Abstract

This paper describes the role of α-subunit VISIT-DG sequence residue αThr-349 in the catalytic sites of Escherichia coli F1Fo ATP synthase. X-ray structures show the highly conserved αThr-349 in the proximity (2.68 Å) of the conserved phosphate binding residue βR182 in the phosphate binding subdomain. αT349A, -D, -Q, and -R mutations caused 90–100-fold losses of oxidative phosphorylation and reduced ATPase activity of F1Fo in membranes. Double mutation αT349R/βR182A was able to partially compensate for the absence of known phosphate binding residue βR182. Azide, fluoroaluminate, and fluoroscandium caused insignificant inhibition of αT349A, -D, and -Q mutants, slight inhibition of the αT349R mutant, partial inhibition of the αT349R/βR182A double mutant, and complete inhibition of the wild type. Whereas NBD-Cl (7-chloro-4-nitrobenzo-2-oxa-1,3-diazole) inhibited wild-type ATPase and its αT349A, -D, -R, and -Q mutants essentially completely, βR182A ATPase and double mutant αT349A/βR182A were inhibited partially. Inhibition characteristics supported the conclusion that NBD-Cl reacts in βE (empty) catalytic sites, as shown previously by X-ray structure analysis. Phosphate protected against NBD-Cl inhibition in the wild type, αT349R, and double mutant αT349R/βR182A but not in αT349A, αT349D, or αT349Q. The results demonstrate that αThr-349 is a supplementary residue involved in phosphate binding and transition state stabilization in ATP synthase catalytic sites through its interaction with βR182.
In a 75 year life span, a typical 70 kg human generates approximately 2.0 million kg of ATP. The cell’s energy currency is generated by converting food into useable energy by oxidation. F1Fo ATP synthase is responsible for the fundamental means of cell energy production in animals, plants, and almost all microorganisms, which occurs by oxidation or photophosphorylation in membranes of bacteria, mitochondria, and chloroplasts. ATP synthase is one of the smallest biological nanomotors and is structurally similar in all species.1−4 In its simplest form, as in Escherichia coli, it contains eight different subunits distributed in the water-soluble F1 sector (subunits α3β3γδε) and the membrane-associated Fo sector (subunits ab2c10). The total molecular size is ∼530 kDa.4 In chloroplasts, there are two isoforms of subunit b. In mitochondria, there are seven to nine additional subunits, depending on the source, but in total, they contribute only a small fraction of additional mass and may have regulatory roles.5−7
The membrane-bound F1Fo ATP synthase enzyme is highly conserved and structurally identical among different species. X-ray structures of bovine enzyme8 established the presence of three catalytic sites at α-subunit−β-subunit interfaces of the α3β3 hexamer. ATP hydrolysis and synthesis occur in the F1 sector, whereas proton transport occurs through the membrane-embedded Fo.8,9 ATP synthesis is a result of proton gradient-driven clockwise rotation of γ (as viewed from the outer membrane), while ATP hydrolysis results in anticlockwise rotation of the γ-subunit. Detailed reviews of ATP synthase structure and function may be found in refs (10−18).
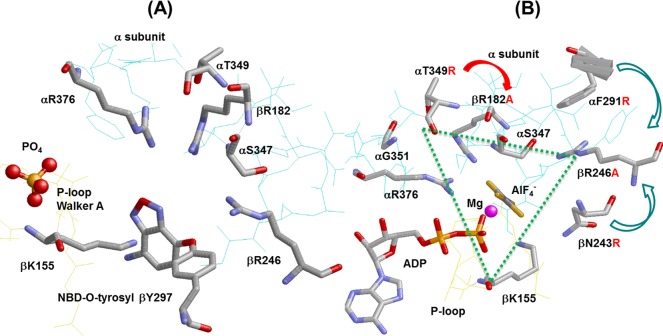
A precise knowledge of Pi (inorganic phosphate) binding is not only essential for following the reaction mechanism of ATP synthesis and hydrolysis but also equally important for understanding the relationship between catalytic mechanism and mechanical rotation in this biological nanomotor. For this reason, we have focused our efforts on determining the role of conserved residues in and around catalytic site Pi binding subdomain.19 Knowledge of Pi binding residues and residues surrounding the Pi binding subdomain is imperative for (i) the molecular modulation of the catalytic site(s) for the improved catalytic and motor function of this enzyme, (ii) an explanation of how ATP synthase binds ADP and Pi within its catalytic sites in the face of a relatively high ATP/ADP concentration ratio, and (iii) understanding the relationship between Pi binding and subunit rotation.20−22 Many earlier attempts to measure Pi binding in purified E. coli F1 failed to detect appreciable Pi binding at physiological Pi concentrations,21,23,24 but modification of the assay devised by Perez et al.25 provides a useful measure of Pi binding. In this assay, protection is afforded by Pi against inhibition of ATPase activity induced by covalent reaction with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl). X-ray crystallography showed that the covalent interaction of NBD-Cl specifically with β297 occurs in the βE catalytic site26 (see Figure 1A); thus, protection afforded by Pi indicates that binding of Pi occurs at the βE catalytic site. Modification of the assay described above for E. coli, purified F1 or F1Fo membranes, previously allowed us to investigate the relationship between Pi binding and catalysis for eight residues, namely, βArg-246, βAsn-243, αArg-376, βLys-155, βArg-182, αPhe-291, αSer-347, and αGly-351.a Although all these residues are situated in the proximity of the phosphate analogues AlF3 or SO42– in X-ray structures of catalytic sites,27,28 we found that four residues, namely, βArg-246, αArg-376, βLys-155, and βArg-182, grouped in a triangular fashion, are directly involved in Pi binding while the fifth residue, αSer-347, is indirectly involved in Pi binding through its interaction with βArg-246 (see Figure 1B).19,29−35
Figure 1.
X-ray structures of catalytic sites in mitochondrial ATP synthase showing the spatial relationship of α-subunit VISIT-DG sequence residue αT349. (A) Reacted NBD-O-tyrosyl-297 in the βE site.26 (B) βDP site in the AlF4–-inhibited enzyme.28E. coli residue numbering is shown. The dotted triangle shows residues βLys-155, βArg-182, βArg-246, αArg-376, αSer-347, and αThr-349 forming a triangular Pi binding site. Rasmol was used to generate these structures.
The mechanism of condensation of Pi with MgADP proposed by Senior et al.9 was strengthened by the X-ray crystallography structure of bovine ATP synthase of Menz et al.28 showing the transition state analogue MgADP·AlF4– trapped in catalytic sites (Figure 1B). It is clear from the geometry of this complex that the fluoroaluminate group occupies the position of the ATP γ-phosphate in the predicted transition state. The first transition state-like structure of F1 from rat liver crystallized with the Pi analogue vanadate (Vi), reported by Pedersen’s group, demonstrated that ADP was not essential, suggesting that the MgVi–F1 complex inhibited the catalytic activity to the same extent as that observed for the MgADP–Vi–F1 complex.36 Neither purified F1 nor membrane-bound F1Fo from E. coli is inhibited by MgVi or MgADP–Vi.30 Consequently, we have relied on inhibition of ATPase activity by fluoroaluminate (or fluoroscandium) to assess the potential to stabilize a transition state complex.19,29−32,35
Cingolani and Duncan18 have resolved the first E. coli F1 sector high-resolution crystal structure in an autoinhibited conformation. This structure divulges a wealth of information about the regulatory features of bacterial and chloroplast ATP synthase. Moreover, the E. coli ATP synthase X-ray structure paves the way for the development of new antimicrobial drugs. For E. coli, ATP synthase is naturally a better candidate for antimicrobial drugs in comparison to mitochondrial ATP synthase.4,18 Newly developed anti-tuberculosis drug targeting bacterial ATP synthase corroborates this assertion.37 Because the E. coli high-resolution structure4 does not contain sulfate, phosphate, fluoroaluminate, or fluoroscandium, we have relied on the mitochondrial ATP synthase structure that is very similar to that of E. coli (∼70% homologous sequence) as this study deals with the analogues described above.27,28,38
Fortunately, by mutagenic analysis along with the NBD-Cl protection assay, as well as ATPase inhibition by transition state analogues, we can investigate the direct or indirect role of residues in Pi binding. In this work, we examine the role of the highly conserved α-subunit VISIT-DG sequence residue αThr-349 in the process of Pi binding. Figure 1B shows the position of αThr-349 with respect to other known Pi binding residues. The strategic position of αThr-349 in the Pi binding subdomain leads to the following basic questions: Is αThr-349 involved in Pi binding directly or indirectly? Do the αT349A, αT349D, αT349Q, and αT349R mutations have any effect on transition state formation? Also, can αT349R compensate for βArg-182, a known Pi binding residue?
Materials and Methods
Construction of Wild-Type and Mutant Strains of E. coli
The strain for wild-type E. coli was pBWU13.4/DK8.39 All the mutants were generated by the method of Vandeyar et al.40 The M13mp18 template containing the HindIII–XbaI fragment from pSN6 was used for oligonucleotide-directed mutagenesis. Plasmid pSN6 contains the βY331W mutation from plasmid pSWM441 introduced on a SacI–EagI fragment into pBWU13.4,39 which expresses all the ATP synthase genes. The following mutagenic oligonucleotides were used: αT349A, GTAATCTCTATAGCCGATGGTCAGATC, where the underlined bases introduce the mutation and a new SfcI restriction site; αT349D, GTAATCTCAATTGACGATGGTCAGATC, where the underlined bases introduce the mutation and a new MfeI restriction site; αT349Q, GTAATCTCCATTCAGGATGGTCAGATC, where the underlined base introduces new mutation αT349Q (ACC → CAG); αT349R, CGTAATCTCCATTCGCGATGGTCAGATC, where the underlined bases introduce the mutation and a new NruI restriction site; βR182A, GGCGTAGGTGAAGCTACTCGTGAGGG, where the underlined bases introduce the mutation and a new AluI restriction site. DNA sequencing was performed to confirm the presence of mutations and the absence of undesired changes in sequence, and the mutations were transferred to pSN6 on a Csp451 (an isoschizomer of BstBI) and the PmlI fragment generating the new plasmids pZA20(αT349A/βY331W), pZA21(αT349D/βY331W), pZA22(αT349Q/βY331W), pZA23(αT349R/βY331W), and pZA24(βR182A/βY331W). Double mutant pZA25 (αT349R/βR182A) was generated by combining the pZA23 fragment on the pZA24 plasmid at the Csp451 and PmlI site. Each plasmid was transformed into strain DK842 containing a deletion of ATP synthase genes for expression of the mutant enzymes. It may be noted that all mutant strains contained the βY331W mutation, which is valuable for measurement of nucleotide binding parameters41 and does not affect function significantly on its own. While the presence of the βY331W mutation was not utilized in this work, the Trp mutation was included for possible future use.
Preparation of E. coli Membranes, Measurement of Growth Yield in Limiting Glucose Medium, and Assay of ATPase Activity of Membranes
E. coli membrane-bound F1Fo were prepared by the method of Senior et al.43 Notably in this procedure, F1Fo-bound membrane initial pellets are washed three times. The first wash is conducted in buffer containing 50 mM TES (pH 7.0), 15% glycerol, 40 mM 6-aminohexanoic acid, and 5 mM p-aminobenzamidine. The next two washes are performed in buffer containing 5 mM TES (pH 7.0), 15% glycerol, 40 mM 6-aminohexanoic acid, 5 mM p-aminobenzamidine, 0.5 mM dithiothreitol (DTT), and 0.5 mM EDTA. Before the experiments, membranes were washed twice more by resuspension and ultracentrifugation in 50 mM TrisSO4 (pH 8.0) and 2.5 mM MgSO4. These extra washes are meant to reduce the null mutant to truly zero activity. Therefore, the low activities with the mutants must be coming from the mutants, not from any other contaminants. The experiments are performed to make sure that growth yield in limiting glucose was measured as described previously.44 Measurement of ATPase activity was performed in 1 mL of assay buffer containing 10 mM NaATP, 4 mM MgCl2, and 50 mM TrisSO4 (pH 8.5) at 37 °C. Reactions were started by the addition of membrane-bound F1Fo and stopped by addition of 1 mL of sodium dodecyl sulfate (SDS) to a final concentration of 3.3%. Release of Pi was measured as described in ref (45). Reaction times for the wild-type F1Fo membrane (20–30 μg of protein) were 5–10 min, while reaction times for F1Fo-bound mutant membranes (40–60 μg of protein) were 30–50 min. All reactions were found to be linear with time and protein concentration. The purity and integrity of membranes were checked by SDS gel electrophoresis on 10% acrylamide gels as described in ref (46) and immunoblotting with rabbit polyclonal anti-F1-α and anti-F1-β antibodies as described in ref (47).
Inhibition of ATPase Activity by NBD-Cl and Protection by MgADP or Pi
A stock solution of NBD-Cl was prepared in dimethyl sulfoxide (DMSO) and protected from light. F1Fo-bound membranes (0.2–0.5 mg/mL) were reacted with NBD-Cl for 60 min in the dark, at room temperature, in T8 [50 mM TrisSO4 (pH 8.0)] and 2.5 mM MgSO4. ATPase activity was determined by adding 50 μL aliquots from the assay described above to 1 mL of ATPase assay buffer. For protection from NBD-Cl inhibition by ADP or Pi, membranes were preincubated for 60 min with a protecting agent at room temperature before the addition of NBD-Cl. MgSO4 and ADP or Pi were present at equimolar concentrations in the reaction assay. Control samples containing the ligand without added NBD-Cl were included. Neither MgADP (up to 10 mM) nor Pi (up to 50 mM) had any inhibitory effect alone.
Reversal of NBD-Cl-Inhibited ATPase Activity by DTT
To determine the DTT-induced reversal of NBD-Cl inhibition, F1Fo-bound membranes were first reacted with NBD-Cl (150 μM) for 1 h at room temperature in the dark, and then DTT (final concentration of 4 mM) was added and incubation continued for 1 h at room temperature before the ATPase assay. Control samples without NBD-Cl and/or DTT were incubated for the same amounts of time.
Inhibition of ATPase Activity by Azide, Fluoroaluminate, or Fluoroscandium
For measurement of azide inhibition, membrane-bound F1Fo was preincubated with varied concentrations of sodium azide for 30 min. Then 1 mL of ATPase assay buffer was added to measure the activity. Measurements of fluoroaluminate or fluoroscandium inhibition were performed by incubating membrane-bound F1Fo for 60 min at room temperature in 50 mM TrisSO4, 2.5 mM MgSO4, 1 mM NaADP, and 10 mM NaF at a protein concentration of 0.2–0.5 mg/mL in the presence of varied concentrations of AlCl3 or ScCl3 (see Results); 50 μL aliquots were then added to 1 mL of ATPase assay buffer, and activity was measured as described above. It was confirmed in control experiments that no inhibition was seen if MgSO4, NaADP, or NaF was omitted.
Inhibition of ATPase Activity by Dicyclohexylcarbodiimide (DCCD)
The method of Weber et al.48 was used to covalently modify the wild-type and mutant F1Fo membrane by DCCD. Measurement of ATPase activity was done by adding 1 mL of ATPase assay buffer containing 10 mM NaATP, 4 mM MgCl2, and 50 mM TrisSO4 (pH 8.5) at 37 °C to the 100 μL aliquots of 16 h DCCD-modified ATP synthase.
Results
Growth Properties of αT349A, αT349D, αT349Q, αT349R, βR182A, and αT349R/βR182A Mutants of E. coli ATP Synthase
Five new single mutants, αT349A, αT349D, αT349Q, αT349R, and βR182A, and one double mutant, αT349R/βR182A, were generated. Residue αThr-349 was chosen for mutagenesis because of its high level of conservation in the α-subunit VISIT-DG sequence and proximity to the Pi binding pocket. The αT349A mutant was used to appreciate the role of the Thr-OH side chain in Pi binding and the transition state. The αT349Q mutant was designed to understand the impact of the larger side chain of Gln on αThr-349. αT349D and αT349R were constructed to establish the impact of negative and positive charge on the nearby βR182, a known Pi binding residue. It should be noted here that the growth properties of βR182A were in excellent agreement with those published previously9,33 for the purified F1E. coli ATP synthase. The motivation behind double mutant αT349R/βR182A was to determine if Arg on αT349 could compensate for the absence of Arg on βR182A.
Table 1 shows that introduction of Ala, Asp, Gln, or Arg as αT349A, αT349D, αT349Q, αT349R, and βR182A resulted in a loss of oxidative phosphorylation. All mutations barred growth on succinate-containing medium, and growth yields in limiting glucose medium were reduced close to those of the ATP synthase null control. Substantial oxidative phosphorylation was retained by double mutant αT349R/βR182A. Specific ATPase activities of membrane-bound F1Fo preparations containing mutant enzymes were compared with wild-type and null control values, and the values are listed in Table 1. Mutations αT349A, αT349D, αT349Q, αT349R, and βR182A reduced the ATPase activity by 90–100-fold, while double mutant αT349R/βR182A reduced ATPase activity ∼6-fold. SDS gel electrophoresis and immunoblotting experiments yielded results that were in excellent agreement with previously published data and confirmed that the same amounts of α- and β-subunits were present in membrane-bound F1Fo as in the wild type;19,29 therefore, reduced ATPase is not due to impaired assembly of ATP synthase or loss of F1 during membrane preparation. Moreover, three extra washes reduce the activity of the null mutant to truly zero. Thus, the low mutant activities can be attributed only to mutant F1.
Table 1. Effects of αT349A, -D, -Q, and -R and αT349R/βR182A Mutations on Cell Growth and ATPase Activity.
| speciesa | growth on succinateb | growth yield in limiting glucose (%) | ATPase activityc (μmol min–1 mg–1) |
|---|---|---|---|
| wild type | ++++ | 100 | 28 |
| null | – | 46 | 0 |
| βY331W | ++++ | 95 | 26 |
| αT349A | ± | 51 | 0.30 |
| αT349D | ± | 49 | 0.28 |
| αT349Q | ± | 47 | 0.29 |
| αT349R | ± | 50 | 0.31 |
| αT349R/βR182A | ++ | 66 | 4.40 |
Wild type, pBWU13.4/DK8; null, pUC118/DK8. αT349A, -D, -Q, and -R and αT349R/βR182A mutants were expressed with the βY331W mutation also present, which does not significantly affect growth. Data are means of four to six experiments each.
Growth on succinate plates after 3 days estimated by eye: ++++, heavy growth; ++, substantial growth; ±, very light growth; −, no growth.
ATPase activity measured at 37 °C and expressed as micromoles of ATP hydrolyzed per minute per milligram of membrane protein. Each individual experimental point is itself the mean of duplicate assay tubes. Data are derived from two separate membrane preparations. Results from separate membrane preparations were in excellent agreement within ±10%.
Inhibition of ATPase Activity of ATP Synthase in Membranes by NBD-Cl and Reversal by Dithiothreitol
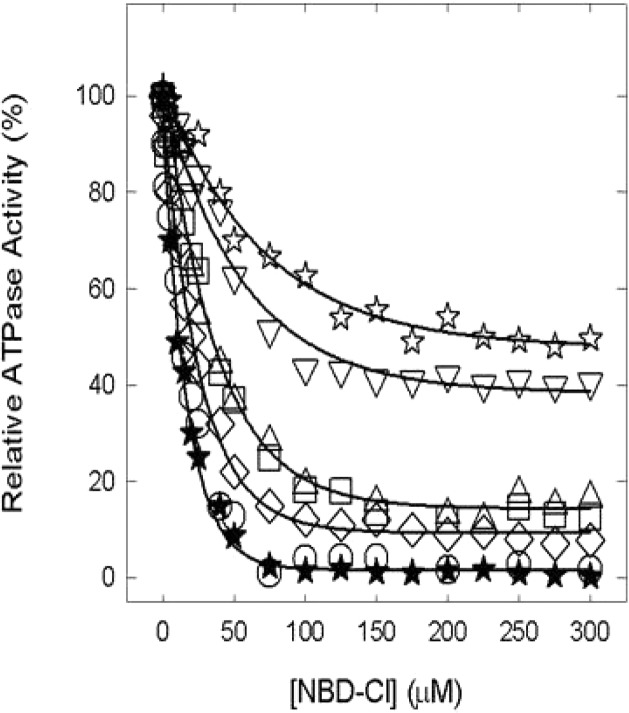
All the inhibition assays were conducted using membrane-bound F1Fo for both membrane preparations and the purified F1 preparation, provide equivalent assay results, and are highly convenient and less time-consuming.19,29,30,35,49−52 Figure 2 shows NBD-Cl-induced inhibition of wild-type and mutant membranes in the presence of varied concentrations of NBD-Cl. NBD-Cl caused potent inhibition of the wild type with no residual activity, and this is consistent with previous studies.19,29−33,35 The αT349A mutant was also almost completely inhibited, while αT349D, αT349Q, αT349R, βR182A, and αT349R/βR182A were inhibited by ∼85, 90, 90, 60, and 50%, respectively, with ∼10–50% residual activity. In previous studies, we have noted several instances in which mutant or wild-type ATP synthase was incompletely inhibited by inhibitors like fluoroaluminate, fluoroscandium, sodium azide, NBD-Cl, polyphenols, and peptides.19,29−33,35,49−52 To authenticate that maximal reaction with NBD-Cl had been achieved, we incubated each membrane-bound F1Fo preparation with 150 μM NBD-Cl for 1 h as in Figure 2, followed by a supplementary amount of 200 μM NBD-Cl (totaling 350 μM) and continuing the incubation for an extra hour before assaying ATPase activity. As expected, very little or no additional inhibition occurred (Figure 3A). This demonstrates that the reaction of NBD-Cl was complete and that fully reacted αT349D, αT349Q, αT349R, βR182A, and αT349R/βR182A mutant F1Fo membranes retained residual activity. Subsequently, we checked if inactivation by NBD-Cl could be reversed by addition of the reducing agent DTT because reversibility by DTT was indicative of specificity of reaction in previous studies. In this case, wild-type and mutant enzymes were preincubated with 150 μM NBD-Cl as in Figure 2 and then 4 mM DTT was added and incubation continued for 1 h before the ATPase assay. It can be seen in Figure 3B that DTT completely restored full activity in all cases. This proves that NBD-Cl reacts specifically with residue βTyr-297 in the wild type as well as in six other mutants.53,54
Figure 2.
Inhibition of membrane-bound wild-type and mutant ATP synthase by NBD-Cl. Membranes were preincubated for 60 min at room temperature with varied concentrations of NBD-Cl; then aliquots were added to 1 mL of assay buffer, and ATPase activity was determined. Details are given in Materials and Methods. Symbols: (★) wild type, (○) αT349A, (□) αT349D, (◇) αT349Q, (△) αT349R, (▽) βR182A, and (☆) αT349R/βR182A. Each data point represents an average of at least four experiments, using two or three independent membrane preparations of each mutant. Results agreed within ±10%.
Figure 3.
Results of an extra pulse of NBD-Cl in mutants and reversal of NBD-Cl effects by DTT. (A) Membrane ATP synthase (Mbr) was inhibited with 150 μM NBD-Cl for 60 min under the conditions described in the legend of Figure 2. Then, a further pulse of 200 μM NBD-Cl was added and incubation continued for 1 h before the assay. (B) Membrane ATP synthase (Mbr) was incubated with or without 150 μM NBD-Cl for 60 min under the conditions described in the legend of Figure 2. The degree of inhibition was assayed. In parallel samples, 4 mM DTT was then added, and incubation was continued for a further 60 min before the assay. Each bar graph represents wild type, αT349A, αT349D, αT349Q, αT349R, βR182A, and αT349R/βR182A from left to right.
Protection against NBD-Cl Inhibition of ATPase Activity by MgADP or Pi
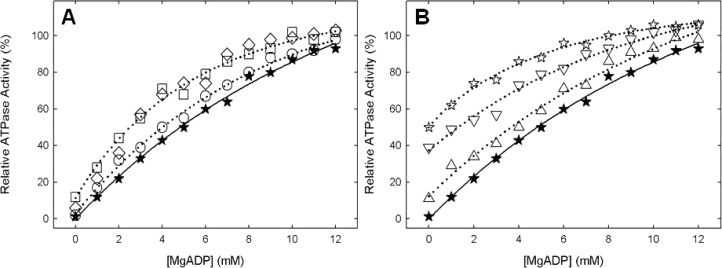
Panels A and B of Figure 4 show the MgADP protection data against NBD-Cl in wild-type and membrane-bound F1Fo enzymes. It is seen that wild-type and mutant membranes were similarly protected against NBD-Cl inhibition. Earlier, we have shown that MgADP protects against NBD-Cl inhibition of wild-type soluble F1 as well as membrane preparations of F1Fo; however, protection occurred only at high concentrations (EC50 ∼ 4.5 mM MgADP). In this study, the EC50 values were 4.2, 3.1, 3.1, 4.4, 3.6, 2.5, and 4.8 mM for αT349A, αT349D, αT349Q, αT349R, βR182A, αT349R/βR182A, and the wild type, respectively. We surmise that high concentrations are required to effectively keep the βE site occupied by MgADP in time average and thus hold back the access to NBD-Cl by sterically obstructing the site.19,29−35 This scheme is consistent with the conclusion of Orris et al.,26 who provided X-ray crystallographic proof that NBD-Cl reacts specifically in the βE catalytic site. Therefore, we conclude that NBD-Cl is reacting in βE in the mutants and that the ATPase activities measured in the mutants can be attributed to the ATP synthase enzyme and not to a contaminant.
Figure 4.
Protection against NBD-Cl reaction by MgADP. Wild-type and mutant membrane were preincubated for 1 h at room temperature with varied concentrations of MgADP as shown, and then 150 μM NBD-Cl was added and incubation continued at room temperature in the dark for 1 h. Aliquots were then assayed for ATPase activity. Symbols: (★) wild type, (○) αT349A, (□) αT349D, (◇) αT349Q, (△) αT349R, (▽) βR182A, and (☆) αT349R/βR182A. Results are means of quadruplicate experiments that agreed within ±10%.
Figure 5 shows the MgPi protection against the NBD-Cl reaction. It is obvious that Pi protected well against NBD-Cl inhibition of ATPase activity in the wild type, αT349R, and αT349R/βR182A but did not protect at all against NBD-Cl inactivation in αT349A, αT349D, αT349Q, or βR182A.
Figure 5.
Protection by Pi of ATPase activity in wild-type and mutant membranes from inactivation by NBD-Cl. Membranes were preincubated with MgPi at 0, 2.5, 5, or 10 mM as shown, for 60 min at room temperature. Then NBD-Cl (150 μM) was added, and aliquots were withdrawn for the assay at time intervals as shown. The ATPase activity remaining is plotted vs time of incubation with NBD-Cl: (○) no Pi added, (◊) 2.5 mM Pi, (□) 5 mM Pi, and (△) 10 mM Pi. Each data point represents the average of four different experiments using two or three independent membrane preparations of each mutant.
Inhibition of ATPase Activity by Fluoroaluminate, Fluoroscandium, and Azide
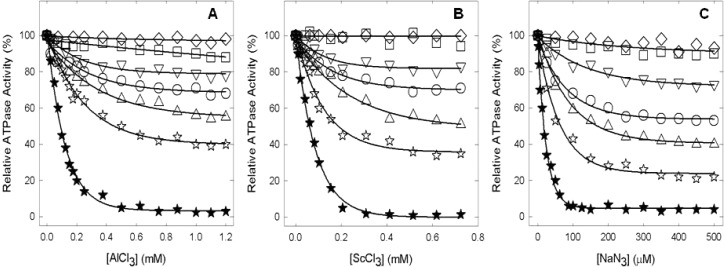
Subsequently, we examined the effects of transition state and ground state analogues. Panels A and B of Figure 6 show inhibition of wild-type and mutant enzymes by MgADP-fluoroaluminate and MgADP-fluoroscandium, respectively. The wild type was completely inhibited. Levels of AlFx- and ScFx-induced inhibition of mutants were ∼25 and 32% (αT349A), 5 and 12% (αT349D), 49 and 46% (αT349R), 17 and 22% (βR182A), and 65 and 61% (αT349R/βR182A), respectively. In contrast, mutant αT349Q was particularly resistant to inhibition by either MgADP-fluoroaluminate or MgADP-fluoroscandium. Figure 6C shows that azide, another potent inhibitor of ATPase in ATP synthase, strongly inhibited the wild type but showed varied residual activity of ∼53% (αT349A), ∼90% (αT349D), ∼90% (αT349Q), ∼40% (αT349R), ∼72% (βR182A), and ∼21% (αT349R/βR182A) in mutants.
Figure 6.
Inhibition of membrane ATPase activity from mutant and wild-type ATP synthase enzymes by fluoroaluminate, fluoroscandium, and azide. Membranes were preincubated for 60 min at room temperature with 1 mM MgADP, 10 mM NaF, and the indicated concentration of AlCl3 (A) or ScCl3 (B). Then aliquots were added to 1 mL of assay buffer, and ATPase activity was determined. Sodium azide was added directly to the membranes and incubated for 30 min before the assay (C) (for details, see Materials and Methods). Symbols: (★) wild type, (○) αT349A, (□) αT349D, (◇) αT349Q, (△) αT349R, (▽) βR182A, and (☆) αT349R/βR182A. All the data points are means of at least four different experiments using two or three independent membrane preparations of each mutant. The variation was ±10% between different experiments.
Inhibition of ATPase Activity by DCCD
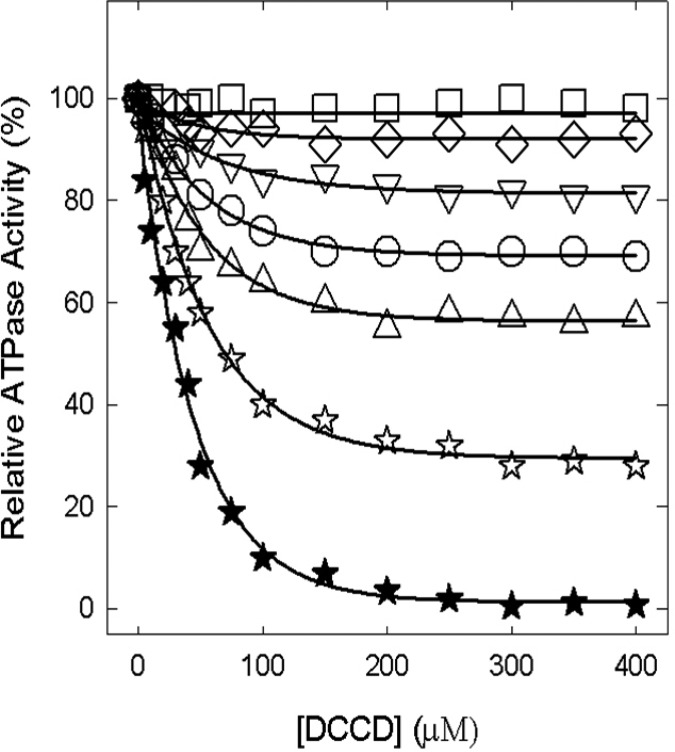
Figure 7 shows the wild-type, αT349A, αT349D, αT349Q, αT349R, βR182A, and αT349R/βR182A enzymes inactivated by DCCD. While the wild type is completely inhibited by 200 μM DCCD after incubation for 16 h at room temperature, mutants show varied degrees of inhibition. αT349A is inhibited ∼31%, αT349Q ∼7%, αT349R ∼43%, βR182A ∼19%, and αT349R/βR182A ∼72%, while αT349D is not inhibited at all. In another set of experiments with 2 or 5 h incubations using the same DCCD concentrations and reaction conditions, we found that the wild type was still fully inhibited, αT349A, αT349D, αT349Q, αT349R, and βR182A showed no inhibition, but double mutant αT349R/βR182A was inhibited maximally by 25% (2 h) and 55% (5 h).
Figure 7.
Inhibition of membrane ATPase activity from mutant and wild-type ATP synthase enzymes by DCCD. Membranes were preincubated for 16 h at room temperature with the varied concentrations of DCCD indicated in the figure. Then 1 mL oof ATPase assay buffer was added to determine the activity. Symbols: (★) wild type, (○) αT349A, (□) αT349D, (◇) αT349Q, (△) αT349R, (▽) βR182A, and (☆) αT349R/βR182A. All the data points are means of at least four different experiments using two or three independent membrane preparations of each mutant. The variation was ±10% between different experiments.
Discussion
The objective of this study was to examine the functional role(s) of residue αThr-349 of E. coli ATP synthase. This residue is part of the strongly conserved α-subunit VISIT-DG sequence. The VISIT-DG sequence residues are located in the proximity of the α-subunit−β-subunit interface bordering the Pi binding pocket (Figure 1B). X-ray crystal structures of the AlF3-inhibited enzyme27 as well as the AlF4–-inhibited enzyme (which also contained SO42– in a second catalytic site)28 show that the side chain of residue αThr-349 is very close to these bound Pi analogues (Figure 1). Pi binding is fundamental for ATP synthesis by ATP synthase. Therefore, the process of Pi binding can divulge a wealth of information about ATP synthesis. Mutagenic analysis and molecular modulation of Pi binding residues are some of the best ways to examine and appreciate the functional role of residues in the catalytic site.
Earlier studies established that mutagenesis combined with the use of the Pi protection assay against NBD-Cl inhibition, as well as the use of inhibitory analogues, allowed the characterization of functional role(s) of residues suspected to be involved in Pi binding.19,29−35 From analysis of eight such catalytic site residues, we determined that five residues, namely, αArg-376, βArg-182, βArg-246, βLys-155, and αSer-347, are critical for Pi binding and form a triangular subdomain within the catalytic site. While four residues, αArg-376, βArg-182, βArg-246, and βLys-155, were directly involved in Pi binding, the fifth residue, αSer-347, supported Pi binding and transition state stabilization through its interaction with βArg-246 (and possibly with βArg-182, too)19,29−35 (Figure 1B). Earlier, we also established that introduction of negative or positive charge at this location resulted in strong alteration of Pi binding,29,31,35 indicating that negative charge within the triangular subdomain was an important determinant of Pi binding. Here we used the same approaches to study the αThr-349 residue.
Generation of the αT349A, αT349D, αT349Q, αT349R, βR182A, or αT349R/βR182A mutant did not affect the assembly or structural integrity of the membrane ATP synthase. Membrane-bound F1Fo showed similar contents of F1 α- and β-subunits compared to the wild type. The αT349A, αT349D, αT349Q, or βR182A mutation caused severe loss of oxidative phosphorylation as judged by growth on succinate or limiting glucose medium. Also, strong inhibition of ATPase activity was observed along with abrogation of Pi binding. The αT349R/βR182A double mutant allowed Pi binding with substantial oxidative phosphorylation and ATPase activity. The αT349R mutant was interesting for it has very little ATPase activity with no oxidative phosphorylation and still allowed Pi binding just like double mutant αT349R/βR182A (Table 1 and Figure 5).
Fluoroaluminate and fluoroscandium in combination with MgADP potently inhibit wild-type E. coli ATP synthase,19,29−33,35,55,56 and both are believed to mimic the chemical transition state. Transition state-like structures involving the bound MgADP–AlF4– complex have been seen in catalytic sites in ATP synthase by X-ray crystallography.28 MgADP-fluoroaluminate or MgADP-fluoroscandium failed to inhibit αT349D and αT349Q mutants, indicating strong destabilization of the transition state, while partial inhibition occurred in αT349A, and βArg-182, αT349R, and double mutant αT349R/βR182A, representative of the partial destabilization of the transition state (Figure 6A,B). These results are in agreement with the amount of oxidative phosphorylation and ATPase activity found in each of the mutants. Evidently, α-subunit VISIT-DG sequence residue αThr-349 is involved directly in the transition state and in catalysis and therefore should be considered as a sixth member of the group of Pi binding residues that make up the triangular Pi binding pocket.
All mutations affected the degree of inhibition by azide, with double mutant αT349R/βR182A reducing it substantially (by ∼80%) and αT349A (∼47%), αT349D (∼10%), αT349Q (∼10%), αT349R (∼60%), and βR182A (∼28%) reducing it less severely (Figure 6C). An X-ray crystallographic study of azide-induced inhibition of ATP hydrolysis57 showed that azide inhibits ATP synthase by forming a tight binding MgADP–azide complex in βDP catalytic sites, which resembles that formed by MgADP-beryllium fluoride, and may therefore be considered an analogue of the MgATP ground state. In the MgADP–azide complex, the azide occupies a position equivalent to that of the γ-phosphate of MgATP. Thus, all mutants also had effects on substrate binding by virtue of an effect at the γ-P position.
DCCD inhibits wild-type E. coli F1 by reacting with residue βGlu-19258 and/or cAsp-61,59 with the latter predominating at lower DCCD concentrations and/or shorter incubation times. As expected, wild-type ATP synthase was inhibited almost 100%. The αT349D mutant was not inhibited at all, and αT349A was inhibited ∼30%, αT349Q ∼7%, αT349R ∼43%, βArg-182 ∼20%, and double mutant αT349R/βArg-182 ∼72% (Figure 7). Notably, at shorter incubation times, while double mutant αT349R/βArg-182 showed substantial inhibition, all the single mutations resisted inhibition (see Results). The data therefore indicate that in the αT349R/βArg-182 double mutant ATPase activity on F1 is partly coupled to proton translocation in Fo, which explains why double mutant αT349R/βArg-182 retains some growth on succinate and in limiting glucose (Table 1).
It is interesting to note here that Pi binding and release events have been shown to be directly linked to rotation of the central stalk in single-molecule experiments.60 Perturbation of the Pi binding site might well be anticipated to perturb the integrity of the link between Pi binding and rotation and manifest as uncoupling. Thus, the data for the αThr-349 mutation strongly suggest that the Thr-OH group is needed for transition state stabilization and Pi binding.
It is established that Arg residues occur frequently in Pi binding sites in proteins;30 therefore, varying the number of Arg residues in the Pi binding site of ATP synthase seemed to be a useful approach. Residue αThr-349 lies 2.68 and 4.38 Å, 2.86 and 4.01 Å, and 3.61 and 3.40 Å from known Pi binding residues βArg-182 and αArg-376, respectively, in AlF3-, AlF4-, and SO42–-containing catalytic sites (nearest atom distances). Thus, one experimental approach we used was to introduce mutation αT349R into the wild-type background (with βArg-182) in the presence of the βR182A mutation. The location of residue αThr-349 at the end of the Pi binding pocket across the catalytic α-subunit−β-subunit interface with its side chain pointing toward the bound Pi analogues also appeared to be a suitable location for the introduction of a new Arg. Apparently, the αT349R mutation would place extra positive charge relatively close to Pi, and double mutation αT349R/βR182A will allow the αT349R mutant Arg to fit into the large “hole” generated by the βAla-182 mutation. The βR182A mutant did not show Pi binding, but the αT349R mutation “rescued” Pi binding in combination with βR182A (Figure 5). On the basis of the loss of oxidative phosphorylation that was made evident by growth on succinate or limiting glucose medium along with very low ATPase activity, αArg-349 could be expected to assume the same exact stereochemical interactions achieved by βArg-182. Thus, electrostatic interaction per se is therefore important, and we conclude that the presence of at least one positive charge at this general location is a requisite determinant of initial Pi binding in catalytic site βE. In addition, the αT349R mutation in the wild-type background totaling one extra positive charge did not prevent Pi binding (Figure 5), but the presence of negative charge in the form of αT349D resulted in abrogation of Pi binding. Presumably, the presence of Asp negated the positive charge of nearby residue βArg-182, resulting in the abrogation of Pi binding.
αThr-349 is positioned close to bound AlF4– in catalytic sites (see Figure 1B).28 The Thr-OH lies 5.46 Å from the F3 atom in AlF4– and thus may contribute to transition state stabilization by direct interaction. It may be mentioned that a similar conclusion was reached regarding the -OH group contributed by αSer-347 of the E. coli VISIT-DG sequence19 and Ser-OH of the highly conserved “LSGGQ” ABC signature sequence in P-glycoprotein.61 Considering how Pi binding is affected, αThr-349-OH lies 6.44 Å from O2 in SO42–28 and 5.56 Å from F1 of AlF3 in the respective catalytic sites.27 Thus, some direct interaction may be operative. However, more important than the findings described above may be the fact that the Thr-OH lies 2.86 Å from NH2 of βArg-182 (in the AlF4– site) and 2.68 Å from NH2 of βArg-182 in the AlF3-occupied site. βArg-182 is strongly conserved and critical for Pi binding and transition state stabilization.9,33 Further, the carbonyl O of αThr-349 lies 3.40 and 3.63 Å from NH1 and NH2, respectively, of αArg-376, another Pi binding residue. The likely H-bond interaction between αThr-349 and βArg-182 (and αArg-376) suggests these residues act together to support Pi binding and transition state stabilization.
In summary, the αThr-349 residue of the conserved VISIT-DG sequence in the ATP synthase α-subunit is required for catalysis, Pi binding, and transition state stabilization. Introduction of Arg at this site can compensate for the absence of Arg at the known Pi binding residue βArg-182 site. Furthermore, arrangement of positive charges with respect to one another is of paramount importance for oxidative phosphorylation and Pi binding.
Acknowledgments
We are thankful to Dr. Alan Senior (Professor Emeritus, Department of Biochemistry and Biophysics, University of Rochester Medical Center, Rochester, NY) for his suggestions and comments on the manuscript.
This work was supported by National Institutes of Health Grant GM085771 to Z.A.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Footnotes
E. coli residue numbering used throughout.
References
- Senior A. E. (2012) Two ATPases. J. Biol. Chem. 287, 30049–30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z.; Cox J. L. (2014) ATP Synthase: The Right Size Base Model for Nanomotors in Nanomedicine. Sci. World J. 2014, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y.; Sambongi Y.; Futai M. (2000) Biological nano motor, ATP synthase FoF1: From catalysis to γεc(10–12) subunit assembly rotation. Biochim. Biophys. Acta 1459, 499–505. [DOI] [PubMed] [Google Scholar]
- Roy A.; Hutcheon M. L.; Duncan T. M.; Cingolani G. (2012) Improved crystallization of Escherichia coli ATP synthase catalytic complex (F1) by introducing a phosphomimetic mutation in subunit ε. Acta Crystallogr. F68, 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. (1988) ATP synthesis by oxidative phosphorylation. Physiol. Rev. 68, 177–231. [DOI] [PubMed] [Google Scholar]
- Karrasch S.; Walker J. E. (1999) Novel features in the structure of bovine ATP synthase. J. Mol. Biol. 290, 379–384. [DOI] [PubMed] [Google Scholar]
- Devenish R. J.; Prescott M.; Roucou X.; Nagley P. (2000) Insights into ATP synthase assembly and function through the molecular genetic manipulation of subunits of the yeast mitochondrial enzyme complex. Biochim. Biophys. Acta 1458, 428–442. [DOI] [PubMed] [Google Scholar]
- Abrahams J. P.; Leslie A. G. W.; Lutter R.; Walker J. E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628. [DOI] [PubMed] [Google Scholar]
- Senior A. E.; Nadanaciva S.; Weber J. (2002) The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta 1553, 188–211. [DOI] [PubMed] [Google Scholar]
- Noji H.; Yoshida M. (2001) The rotary machine in the cell, ATP synthase. J. Biol. Chem. 276, 1665–1668. [DOI] [PubMed] [Google Scholar]
- Weber J.; Senior A. E. (2003) ATP synthesis driven by proton transport in F1F0-ATP synthase. FEBS Lett. 545, 61–70. [DOI] [PubMed] [Google Scholar]
- Frasch W. D. (2000) The participation of metals in the mechanism of the F1-ATPase. Biochim. Biophys. Acta 1458, 310–325. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. (2007) Transport ATPases into the year 2008: A brief overview related to types, structures, functions and roles in health and disease. J. Bioenerg. Biomembr. 39, 349–355. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Okafor F.; Laughlin T. F. (2011) Role of Charged Residues in the Catalytic Sites of Escherichia coli ATP Synthase. J. Amino Acids 2011, 785741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z.; Laughlin T. F. (2010) Medicinal chemistry of ATP synthase: A potential drug target of dietary polyphenols and amphibian antimicrobial peptides. Curr. Med. Chem. 17, 2822–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. (2007) ATP synthase: Motoring to the finish line. Cell 130, 220–221. [DOI] [PubMed] [Google Scholar]
- Martin J. L.; Ishmukhametov R.; Hornung T.; Ahmad Z.; Frasch W. D. (2014) Anatomy of F1-ATPase powered rotation. Proc. Natl. Acad. Sci. U.S.A. 111, 3715–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani G.; Duncan T. M. (2011) Structure of the ATP synthase catalytic complex (F(1)) from Escherichia coli in an autoinhibited conformation. Nat. Struct. Mol. Biol. 18, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Brudecki L. E.; Senior A. E.; Ahmad Z. (2009) Role of α-subunit VISIT-DG sequence residues Ser-347 and Gly-351 in the catalytic sites of Escherichia coli ATP synthase. J. Biol. Chem. 284, 10747–10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. D. (1989) A perspective of the binding change mechanism for ATP synthesis. FASEB J. 3, 2164–2178. [DOI] [PubMed] [Google Scholar]
- al-Shawi M. K.; Senior A. E. (1992) Effects of dimethyl sulfoxide on catalysis in Escherichia coli F1-ATPase. Biochemistry 31, 886–891. [DOI] [PubMed] [Google Scholar]
- Al-Shawi M. K.; Ketchum C. J.; Nakamoto R. K. (1997) The Escherichia coli FOF1 γM23K uncoupling mutant has a higher K0.5 for Pi. Transition state analysis of this mutant and others reveals that synthesis and hydrolysis utilize the same kinetic pathway. Biochemistry 36, 12961–12969. [DOI] [PubMed] [Google Scholar]
- Lobau S.; Weber J.; Senior A. E. (1998) Catalytic site nucleotide binding and hydrolysis in F1F0-ATP synthase. Biochemistry 37, 10846–10853. [DOI] [PubMed] [Google Scholar]
- Weber J.; Senior A. E. (1995) Location and properties of pyrophosphate-binding sites in Escherichia coli F1-ATPase. J. Biol. Chem. 270, 12653–12658. [DOI] [PubMed] [Google Scholar]
- Perez J. A.; Greenfield A. J.; Sutton R.; Ferguson S. J. (1986) Characterisation of phosphate binding to mitochondrial and bacterial membrane-bound ATP synthase by studies of inhibition with 4-chloro-7-nitrobenzofurazan. FEBS Lett. 198, 113–118. [DOI] [PubMed] [Google Scholar]
- Orriss G. L.; Leslie A. G.; Braig K.; Walker J. E. (1998) Bovine F1-ATPase covalently inhibited with 4-chloro-7-nitrobenzofurazan: The structure provides further support for a rotary catalytic mechanism. Structure 6, 831–837. [DOI] [PubMed] [Google Scholar]
- Braig K.; Menz R. I.; Montgomery M. G.; Leslie A. G.; Walker J. E. (2000) Structure of bovine mitochondrial F1-ATPase inhibited by Mg2+ADP and aluminium fluoride. Structure 8, 567–573. [DOI] [PubMed] [Google Scholar]
- Menz R. I.; Walker J. E.; Leslie A. G. (2001) Structure of bovine mitochondrial F1-ATPase with nucleotide bound to all three catalytic sites: Implications for the mechanism of rotary catalysis. Cell 106, 331–341. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2005) Modulation of charge in the phosphate binding site of Escherichia coli ATP synthase. J. Biol. Chem. 280, 27981–27989. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2004) Mutagenesis of residue βArg-246 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F1-ATPase. J. Biol. Chem. 279, 31505–31513. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2004) Role of βAsn-243 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F1-ATPase. J. Biol. Chem. 279, 46057–46064. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2005) Identification of phosphate binding residues of Escherichia coli ATP synthase. J. Bioenerg. Biomembr. 37, 437–440. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2005) Involvement of ATP synthase residues αArg-376, βArg-182, and βLys-155 in Pi binding. FEBS Lett. 579, 523–528. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Senior A. E. (2006) Inhibition of the ATPase activity of Escherichia coli ATP synthase by magnesium fluoride. FEBS Lett. 580, 517–520. [DOI] [PubMed] [Google Scholar]
- Brudecki L. E.; Grindstaff J. J.; Ahmad Z. (2008) Role of αPhe-291 residue in the phosphate-binding subdomain of catalytic sites of Escherichia coli ATP synthase. Arch. Biochem. Biophys. 471, 168–175. [DOI] [PubMed] [Google Scholar]
- Chen C.; Saxena A. K.; Simcoke W. N.; Garboczi D. N.; Pedersen P. L.; Ko Y. H. (2006) Mitochondrial ATP synthase. Crystal structure of the catalytic F1 unit in a vanadate-induced transition-like state and implications for mechanism. J. Biol. Chem. 281, 13777–13783. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227. [DOI] [PubMed] [Google Scholar]
- Walker J. E.; Saraste M.; Runswick M. J.; Gay N. J. (1982) Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum C. J.; Al-Shawi M. K.; Nakamoto R. K. (1998) Intergenic suppression of the γM23K uncoupling mutation in F0F1 ATP synthase by βGlu-381 substitutions: The role of the β380DELSEED386 segment in energy coupling. Biochem. J. 330, 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A.; Weiner M. P.; Hutton C. J.; Batt C. A. (1988) A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene 65, 129–133. [DOI] [PubMed] [Google Scholar]
- Weber J.; Wilke-Mounts S.; Lee R. S.; Grell E.; Senior A. E. (1993) Specific placement of tryptophan in the catalytic sites of Escherichia coli F1-ATPase provides a direct probe of nucleotide binding: Maximal ATP hydrolysis occurs with three sites occupied. J. Biol. Chem. 268, 20126–20133. [PubMed] [Google Scholar]
- Klionsky D. J.; Brusilow W. S.; Simoni R. D. (1984) In vivo evidence for the role of the ε subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J. Bacteriol. 160, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E.; Langman L.; Cox G. B.; Gibson F. (1983) Oxidative phosphorylation in Escherichia coli. Characterization of mutant strains in which F1-ATPase contains abnormal β-subunits. Biochem. J. 210, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E.; Latchney L. R.; Ferguson A. M.; Wise J. G. (1984) Purification of F1-ATPase with impaired catalytic activity from partial revertants of Escherichia coli uncA mutant strains. Arch. Biochem. Biophys. 228, 49–53. [DOI] [PubMed] [Google Scholar]
- Taussky H. H.; Shorr E. (1953) A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 202, 675–685. [PubMed] [Google Scholar]
- Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Rao R.; Perlin D. S.; Senior A. E. (1987) The defective proton-ATPase of uncA mutants of Escherichia coli: ATP-binding and ATP-induced conformational change in mutant α-subunits. Arch. Biochem. Biophys. 255, 309–315. [DOI] [PubMed] [Google Scholar]
- Weber J.; Wilke-Mounts S.; Senior A. E. (1994) Cooperativity and stoichiometry of substrate binding to the catalytic sites of Escherichia coli F1-ATPase. Effects of magnesium, inhibitors, and mutation. J. Biol. Chem. 269, 20462–20467. [PubMed] [Google Scholar]
- Chinnam N.; Dadi P. K.; Sabri S. A.; Ahmad M.; Kabir M. A.; Ahmad Z. (2010) Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 46, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadi P. K.; Ahmad M.; Ahmad Z. (2009) Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 45, 72–79. [DOI] [PubMed] [Google Scholar]
- Laughlin T. F.; Ahmad Z. (2010) Inhibition of Escherichia coli ATP synthase by amphibian antimicrobial peptides. Int. J. Biol. Macromol. 46, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z.; Ahmad M.; Okafor F.; Jones J.; Abunameh A.; Cheniya R. P.; Kady I. O. (2012) Effect of structural modulation of polyphenolic compounds on the inhibition of Escherichia coli ATP synthase. Int. J. Biol. Macromol. 50, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. J.; Lloyd W. J.; Radda G. K. (1975) The mitochondrial ATPase. Selective modification of a nitrogen residue in the β subunit. Eur. J. Biochem. 54, 127–133. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J.; Lloyd W. J.; Lyons M. H.; Radda G. K. (1975) The mitochondrial ATPase. Evidence for a single essential tyrosine residue. Eur. J. Biochem. 54, 117–126. [DOI] [PubMed] [Google Scholar]
- Nadanaciva S.; Weber J.; Senior A. E. (2000) New probes of the F1-ATPase catalytic transition state reveal that two of the three catalytic sites can assume a transition state conformation simultaneously. Biochemistry 39, 9583–9590. [DOI] [PubMed] [Google Scholar]
- Nadanaciva S.; Weber J.; Senior A. E. (1999) Binding of the transition state analog MgADP-fluoroaluminate to F1-ATPase. J. Biol. Chem. 274, 7052–7058. [DOI] [PubMed] [Google Scholar]
- Bowler M. W.; Montgomery M. G.; Leslie A. G.; Walker J. E. (2006) How azide inhibits ATP hydrolysis by the F-ATPases. Proc. Natl. Acad. Sci. U.S.A. 103, 8646–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M.; Allison W. S.; Esch F. S.; Futai M. (1982) The specificity of carboxyl group modification during the inactivation of the Escherichia coli F1-ATPase with dicyclohexyl[14C]carbodiimide. J. Biol. Chem. 257, 10033–10037. [PubMed] [Google Scholar]
- Hermolin J.; Fillingame R. H. (1989) H+-ATPase activity of Escherichia coli F1F0 is blocked after reaction of dicyclohexylcarbodiimide with a single proteolipid (subunit c) of the F0 complex. J. Biol. Chem. 264, 3896–3903. [PubMed] [Google Scholar]
- Adachi K.; Oiwa K.; Nishizaka T.; Furuike S.; Noji H.; Itoh H.; Yoshida M.; Kinosita K. Jr. (2007) Coupling of rotation and catalysis in F1-ATPase revealed by single-molecule imaging and manipulation. Cell 130, 309–321. [DOI] [PubMed] [Google Scholar]
- Tombline G.; Bartholomew L.; Gimi K.; Tyndall G. A.; Senior A. E. (2004) Synergy between conserved ABC signature Ser residues in P-glycoprotein catalysis. J. Biol. Chem. 279, 5363–5373. [DOI] [PubMed] [Google Scholar]