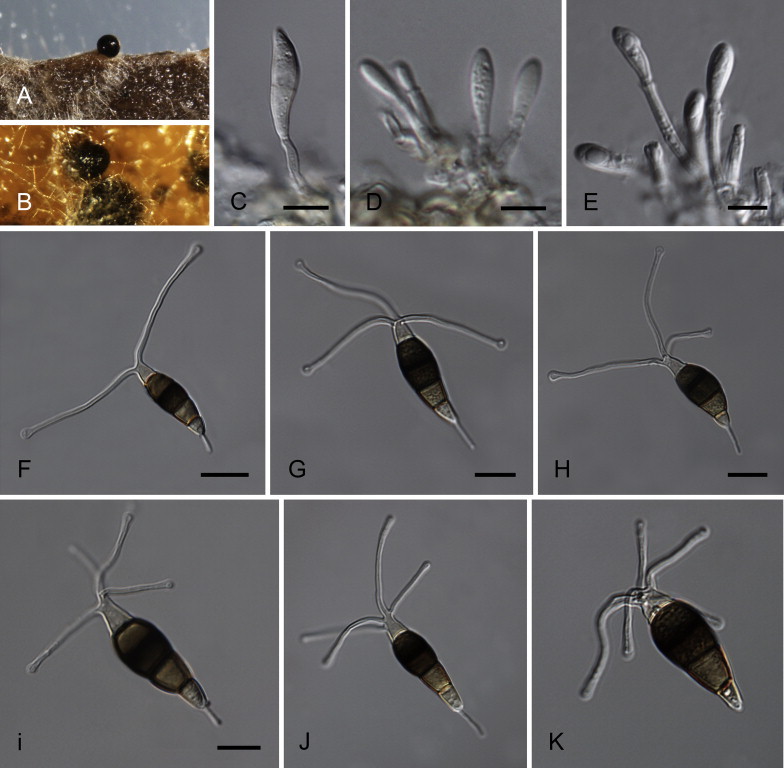
Pestalotiopsis spathulata CBS 356.86T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: The species epithet refers to the knobbed nature of its apical appendages.
Conidiomata pycnidial, globose, aggregated or scattered, semi-immersed to erumpent or embedded on PDA, dark brown to black, 100–400 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores 0–2-septate, branched at base, subcylindrical, often reduced to conidiogenous cells, hyaline, smooth-walled up to 20 μm long. Conidiogenous cells discrete, ampulliform to lageniform or cylindrical, proliferating 2–5 times percurrently, wide at the base, tapering to a long, thin neck, 5–40 × 2–8 μm, prominent periclinal thickening with flaring collarettes. Conidia fusoid, straight to slightly curved, 4-septate, (24–)25–31(–32) × 7.5–9.5 μm, ± SD = 27.7 ± 2 × 8.6 ± 0.3 μm, slightly constricted at septa; basal cell conic to obconic with a truncate base, rugose and thin-walled, 5–7.5 μm long; three median cells, (13–)14–19.5(–20) μm long, ± SD = 17.1 ± 1.8 μm, doliiform, verruculose, dark brown to olivaceous, versicoloured (second cell from base pale brown to olivaceous, 4.5–7 μm, third cell honey brown, 4.5–6 μm long; fourth cell honey brown, 5.5–7 μm long); apical cell cylindrical, hyaline, thin and smooth-walled, 5–6 μm long; with 2–5 tubular apical appendages, arising not in an apical crest, but each inserted at a different locus in the upper half of the cell, swollen at the tip, filiform, flexuous, some appendages branched, (17–)18–24(–25) μm, ± SD = 21.1 ± 1.7 μm; basal appendage single, tubular, unbranched, centric, 4–7 μm long.
Culture characteristics: Colonies on PDA reaching 50–60 mm diam after 7 d at 25 °C, with undulate edge, whitish, with dense, aerial mycelium on surface, conidiomata black, gregarious; reverse similar in colour.
Habitat: On leaf spot on Gevuina avellana.
Known distribution: Chile.
Material examined: Chile, leaf spot on Gevuina avellana, Sep. 1961, unknown collector (CBS H-21751, holotype, ex-type culture CBS 356.86).
Notes: Pestalotiopsis spathulata (clade 8; Fig. 5) is morphologically and phylogenetically distinct (Fig. 5). The two upper median cells in P. spathulata are especially darker than the lower median cell. This is also found in its sister species P. gaultheria (clade 9; Fig. 5). Pestalotiopsis gaultheria differs from P. spathulata in having fewer (–3), and longer apical appendages (13–54 μm).
Pestalotiopsis telopeae Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809752. Fig. 44.
Fig. 44.
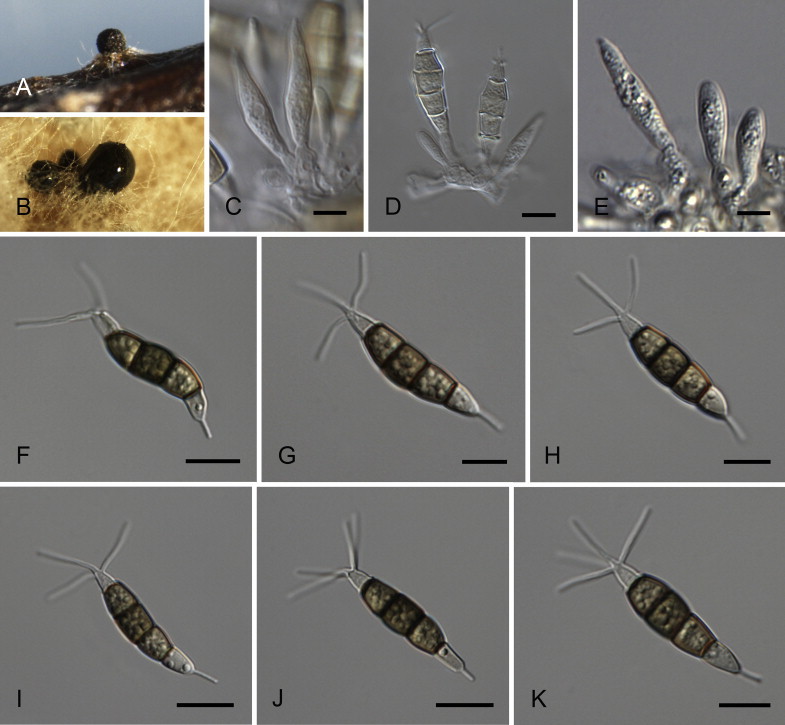
Pestalotiopsis telopeae CBS 114161T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus, Telopea.
Leaf spots on Telopea sp. circular to subcircular, up to 2 cm diam, amphigenous, pale to medium brown with a broad, dark brown border, which can be conspicuously raised in some leaf spots. Conidiomata pycnidial in culture on PDA, globose, aggregated or scattered, semi-immersed, dark brown to black, up to 500 μm diam; exuding globose, dark brown to black conidial masses. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform or lageniform, hyaline, smooth, proliferating 2–4 times percurrently, 5–15 × 2–9 μm, collarette present and not flared. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, (24–)24.5–31(–32) × 6–8 μm, ± SD = 27 ± 1.5 × 7 ± 0.3 μm; basal cell obconic, hyaline, verruculose and thin-walled, 4.5–7 μm long; three median cells doliiform, (15–)16–18.5(–19) μm long, ± SD = 17.1 ± 1 μm, wall verruculose, concolourous, brown to olivaceous (second cell from the base 4.5–7 μm long; third cell 5–7.5 μm long; fourth cell 5–7 μm long); apical cell 3.5–5.5 μm long, hyaline, subcylindrical; with 2–4 tubular apical appendages (mostly 3), arising from an apical crest, unbranched, filiform, (7–)8–15(–16) μm long, ± SD = 12.6 ± 1.7 μm; basal appendage single, tubular, unbranched, centric, 3.5–7 μm long.
Culture characteristics: Colonies on PDA reaching 40–50 mm diam after 7 d at 25 °C, with undulate edge, whitish, with dense, aerial mycelium on surface, conidiomata black, gregarious; reverse similar in colour.
Habitat: On leaves of Telopea sp.
Known distribution: Australia.
Materials examined: Australia, New South Wales, Mount Annan, on leaves of Telopea sp., Aug. 1999, P.W. Crous, JT 975 (CBS H-21750, holotype, ex-type culture CBS 114161 = STE-U 3083); ibid., JT 975, culture CBS 113606 = STE-U 3082; Protea neriifolia × susannae cv. ‘Pink Ice’, 12 Oct. 1999, P.W. Crous, culture CBS 114137 = STE-U 2952.
Notes: The two collections of P. telopeae (clade 40; Fig. 5) are morphologically most similar to P. australasiae (clade 39; Fig. 5), but differ in having shorter conidiogenous cells. Furthermore, in the phylogenetic analyses, P. telopeae represents a distinct clade. Although no pathogenicity tests were conducted, P. telopeae is consistently associated with a prominent leaf spot disease of Telopea in Australia.
Pestalotiopsis trachicarpicola Y.M. Zhang & K.D. Hyde
Materials examined: China, Hunan Province, Yizhang County, Mangshan, on living leaves of Schima sp., 12 Apr. 2002, W.P. Wu, culture NN0469830 = MFLUCC 12-0265; Hunan Province, Yizhang County, Mangshan, on living leaves of Sympolocos sp., 12 Apr. 2002, W.P. Wu, culture NN0469780 = MFLUCC 12-0266; Hunan Province, Yizhang County, Mangshan, on living leaves of unidentified tree, 12 Apr. 2002, W.P. Wu, cultures NN0470990 = MFLUCC 12-0267, NN0470720 = MFLUCC 12-0263; Yunnan Province, Dehong, Mangshi, leaf spots on Podocarous macrophyllus, Sep. 2011, Y.M. Zhang, IFRD 411-018, culture IFRDCC 2403; Yunnan Province, Kunming, Kunming Botanical Gardens, leaf spots on Trachycarpus fortunei, Mar. 2011, K.D. Hyde OP068 (IFRD 9026, holotype, ex-type culture IFRDCC 2440); Yunnan Province, Kunming, on living leaves of Chrysophullum sp., 19 Mar. 2002, W.P. Wu, culture NN0471960 = MFLUCC 12-0264.
Note: This species (clade 43; Fig. 5) was treated in detail by Zhang et al. (2012a).
Pestalotiopsis unicolor Maharachch. & K.D. Hyde
Materials examined: China, Hunan Province, Yizhang County, Mangshan, on living leaves of Rhododendron sp., 12 Apr. 2002, W.P. Wu (HMAS046974, holotype, MFLU 12-0417, isotype, ex-type culture NN0469740 = MFLUCC 12-0276); Hunan Province, on living leaves of unidentified tree, 12 Apr. 2002, W.P. Wu, culture NN0473080 = MFLUCC 12-0275.
Note: This species (clade 24; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pestalotiopsis verruculosa Maharachch. & K.D. Hyde
Material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on living leaves of Rhododendron sp., 19 Mar. 2002, W.P. Wu (HMAS047309, holotype, MFLU 12-0416, isotype, ex-type culture NN0473090 = MFLUCC 12-0274).
Note: This species (clade 20; Fig. 5) was treated in detail by Maharachchikumbura et al. (2012).
Pseudopestalotiopsis Maharachch., K.D. Hyde & Crous, gen. nov. MycoBank MB809753.
Etymology: Named after its morphological similarity to Pestalotiopsis.
Conidiomata acervular or pycnidial, subglobose, globose, clavate, solitary or aggregated, dark brown to black, immersed to erumpent, unilocular; exuding dark brown to black conidia in a slimy, globose mass. Conidiophores indistinct, reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical, ampulliform to lageniform, hyaline, smooth- and thin-walled; conidiogenesis initially holoblastic, percurrent proliferations to produce additional conidia at slightly higher levels. Conidia fusoid, ellipsoid, subcylindrical, straight to slightly curved, 4-septate, slightly constricted at septa; basal cell conical to cylindric with a truncate base; three median cells doliiform, concolourous, brown to dark brown or olivaceous, wall rugose to verruculose, septa darker than the rest of the cell; apical cell conic to cylindrical, thin- and smooth-walled; with tubular apical appendages, one to many, filiform or attenuated, flexuous, branched or unbranched, with or without spatulate tips; basal appendage single, tubular, unbranched, centric.
Type species: Pseudopestalotiopsis theae (Sawada) Maharachch., K.D. Hyde & Crous (see below).
Notes: In most studies (Jeewon et al. 2003, Liu et al. 2010, Hu et al. 2007, Maharachchikumbura et al. 2011, 2012), species with dark concolourous median cells with knobbed apical appendages formed a distinct clade with high support, which is defined here as a novel genus, Pseudopestalotiopsis. Partial LSU sequence data confirm that Pseudopestalotiopsis is phylogenetically related to Neopestalotiopsis (Fig. 3), but these genera are also morphologically distinct. In Pseudopestalotiopsis the three median cells are the same colour (concolourous), whereas in Neopestalotiopsis these are versicoloured.
Pseudopestalotiopsis cocos Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809754. Fig. 45.
Fig. 45.

Pseudopestalotiopsis cocos CBS 272.29T. A. Conidioma sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–J. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was isolated, Cocos.
Conidiomata pycnidial, 100–300 μm diam, globose, dark brown, semi-immersed on host substrate on PDA; exuding black conidia in a slimy, globose, glistening mass. Conidiophores indistinct, often reduced to conidiogenous cells. Conidiogenous cells discrete, hyaline, smooth-walled, simple, filiform, sometimes slightly wide at the base, truncate at apex, proliferating 2–3 times percurrently, 12–15 × 1–3 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, constricted at septum, (20–)21–25(–26.5) × 6–7.5 μm, ± SD = 23.0 ± 1.6 × 6.5 ± 0.4 μm; basal cell obconic with a truncate base, hyaline, thin- and smooth-walled, granular, 3.5–5 μm long; three median cells (13.5–)14–16.5(–17.5) μm long, ± SD = 15.5 ± 1.2 μm, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 5.5–6.5 μm long; third cell 4.5–5.5 μm long; fourth cell 5.5–6 μm long); apical cell 3.5–5 μm long, hyaline, cylindrical; with 2–4 tubular apical appendages (mostly 3), arising in an apical crest, but each inserted at a different locus, flexuous, unbranched, (12–)14–21(–23) μm long, ± SD = 17.6 ± 3.2 μm; basal appendage single, tubular, unbranched, centric, 5–8 μm long.
Culture characteristics: Colonies on PDA attaining 50–60 mm diam after 7 d at 25 °C, with smooth edge, whitish to grey, with black, gregarious conidiomata; reverse similar in colour.
Habitat: On Cocos nucifera.
Known distribution: Indonesia (Java).
Material examined: Indonesia, Java, Bogor (Buitenzorg), from Cocos nucifera, unknown collection date, C.M. Doyer (CBS H-15666, holotype, ex-type culture CBS 272.29).
Notes: Pseudopestalotiopsis cocos is a distinct species based on its morphology and phylogeny (Figs 3, 4). It can clearly be differentiated from its sibling species, Ps. indica (31.5–37 × 6.5–9 μm; Fig. 4) by relatively smaller conidia (20–26.5 × 6–7.5 μm), and shorter apical appendages (12–23 μm), whereas in Ps. indica appendages are longer (30–40 μm). Furthermore, the three median cells in Ps. cocos are paler in colour than in Ps. indica. This species is sister to a clade that contains Ps. theae (22–32 × 5–8 μm; Fig. 4) and they have overlapping morphometric characters. However, in Ps. theae the apical appendages are knobbed, which is a feature absent in Ps. cocos.
Pseudopestalotiopsis indica Maharachch., K.D. Hyde & Crous, sp. nov. MycoBank MB809755. Fig. 46.
Fig. 46.
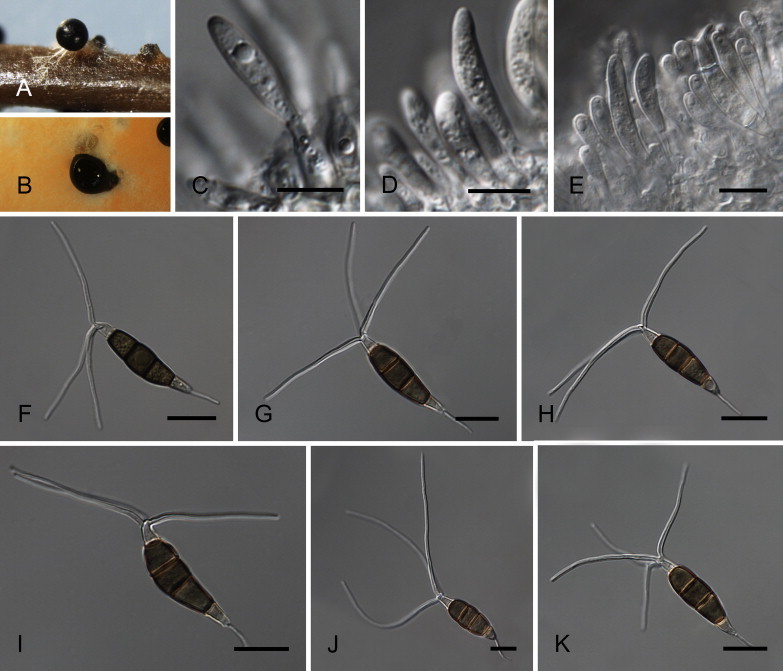
Pseudopestalotiopsis indica CBS 459.78T. A. Conidiomata sporulating on PNA. B. Conidiomata on PDA. C–E. Conidiogenous cells. F–K. Conidia. Scale bars = 10 μm.
Etymology: Named after the country where it was collected, India.
Conidiomata (on PDA) pycnidial, globose to clavate, solitary or aggregated, dark brown, semi-immersed or partly erumpent, 200–500 μm diam; exuding brown to black conidial masses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, ampulliform to lageniform, 5–18 × 2–7 μm, hyaline, smooth- and thin-walled, sometimes percurrently proliferating 1–2 times, periclinal thickening in the apical region, collarette present and flared. Conidia fusoid to ellipsoid, straight to slightly curved, 4-septate, slightly constricted at septa, (31.5–)32.5–36(–37) × 6.5–9 μm, ± SD = 34.5 ± 1.6 × 7.5 ± 0.5 μm; basal cell conic with truncate base, rugose and thin-walled, 5.5–7 μm long; three median cells (19.5–)20–22(–22.5) μm long, ± SD = 21.6 ± 1.0 μm, doliiform, verrucose, concolourous, dark brown, septa darker than the rest of the cell (second cell from base 6.5–8.5 long; third cell 5.5–8 μm long; fourth cell 6.5–8.5 μm long); apical cell subcylindrical, hyaline, thin and smooth-walled, 5.5–7 μm long; with 3–4 tubular apical appendages (mostly 3) arising from the apical crest, flexuous, unbranched, (30–)33–39(–40) μm long, ± SD = 35 ± 2.8 μm; basal appendage single, tubular, unbranched, centric, 6–10 μm long.
Culture characteristics: Colonies on PDA reaching 60–80 mm diam after 7 d at 25 °C, undulate at the edge, whitish to pale honey-coloured, with black, gregarious conidiomata; reverse pale honey-coloured.
Habitat: On Hibiscus rosa-sinensis.
Known distribution: India.
Material examined: India, Bangalore, on Hibiscus rosa-sinensis, Aug. 1978, H.C. Govindu (CBS H-21749, holotype, ex-type culture CBS 459.78).
Notes: This species is characterised by large conidia (32.5–36 × 7–8.5 μm) with three median cells that are dark in colour. It forms a sister group (Fig. 4) with Ps. cocos and Ps. theae. Pseudopestalotiopsis indica differs from Ps. cocos (20–26.5 × 6–7.5 μm) and Ps. theae (22–32 × 5–8 μm) in its larger conidia.
Pseudoestalotiopsis theae (Sawada) Maharachch., K.D. Hyde & Crous, comb. nov. MycoBank MB809756.
Basionym: Pestalotia theae Sawada, Spec. Report Agric. Exp. Station Formosa 11: 113. 1915. as “Pestalozzia”
≡ Pestalotiopsis theae (Sawada) Steyaert, Bull. Jard. bot. État Brux. 19: 327. 1949.
Materials examined: Taiwan, Republic of China, Taipei, on living leaves of Camellia sinensis, 13 Jul. 1908, Y. Fujikiro, det. K. Sawada (BPI 406804, lectotype). Thailand, Chiang Mai Prov., Mae Taeng Distr., Ban Pha Deng, Mushroom Research Centre, 19°17.123′N 98°44.009′E, 900 m, rainforest, on living leaves of Camellia sinensis, 20 Jan. 2010, S.S.N. Maharachchikumbura (MFLU 12-0116, epitype, ex-epitype culture MFLUCC 12-0055 = CPC 20281); on living leaves of Camellia sinensis, unknown collection date and collector, culture SC011.
Discussion
Winter (1887) established the Amphisphaeriaceae, which is characterised by having immersed ascomata in the host and with dark peridial walls and ascal apices that are usually amyloid (Barr 1975). The Amphisphaeriaceae is a large heterogeneous family, which mainly possesses pestalotiopsis-like asexual states (Jeewon et al. 2002). These conidial forms are generally characterised by septate conidia with filiform apical appendages (Barr 1990, Nag Raj 1993) and with the exception of Bartalinia, Discosia and Monochaetia, most genera are linked to a sexual morph. Conidial septation appears to be effective in placement of taxa in genera of Amphisphaeriaceae. Sequence data generated to date reveal Truncatella, Pestalotiopsis and Seiridium to represent three distinct genera, which are characterised by 4-celled, 5-celled and 6-celled conidia, respectively. However, it has not been established whether, as defined, Pestalotia differs from Pestalotiopsis based on molecular evidence. Although they are clearly distinct in conidial morphology, Pestalotiopsis has 5-celled conidia while Pestalotia has 6-celled conidia. From a phenotypic viewpoint, Pestalotia species are more similar to Seiridium species, as both have 6-celled conidial forms. The type species of Pestalotia, P. pezizoides, can be distinguished from Seiridium species by its more numerous appendages, which are branched, while in Seiridium appendages are fewer and generally unbranched. However, branched apical appendages typical of Pestalotia are also found in S. corni and S. venetum (Nag Raj 1993), and thus Pestalotia could potentially prove to be congeneric with Seiridium. Appendage morphology appears to be highly informative at the species level, even though conidial appendages alone cannot be used as a useful character for generic separation (Crous et al. 2012). The monotypic genus Pestalotia (1839) may therefore be a synonym of Seiridium (1816), since both genera have similar morphologies. However, Guba's (1961) treatment of Monochaetia as a distinct genus has proved valid. LSU phylogenetic analyses reveal Monochaetia to represent a genus that is distinct from Pestalotiopsis, Seiridium and Truncatella (Fig 3). However, it is essential to incorporate molecular data and more taxon sampling in future analyses as Monochaetia includes 3-, 4-, and 6-celled conidial forms.
Pestalotiopsis species are morphologically diverse in conidial morphology, and phylogenetic analyses of different gene regions have established that Pestalotiopsis comprises three distinct lineages (Jeewon et al. 2003, Maharachchikumbura et al. 2011, 2012). Based on these findings, we divided Pestalotiopsis into three genera: Pestalotiopsis, Neopestalotiopsis and Pseudopestalotiopsis. However, our phylogenetic analyses disagree with Nag Raj's (1993) broad concept of Pestalotiopsis, which included 3-celled, and 4-celled conidial forms. All species within Neopestalotiopsis, Pestalotiopsis and Pseudopestalotiopsis contain only 4-celled conidial forms. Pestalotiopsis maculans, which is the type species of Pestalotiopsis, commonly occurs on Camellia and provides a stable generic concept for Pestalotiopsis. In P. maculans conidiophores are septate, unbranched and often reduced to conidiogenous cells; conidiogenous cells are ampulliform to lageniform or cylindrical to subcylindrical phialides, and conidia have concolourous median cells. Neopestalotiopsis has versicolourous median cells with indistinct conidiophores, while Pseudopestalotiopsis can be distinguished from Pestalotiopsis by sequence data and generally dark-coloured concolourous median cells with indistinct conidiophores. The three genera can also be roughly assigned to distinct groups based on the total number of base pairs in the ITS region.
Pestalotiopsis is a species-rich asexual morph-typified genus with only 13 known sexual states, as compared to the possible 253 asexual names (Zhang et al. 2012a, Maharachchikumbra et al. 2013d). Of the 13 sexually reproducing species, nine are linked to named Pestalotiopsis species and eight have concolourous median cells typical of Pestalotiopsis. Pestalosphaeria maculiformans is linked to Pestalotiopsis maculiformans (Marincowitz et al. 2008), which has versicolourous median cells, hence, belongs in Neopestalotiopsis. However, based on a megablast search of NCBIs GenBank nucleotide database for this species (CBS 122683, GenBank EU552147), the closest hits using the ITS sequence had highest similarity to Pestalotiopsis (species with concolourous median cells). Therefore, presently the known asexual states of Pestalosphaeria are Pestalotiopsis species. Because only one name can be applied to any fungal species (Hawksworth et al. 2011, Taylor 2011, Wingfield et al. 2012) and since Pestalotiopsis is the oldest and the most common name; Maharachchikumbura et al. (2011) suggested that Pestalotiopsis should be adopted for this genus. This has been followed in subsequent publications and is followed in this paper (Maharachchikumbura et al. 2012, Zhang et al. 2012a).
Conidial morphology is the most widely used taxonomic character for inter-specific delineation of Pestalotiopsis (Steyaert 1949, Guba 1961, Nag Raj 1993). However, there are considerable overlapping phenotypic characteristics that make it difficult to segregate morphologically equivocal taxa (Tejesvi et al. 2009). Conidial length and width have been emphasised as crucial characters for species identification, and many contemporary researchers have used length and width to segregate taxa (Steyaert 1949, Guba 1961, Mordue 1985). In the present study, however, species sharing similar conidial dimensions did not necessarily group together. As an example, P. malayana (clade 30; Fig. 5) and P. biciliata (clade 38; Fig. 5) have similar conidial dimensions, but cluster in distinct clades. Therefore, the continued use of conidium length and width in classification for Pestalotiopsis species is unwise. A similar observation was made by Dube & Bilgrami (1965) who showed that conidial size is a homoplasious character and species sharing similar spore sizes may not be closely related (Jeewon et al. 2003).
Various features/aspects of conidial appendages are taxonomically informative at the species level in many coelomycetous genera (Nag Raj 1993, Crous et al. 2012). The function of appendages should not be considered in isolation since appendages usually relate to an ecological function linked to spore dispersal, liberation, deposition and the colonisation of new substrates or niches (Gregory 1952, Crous et al. 2012). Watanabe et al. (2000) investigated the conidial adhesion and germination of Pestalotiopsis neglecta and observed that apical appendages firmly attached conidia to the substrate during the infection process. Generally in Neopestalotiopsis, Pestalotiopsis and Pseudopestalotiopsis apical appendages arise as tubular extensions and maintain protoplasmic continuity with the conidium body. Appendage morphology has been widely used in Pestalotiopsis taxonomy to introduce novel taxa (Steyaert 1949, Guba 1961, Nag Raj 1993, Maharachchikumbura et al. 2013a, Zhang et al. 2012b). Among the appendage-bearing coelomycetes, Pestalotiopsis shows high variation in appendage morphology. These apical appendage characters vary in length of the apical appendage, appendage number, shape, branched or unbranched nature, presence or absence of knobbed tips and the position of the apical appendage attached to the conidial body.
The ecology of species of Pestalotiopsis is poorly understood, especially now that species have been recircumscribed using molecular data. There is little data on geographical distribution and even host range. Since our data set is not robust, it is not clear whether the geographic influences or hosts range or allopatry play a key role in species circumscription and delineation. Therefore, much research is needed and it might be useful to account for substrate, geographic influences, host ranges, and morphological characters when incorporating molecular sequence data to define species borders within Neopestalotiopsis, Pestalotiopsis and Pseudopestalotiopsis. This kind of approach has been successfully used in the past to investigate species in for example Cladosporium (Bensch et al. 2012), Colletotrichum (Damm et al. 2012), Diaporthe (Gomes et al. 2013) and Teratosphaeriaceae (Quaedvlieg et al. 2014).
Common problems in Pestalotiopsis taxonomy are that new species (e.g. P. alpiniae, P. oenotherae and P. nelumbinis) have been defined without accompanying sequence data. In fact, in 2011 there were only four ex-type cultures available for this study on Pestalotiopsis phylogeny. In the first inclusive phylogenetic study of Pestalotiopsis, Jeewon et al. (2003) used ITS sequence data to evaluate the phylogenetic significance of Pestalotiopsis morphological characters in taxonomy. In differentiating endophytic species of Pestalotiopsis in Pinus armandii and Ribes spp., Hu et al. (2007) pointed out that the TUB gene better resolved Pestalotiopsis phylogeny. A combination of both the TUB and ITS genes gave better phylogenetic resolution, and they suggested that at least two genes should be used to resolve the phylogeny of species of Pestalotiopsis. Maharachchikumbura et al. (2012) tested 10 gene regions to resolve species boundaries in the Pestalotiopsis (actin, calmodulin, glutamine synthase, glyceraldehyde-3-phosphate dehydrogenase, ITS, LSU, 18S nrDNA, RNA polymerase II, TEF and TUB). The authors compared the morphological versus sequence data from each gene to establish which characters satisfactorily resolved species limits and ITS, TUB and TEF proved to be the better molecular markers. In the present study, phylogenetic species recognition based on combined ITS, TUB and TEF gene regions gave a high number of strongly supported nodes at the terminal clades. In Neopestalotiopsis however, overall branch-length support values were lower, when compared to Pestalotiopsis. Future studies of Neopestalotiopsis may require additional loci to obtain a better separation of species.
The genus Pestalotiopsis has been shown to produce numerous secondary metabolites with diverse structural features, with antitumour, antifungal, antimicrobial and other activities (Xu et al. 2010, 2014). Three reviews have been recently published and reveal the chemistry of Pestalotiopsis species and related genera. Species belonging to these genera are a rich source for bioprospecting when compared to other fungal genera (Aly et al. 2010, Xu et al. 2010, 2014). Xu et al. (2010) discussed 130 diverse compounds isolated from species of Pestalotiopsis in the past 10 years, while Xu et al. (2014) discussed a further 160 compounds. These biochemicals may have significance in pharmaceutical, agricultural and industrial applications. The names assigned to Pestalotiopsis species producing novel compounds lacked a phylogenetic basis (Maharachchikumbura et al. 2012, 2013c). It would be interesting to establish if different species of Pestalotiopsis were chemically more creative than others and also to establish if Neopestalotiopsis and Pseudopestalotiopsis species are different from Pestalotiopsis species in this regard.
Pestalotiopsis species are important causal agents of plant disease (Keith et al. 2006, Joshi et al. 2009, Keith & Zee 2010, Chen et al. 2011, Evidente et al. 2012, Maharachchikumbura et al. 2013a,b,c), chemically highly diverse (Aly et al. 2010, Xu et al. 2014), extremely common in most habitats (Bate-Smith & Metcalfe 1957, Jeewon et al. 2004, Maharachchikumbura et al. 2011) and are fascinating because of their distinct conidial morphology (Sutton 1980, Nag Raj 1993); thus they are a remarkable group of fungi that have been well-studied morphologically in the past (Steyaert 1949, Maharachchikumbura et al. 2013a,b). In this study we advance the understanding of this group using morphology and multilocus sequence analyses and introduce two new genera, Neopestalotiopsis and Pseudopestalotiopsis, to accommodate seggregates of Pestalotiopsis. Phenotypic analyses of conidial characters coupled with phylogenetic analyses of sequence data were used to clarify species boundaries in the three genera. Although genetic differences exist, several isolates were not assigned to species because of sterile cultures and lack of data on geographical differences; thus the data were insufficient to determine species boundaries in those cases. Sequence data are provided for 24 species of Neopestalotiopsis, 43 species of Pestalotiopsis and three species of Pseudopestalotiopsis and can be used in future studies to increase the understanding of this group. We predict that future studies will reveal numerous distinct and new taxa in this generic complex.
Acknowledgements
We thank the Kunming Institute of Botany, Chinese Academy of Sciences for providing facilities and the World Agroforestry Centre, East and Central Asia office for hosting us, we would also like to thank Humidtropics, a CGIAR Research Program that aims to develop new opportunities for improved livelihoods in a sustainable environment, for partially funding this work, and the National Research Council of Thailand (grant for Pestalotiopsis No: 55201020008). We thank the CBS-KNAW Fungal Biodiversity Centre for funding, and the technical staff, Arien van Iperen (cultures) and Mieke Starink-Willemse (DNA isolation, amplification, and sequencing) for their invaluable assistance.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Aly A.H., Debbab A., Kjer J. Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Diversity. 2010;41:1–16. [Google Scholar]
- Barr M.E. Pestalosphaeria, a new genus in the Amphisphaeriaceae. Mycologia. 1975;67:187–194. [Google Scholar]
- Barr M.E. Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon. 1990;39:43–184. [Google Scholar]
- Bate-Smith E.C., Metcalfe C.R. Leucanthocyanins. 3. The nature and systematic distribution of tannin in dicotyledonous plants. The Journal of the Linnean Society, Botany. 1957;55:669–705. [Google Scholar]
- Bensch K., Braun U., Groenewald J.Z. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyma F.H. van. Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland), VI. Mitteilung. Antonie van Leeuwenhoek. 1940;6:263–290. [PubMed] [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chen C.Q., Zhang B., Yang L.N. Identification and biological characteristics of round leaf spot on blueberry caused by Pestalotiopsis photiniae. Journal of Northeast Forestry University. 2011;39:95–98. (in Chinese) [Google Scholar]
- Corda A.C.J. Vol. 3. 1839. pp. 1–55. (Icones fungorum hucusque cognitorum). [Google Scholar]
- Crous P.W., Braun U., Hunter G.C. Phylogenetic lineages in Pseudocercospora. Studies in Mycology. 2013;75:37–114. doi: 10.3114/sim0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Summerell B.A., Swart L. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Christensen M. How important are conidial appendages? Persoonia. 2012;28:126–137. doi: 10.3767/003158512X652624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. Eucalyptus microfungi known from culture. 1. Cladoriella and Fulvoflamma genera nova, with notes on some other poorly known taxa. Studies in Mycology. 2006;55:53–63. doi: 10.3114/sim.55.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., editors. Fungal biodiversity. Vol. 1. Centraalbureau voor Schimmelcultures; Utrecht, Netherlands: 2009. pp. 1–269. (CBS laboratory manual series). [Google Scholar]
- Damm U., Cannon P.F., Woudenberg J.H.C. The Colletotrichum boninense species complex. Studies in Mycology. 2012;73:1–36. doi: 10.3114/sim0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbab A., Aly A.H., Proksch P. Mangrove derived fungal endophytes – a chemical and biological perception. Fungal Diversity. 2013;61:1–27. [Google Scholar]
- Dube H.C., Bilgrami K.S. Pestalotia or Pestalotiopsis? Mycopathologia et Mycologia Applicata. 1965;29:33–54. [Google Scholar]
- Ellis M.B., Ellis J.P. Richmond Publishing; England: 1997. Microfungi on land plants – an identification handbook. [Google Scholar]
- Evidente A., Zonno M.C., Andolfi A. Phytotoxic a-pyrones produced by Pestalotiopsis guepinii, the causal agent of hazelnut twig blight. The Journal of Antibiotics. 2012;65:203–206. doi: 10.1038/ja.2011.134. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R.R., Glienke C., Videira S.I.R. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory P.H. Fungus spores. Transactions of the British Mycological Society. 1952;35:1–18. [Google Scholar]
- Griffiths D.A., Swart H.J. Conidial structure in two species of Pestalotiopsis. Transactions of the British Mycological Society. 1974;62:295–304. [Google Scholar]
- Griffiths D.A., Swart H.J. Conidial structure in Pestalotia pezizoides. Transactions of the British Mycological Society. 1974;63:169–173. [Google Scholar]
- Guba E.F. Monochaetia and Pestalotia vs. Truncatella, Pestalotiopsis and Pestalotia. Annals of Microbiology. 1956;7:74–76. [Google Scholar]
- Guba E.F. Harvard University Press; Cambridge: 1961. Monograph of Pestalotia and Monochaetia. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hawksworth D.L., Crous P.W., Redhead S.A. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.L., Jeewon R., Zhou D.Q. Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: evidence from rDNA and β-tubulin gene phylogenies. Fungal Diversity. 2007;24:1–22. [Google Scholar]
- Hughes S.J. Revisiones Hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Ismail A.M., Cirvilleri G., Polizzi G. Characterisation and pathogenicity of Pestalotiopsis uvicola and Pestalotiopsis clavispora causing grey leaf spot of mango (Mangifera indica L.) in Italy. European Journal of Plant Pathology. 2013;135:619–625. [Google Scholar]
- Jeewon R., Liew E.C.Y., Hyde K.D. Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Molecular Phylogenetics and Evolution. 2002;25:378–392. doi: 10.1016/s1055-7903(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Jeewon R., Liew E.C.Y., Hyde K.D. Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity. 2004;17:39–55. [Google Scholar]
- Jeewon R., Liew E.C.Y., Simpson J.A. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution. 2003;27:372–383. doi: 10.1016/s1055-7903(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Joshi S.D., Sanjay R., Baby U.I. Molecular characterization of Pestalotiopsis spp. associated with tea (Camellia sinensis) in southern India using RAPD and ISSR markers. Indian Journal of Biotechnology. 2009;8:377–383. [Google Scholar]
- Kang J.C., Hyde K.D., Kong R.Y.C. Studies on the Amphisphaeriales. The Amphisphaeriaceae (sensu stricto) Mycological Research. 1999;103:53–64. [Google Scholar]
- Keith L.M., Velasquez M.E., Zee F.T. Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava in Hawaii. Plant Disease. 2006;90:16–23. doi: 10.1094/PD-90-0016. [DOI] [PubMed] [Google Scholar]
- Keith L.M., Zee F.T. Guava disease in Hawaii and the characterization of Pestalotiopsis spp. affecting guava. Acta Horticulturae (ISHS) 2010;849:269–276. [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data. Journal of Molecular Evolution. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Peterson D. MEGA-CC: Computing Core of Molecular Evolutionary Genetics Analysis Program for Automated and Iterative Data Analysis. Bioinformatics. 2012;28:2685–2686. doi: 10.1093/bioinformatics/bts507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Crous P.W., Wingfield M.J. Pestalotioid fungi from Restionaceae in the Cape Floral Kingdom. Studies in Mycology. 2006;55:175–187. doi: 10.3114/sim.55.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Groenewald J.Z., Crous P.W. Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology. 2004;50:235–252. [Google Scholar]
- Liu A.R., Chen S.C., Wu S.Y. Cultural studies coupled with DNA based sequence analyses and its implication on pigmentation as a phylogenetic marker in Pestalotiopsis taxonomy. Molecular Phylogenetics and Evolution. 2010;57:528–535. doi: 10.1016/j.ympev.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Maddison W.P., Maddison D.R. 2011. Mesquite: a modular system for evolutionary analysis.http://mesquiteproject.org Version 2.75. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Cai L. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Diversity. 2012;56:95–129. [Google Scholar]
- Maharachchikumbura S.S.N., Chukeatirote E., Guo L.D. Pestalotiopsis species associated with Camellia sinensis (tea) Mycotaxon. 2013;123:47–61. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Chukeatirote E. Pestalotiopsis – morphology, phylogeny, biochemistry and diversity. Fungal Diversity. 2011;50:167–187. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Chukeatirote E. Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycological Progress. 2013;13:617–624. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Chukeatirote E. A destructive new disease of Syzygium samarangense in Thailand caused by the new species Pestalotiopsis samarangensis. Tropical Plant Pathology. 2013;38:227–235. [Google Scholar]
- Maharachchikumbura S.S.N., Zhang Y.M., Wang Y. Pestalotiopsis anacardiacearum sp. nov. (Amphisphaeriaceae) has an intricate relationship with Penicillaria jocosatrix, the mango tip borer. Phytotaxa. 2013;99:49–57. [Google Scholar]
- Marincowitz S., Crous P.W., Groenewald J.Z. Micro-fungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series. 2008;7:1–166. [Google Scholar]
- Monden Y., Yamamoto S., Yamakawa R. First case of fungal keratitis caused by Pestalotiopsis clavispora. Clinical Ophthalmology. 2013;7:2261–2264. doi: 10.2147/OPTH.S48732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue J.E.M. An unusual species of Pestalotiopsis: P. steyaertii sp. nov. Transactions of the British Mycological Society. 1985;85:378–380. [Google Scholar]
- Moreau C. Micomycetes africains. I. Revue de Mycologie, Suppliment Colonial (Paris) 1949;14:15–22. [Google Scholar]
- Nag Raj T.R. Redisposals and redescriptions in the Monochaetia-Seiridium, Pestalotia-Pestalotiopsis complexes. II. Pestalotiopsis besseyii (Guba) comb. nov. and Pestalosphaeria varia sp. nov. Mycotaxon. 1985;22:52–63. [Google Scholar]
- Nag Raj T.R. Mycologue Publications; Waterloo, Ontario, Canada: 1993. Coelomycetous anamorphs with appendage-bearing conidia. [Google Scholar]
- Nylander J.A.A. Vol. 2. Evolutionary Biology Centre, Uppsala University; 2004. pp. 1–2. (MrModeltest v2.2. Program distributed by the author). [Google Scholar]
- O'Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- O'Donnell K., Kistler H.C., Cigelnik E. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of United States of America. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research. 1994;98:625–634. [Google Scholar]
- Ren H.Y., Li G., Qi X.J. Identification and characterization of Pestalotiopsis spp. causing twig blight disease of bayberry (Myrica rubra Sieb. & Zucc) in China. European Journal of Plant Pathology. 2013;137:451–461. [Google Scholar]
- Ronquist F., Teslenko M., Mark P. van der. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangchote S., Farungsang U., Farungsang N. Pre and postharvest infection of rambutan by pathogens and effect on postharvest treatments. In: Coates L.M., Hofman P.J., Johnson G.I., editors. Disease control and storage life extension in fruits. Vol. 81. 1998. pp. 87–91. (ACIAR Proceedings). [Google Scholar]
- Silvestro D., Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution. 2011;12:335–337. [Google Scholar]
- Steyaert R.L. Contributions à l'étude monographique de Pestalotia de Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.) Bulletin Jardin Botanique État Bruxelles. 1949;19:285–354. [Google Scholar]
- Steyaert R.L. New and old species of Pestalotiopsis. Transactions of the British Mycological Society. 1953;36:81–89. [Google Scholar]
- Steyaert R.L. Pestalotiopsis from the Gold Coast and Togoland. Transactions of the British Mycological Society. 1953;36:235–242. [Google Scholar]
- Steyaert R.L. Pestalotia, Pestalotiopsis et Truncatella. Bulletin Jardin Botanique État Bruxelles. 1955;25:191–199. [Google Scholar]
- Steyaert R.L. A reply and an appeal to Professor Guba. Mycologia. 1956;48:767–768. [Google Scholar]
- Steyaert R.L. Type specimens of Spegazzini's collections in the Pestalotiopsis and related genera (Fungi Imperfecti: Melanconiales) Darwinia (Buenos Aires) 1961;12:157–190. [Google Scholar]
- Steyaert R.L. Complementary informations concerning Pestalotiopsis guepini (Desmazieres) Steyaert and designation of its lectotype. Bulletin Jardin Botanique l'État Bruxelles. 1963;33:369–373. [Google Scholar]
- Strobel G., Yang X.S., Sears J. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology. 1996;142:435–440. doi: 10.1099/13500872-142-2-435. [DOI] [PubMed] [Google Scholar]
- Sun H.T., Cao R.B. Identification of Pestalotiopsis parasitized on fruit crops. Acta Agriculturae University Zhejiangensis. 1990;16:179–185. (in Chinese) [Google Scholar]
- Sutton B.C. Forest microfungi. III. The heterogeneity of Pestalotia de Not. section Sexloculatae Klebahn sensu Guba. Canadian Journal of Botany. 1969;48:2083–2094. [Google Scholar]
- Sutton B.C. Commonwealth Mycological Institute; Kew, Surrey, UK: 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. [Google Scholar]
- Sutton D.A. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Revista Iberoamericana de Micología. 1999;16:171–179. [PubMed] [Google Scholar]
- Swart L., Taylor J.E., Crous P.W. Pestalotiopsis leaf spot disease of Proteaceae in Zimbabwe. South African Journal of Botany. 1999;65:239–242. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) Version 4. [Google Scholar]
- Taylor J.E. Proteaceae pathogens: the significance of their distribution in relation to recent changes in phytosanitary regulations. Acta Horticulturae. 2001;545:253–264. [Google Scholar]
- Taylor J.W. One Fungus = One Name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus. 2011;2:113–120. doi: 10.5598/imafungus.2011.02.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejesvi M.V., Nalini M.S., Mahesh B. New hopes from endophytic fungal secondary metabolites. Boletín de la Sociedad Química de México. 2007;1:19–26. [Google Scholar]
- Tejesvi M.V., Tamhankar S.A., Kini K.R. Phylogenetic analysis of endophytic Pestalotiopsis species from ethnopharmaceutically important medicinal trees. Fungal Diversity. 2009;38:167–183. [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4239–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Motohashi K., Ono Y. Description of Pestalotiopsis pallidotheae: a new species from Japan. Mycoscience. 2010;51:182–188. [Google Scholar]
- Watanabe K., Parbery D.G., Kobayashi T. Conidial adhesion and germination of Pestalotiopsis neglecta. Mycological Research. 2000;104:962–968. [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, California: 1990. pp. 315–322. [Google Scholar]
- Wingfield M.J., De Beer Z.W., Slippers B. One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology. 2012;13:604–613. doi: 10.1111/j.1364-3703.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G (1887). Pilze; Ascomyceten. In: Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz [i–vi] 1(2): 1–928.
- Xu J., Ebada S.S., Proksch P. Pestalotiopsis a highly creative genus: chemistry and bioactivity of secondary metabolites. Fungal Diversity. 2010;44:15–31. [Google Scholar]
- Xu L., Kusakari S., Hosomi A. Postharvest disease of grape caused by Pestalotiopsis species. Annals of the Phytopathological Society of Japan. 1999;65:305–311. [Google Scholar]
- Xu J., Yang X., Lin Q. Chemistry and biology of Pestalotiopsis-derived natural products. Fungal Diversity. 2014;66:37–68. [Google Scholar]
- Zhang Y.M., Maharachchikumbura S.S.N., McKenzie E.H.C. A novel species of Pestalotiopsis causing leaf spots of Trachycarpus fortunei. Cryptogamie Mycologie. 2012;33:1–8. [Google Scholar]
- Zhang Y.M., Maharachchikumbura S.S.N., Tian Q. Pestalotiopsis species on ornamental plants in Yunnan Province, China. Sydowia. 2013;65:59–74. [Google Scholar]
- Zhang Y.M., Maharachchikumbura S.S.N., Wei J.G. Pestalotiopsis camelliae, a new species associated with grey blight of Camellia japonica in China. Sydowia. 2012;64:335–344. [Google Scholar]


