Abstract
A 63-year-old man was evaluated in consultation for unexplained dyspnea. At the time of the initial clinical encounter at our institution, the patient endorsed a 10-year history of progressive exertional dyspnea, which had become debilitating over the preceding 3 months and was characterized by shortness of breath accompanying subtle physical activities such as tying shoelaces. The patient underwent multiple hospital admissions reportedly for the treatment of congestive heart failure ascribed to impaired left ventricular (LV) diastolic function. Review of systems identified postural dizziness and history of near syncope, possible nocturnal dyspnea, and peripheral neuropathy, but not cardiac angina, orthopnea, nocturia, edema, cough, palpitations, syncope, claudication, or other cardiopulmonary symptoms. He related that he was first noted to have a cardiac murmur detected 4 decades previously during a military service physical examination but that the murmur was not characterized further and that he served in the Vietnam conflict without functional limitation.
The patient’s relevant medical history included rate-controlled atrial fibrillation, 90 pack-year tobacco use (3 packs daily between 21 and 51 years of age), dyslipidemia, systemic hypertension, type 2 diabetes mellitus, moderate chronic obstructive pulmonary disease, obstructive sleep apnea not currently treated, and gastrointestinal bleed caused by colon cancer treated with hemicolectomy 11 years earlier. There was no illicit drug use, but a remote history of heavy alcohol consumption was reported. Family history was unremarkable except that his father died at 55 years of age of myocardial infarction.
Medications included aspirin 81 mg daily, warfarin 2 mg daily, losartan 50 mg daily, metoprolol tartrate 25 mg twice daily, simvastatin 10 mg daily, fenofibrate 48 mg daily, albuterol inhaler 90-μg puffs as needed, and gabapentin 300 mg 3 times daily.
Keywords: atrial septal defect sinus venosus; heart defects, congenital; heart failure; hypertension, pulmonary
Dr Bradley A. Maron: Progressive unexplained breathlessness is a common chief complaint prompting patient referrals to cardiologists and pulmonologists for further evaluation. The differential diagnosis of unexplained dyspnea is broad; thus, determining the trajectory of a diagnostic evaluation hinges on a detailed history and thorough physical examination. A cardiac murmur was appreciated initially in this patient in his third decade of life during military screening, a time when he was ostensibly physically fit. A murmur in early adulthood raises the possibility of primary structural heart disease, including valvular lesion(s), genetic cardiomyopathies, or congenital heart disease. However, many patients with murmurs have no associated heart disease. Near syncope raises the possibility of dysrhythmia, which, if present, may be a consequence of structural heart disease or ischemia. Thus, cardiomyopathy and coronary artery disease are common diagnoses that must be evaluated, particularly given this patient’s numerous risk factors for atherosclerotic disease. Shortness of breath with an extensive tobacco history requires consideration of various lung diseases beyond chronic obstructive pulmonary disease, including malignancy. Less likely diagnoses that require consideration in this case include pulmonary arterial hypertension, which is increasingly identified initially in older patients, and pulmonary hypertension caused by chronic obstructive pulmonary disease or obstructive sleep apnea, which is also common in veteran patients. Cirrhosis from alcohol consumption may cause dyspnea in connection with portopulmonary hypertension, hepatopulmonary syndrome, or even cirrhotic cardiomyopathy. Moreover, anemia should be excluded given the history of colon cancer, colectomy, and gastrointestinal bleed. The patient’s peripheral neuropathy is most likely secondary to diabetes mellitus, although cardioneurological syndromes associated with heart failure such as amyloidosis must remain in the differential diagnosis.
Patient presentation (continued): Physical examination demonstrated an irregularly irregular rhythm at 67 bpm with symmetrical upper-extremity blood pressures of 122/52 mm Hg. The patient’s respiratory rate was 18 breaths per minute; his peripheral oxyhemoglobin saturation (Sao2) level was 96% while breathing ambient air; and his body mass index was 27 kg/m2. He appeared comfortable while lying supine and was able to speak full sentences. Jugular venous pressure was 16 cm H2O, and prominent v waves were observed without a Kussmaul sign. Chest palpation revealed a discrete apical impulse and systolic impulses at the right parasternal border and second left intercostal space. Cardiac auscultation was significant for persistently split S2, II/VI holosystolic murmur at the left lower sternal border, and absence of an S3. The lungs were clear on auscultation without dullness on percussion. The abdominal examination was notable for a pulsatile liver without significant hepatosplenomegaly or ascites. Peripheral examination demonstrated warm extremities with +2 symmetrical carotid, radial, femoral, popliteal, posterior tibial, and dorsalis pedis pulses; minimal varicosities of lower-extremity veins were appreciated without edema, clubbing, cyanosis, or venous stasis dermatopathy. There were no stigmata of chronic liver disease.
Dr Maron: The examination overall suggests dilatation of the right side of the heart and elevated venous pressure. The right parasternal impulse, or right ventricular (RV) lift, is consistent with RV volume overload. In agreement with this is the finding of jugular venous distention and a likely tricuspid regurgitation murmur at the left sternal border. Accentuation of a persistently split S2 by auscultation and the pulmonary artery “tap,” palpated in the second left intercostal space, both suggest increased RV pressure loading. Findings that suggest failure of the left side of the heart as a cause of failure of the right side of the heart, including left-sided S3, enlarged or sustained apical impulse, lung rales, narrow pulse pressure, and evidence of distal malperfusion, are notably absent.
Patient presentation (continued): Laboratory results were notable for total bilirubin 1.5 mg/dL and alkaline phosphatase 185 U/L. Other laboratory studies were within established normal limits, including hemoglobin, platelets, glomerular filtration rate, alanine aminotransferase, and albumin. Troponin and brain natriuretic peptide levels were unavailable. A 12-lead ECG demonstrated atrial fibrillation and right bundle-branch block (Figure 1). Chest roentgenography demonstrated enlargement of the right atrial (RA) and RV silhouettes on the posteroanterior and lateral projections, respectively (Figure 2). The proximal pulmonary arterial branches also appear enlarged. Comparison with a previous roentgenograph from 3 years earlier confirmed progressive enlargement of the RA and pulmonary vessels.
Figure 1.
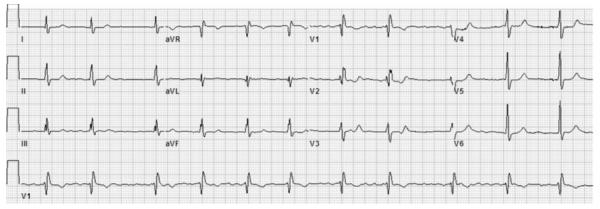
ECG demonstrating atrial fibrillation and right bundle-branch block.
Figure 2.
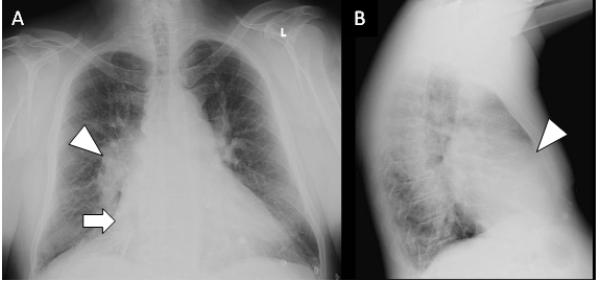
Chest roentgenograms. A, Posteroanterior chest radiography demonstrates enlargement of the right atrial silhouette (arrow) and proximal pulmonary arterial branches (arrowhead). B, On the lateral view, cardiac chamber occupation of the retrosternal space suggests right ventricular enlargement (arrowhead).
Dr Maron: The elevation in alkaline phosphatase and total bilirubin with a normal alanine aminotransferase may suggest a cholestatic pattern of hepatic injury. In the absence of primary liver disease or laboratory patterns indicative of alcohol-induced hepatitis, congestive hepatopathy resulting from elevated right-sided pressure is the likely cause of cholestasis, and this possibility is supported by the examination finding of pulsatile liver, which indicates that the mechanism of congestion is likely related to tricuspid regurgitation.
Right bundle-branch block on ECG may be seen in patients with RV dilatation, in patients with volume overload, or in the setting of certain cardiomyopathies such as sarcoidosis. RA size cannot be assessed electrocardiographically in the setting of atrial fibrillation. The chest radiograph further supports the presence of progressive right-sided heart disease. On the basis of the available data, understanding with greater clarity the cardiac chamber geometry and investigating a cardiac lesion by which to potentially explain the patient’s clinical evidence of right heart failure are a priority. Thus, moving forward, reviewing previous studies evaluating cardiac chamber size, biventricular function, and cardiopulmonary hemodynamics may be useful.
Patient presentation (continued): Medical records that were available for review included summary reports of a transthoracic echocardiogram (TTE) from 12 years earlier and a transesophageal echocardiogram (TEE) from 4 years before our consultation, as well as a pulmonary function test from 2 months previously. Echocardiography demonstrated normal size of the left side of the heart and normal LV function. Assessment of the right side of the heart indicated an estimated RV systolic pressure of 52 mm Hg with RA dilatation, RV dilation, and moderate global RV hypokinesis. Moreover, an enlarged coronary sinus was identified. Interpretation of the pulmonary function test results supported the diagnosis of a moderate obstructive ventilatory defect (forced expiratory volume at 1 second, 59% predicted) with preserved diffusing capacity.
Dr Maron: Previous echocardiography data are consistent with the current physical examination findings suggesting right-sided heart failure caused by right-sided cardiac pathology. Unexplained RA and RV dilatation on TTE should routinely prompt further investigation of an underlying cause,1 particularly if pulmonary hypertension or intracardiac shunt is suspected, as might have been in this case by virtue of normal LV size and systolic function.2 A dilated coronary sinus may be observed in patients with a persistent left-sided superior vena cava (SVC), anomalous pulmonary venous return, RA hypertension or pulmonary hypertension,3 RV dysfunction, unroofed coronary sinus,4 or a coronary artery fistula. Overall, on the basis of this patient’s advanced age and clinical presentation, the most probable anatomic lesion by which to account for right-sided chamber dilation is a secundum atrial septal defect (ASD), although a sinus venosus defect (SVD), isolated anomalous pulmonary venous return, and even tetralogy of Fallot have been reported infrequently as causes of exertional shortness of breath and heart failure in the seventh decade of life or later.2,5 Overall, further characterizing the patient’s cardiopulmonary hemodynamics, including oxyhemoglobin saturation levels, in each of the right heart compartments via cardiac catheterization is indicated to characterize pulmonary vascular remodeling, pulmonary hypertension, and the possibility of intracardiac shunt.
Patient presentation (continued): Resting intracardiac pressures were measured by catheterization of the right side of the heart: RA, 16 mm Hg; RV, 53/12 mm Hg; pulmonary artery, 53/16 mm Hg with mean pulmonary artery pressure 30 mm Hg; and end-expiratory pulmonary capillary wedge pressure, 13 mm Hg, with cardiac output, assessed by the thermodilution technique, elevated at 9.25 L/min. Relevant Sao2 levels are reported in Table 1.
Table 1. Oxyhemoglobin Saturation Level Measurements Obtained at Catheterization of the Right Side of the Heart.
| Chamber | Oxyhemoglobin Saturation, % |
|---|---|
| High SVC | 83 |
| Low SVC | 90 |
| IVC | 77 |
| High RA | 89 |
| RA | 79 |
| Low RA | 77 |
| RV, body | 84 |
| RV outflow tract | 86 |
| Main pulmonary artery | 86 |
| Left upper pulmonary vein | 98 |
| Aorta | 96 |
IVC indicates inferior vena cava; RA, right atrium; RV, right ventricle; and SVC, superior vena cava.
Dr Maron: These data identify 2 pathophysiologies, pulmonary hypertension and intracardiac shunt, that may be interrelated clinically. The approach to cardiopulmonary hemodynamic analysis in patients with pulmonary hypertension varies substantially in clinical practice. The pulmonary capillary wedge pressure of 13 mm Hg suggests that the elevation in right-sided pressure is unlikely to be due to elevated left-sided pressure. Calculation of the pulmonary vascular resistance (PVR) as mean pulmonary artery pressure minus pulmonary capillary wedge pressure divided by cardiac output is a useful measure of pulmonary vascular remodeling. Moreover, PVR associates positively with increased RV pressure loading and contributes to RV–pulmonary circulatory uncoupling, which promotes failure of the right side of the heart. In this case, the calculated PVR is 1.8 Wood units and may be interpreted as normal (<2.5 Wood units) on initial assessment. However, the accuracy of cardiac output measured in patients with intracardiac shunt (or severe tricuspid regurgitation) is controversial and may result in unpredictable overestimations and underestimations of PVR.6 For these reasons, cardiac output calculated from the Fick equation with arteriovenous oxygen difference and volume of oxygen consumption measured directly with pneumotachography7 or by cardiac magnetic resonance imaging is preferable. In this case, pneumotachography was not available, and the Fick method was not used. Nevertheless, these data demonstrate a significant “step-up” between the high and low SVC, and it is reasonable to suspect that increased pulmonary blood flow resulting from a chronic left-to-right shunt promotes a congestive pulmonary vasculopathy, increased pulmonary artery pressure, and adverse RV remodeling, which collectively are contributors to the patient’s clinical syndrome.
Dr Deepak L. Bhatt: The next key element of this case is interpreting the oxyhemoglobin flux between cardiovascular compartments. First, the 7% increase in Sao2 level between the high (proximal) and low (distal) SVC indicates the introduction of oxygenated blood to the right heart, which is consistent with the patient’s (apparently) elevated cardiac output and high pulmonary pressures. As Dr Maron stated, the patient’s Sao2 data suggest left-to-right cardiac shunt at the level of the SVC. It is worthwhile to note that even in the absence of intracardiac shunt, the oxygen saturation of RA blood may be up to 4% greater than SVC blood because of the variability in measurements.8 Nevertheless, an incremental increase in Sao2 of the magnitude observed in this case supports a diagnosis of intracardiac shunt, particularly in the setting of pulmonary hypertension and symptoms and signs indicative of failure of the right side of the heart.
The shunt fraction Qp/Qs can be estimated when information on oxygen consumption is not available: Qp/Qs=(Sao2–Mvo2)/(Pvo2–Pao2), where Sao2 is the systemic arterial oxygen saturation, Mvo2 is the mixed venous saturation, Pvo2 is the pulmonary vein saturation, and Pao2 is the pulmonary artery saturation. Although Sao2, Pvo2, and Pao2 are directly measured, the Mvo2 value in the case of a left-to-right shunt must reflect the mixing of venous blood that occurs before the shunt. For a ventricular septal defect, the venous blood would be fully mixed in the atrium, but for a suspected ASD, the mixing can be estimated with several approaches such as the following formula: 3/4×SVC saturation+1/4×inferior vena cava saturation.9 However, although this formula is applied to shunt calculations when there is mixing within the atrium, in this case, the shunting directly affects the SVC saturation, so another available estimation is to use the inferior vena cava saturation as the Mvo2 saturation. Thus, Qp/Qs=(96%–77%)/(98%–86%)=1.6, consistent with net left-to-right blood flow. There is inherent variability (≈3%–4%) in the oximetry measurements, and it is worth noting that such seemingly small variations in these measurements can affect the magnitude of the calculated Qp/Qs disproportionately.
The differential diagnoses of a left-to-right shunt that would manifest an increase in Sao2 levels from the SVC to RA include primum ASD, secundum ASD, and a superior SVD; anomalous pulmonary venous return to the RA; ventricular septal defect with significant tricuspid regurgitation; Gerbode-type ventricular septal defect (an LV-to-RA connection); coronary artery fistula involving the RA; and ruptured sinus of Valsalva aneurysm. Contrast angiography, particularly left ventriculography, characterizes the anatomy of these shunts effectively, with the notable exception of ASDs and anomalous pulmonary venous return.10
Patient presentation (continued): To better define an anatomic substrate of the shunt, contrast opacification venography and atrialography were performed at the time of catheterization of the right side of the heart. Coronary angiography revealed only a focal 70% stenosis in the middle left anterior descending coronary artery, without anomalous coronary origins, courses, or fistulas. The LV end-diastolic pressure was 16 mm Hg.
Dr Bhatt: In this patient, a multipurpose catheter was maneuvered from the right femoral vein into the left atrium (LA) via the RA (Figure 3). This maneuver identified an interatrial communication that connects the superomedial LA and the SVC-RA junction. This anatomy is consistent with a superior SVD. The communication between the atria as outlined by radiocontrast is most clearly visualized in the angiogram cine images in which contrast is observed to flow from the LA into the RA and toward the RV (Movie I in the online-only Data Supplement). In addition, although the Qp/Qs value of 1.6 reflects the net ratio of left-to-right blood flow between the systemic and pulmonary circulations, there may be dynamic bidirectional flow with a component of right-to-left shunting via this same pathway. Overall, complementary imaging modalities are required to better define the cardiac and vascular anatomic relationships in this patient.
Figure 3.
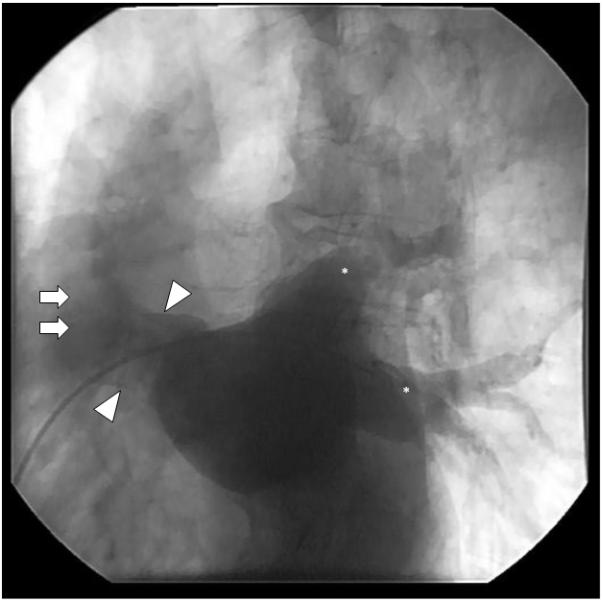
Contrast injection during cardiac catheterization demonstrates an interatrial communication. Atriagram taken in the right angle oblique projection shows a multipurpose catheter extending from the right atrium into the left atrium, as identified by the characteristic radiocontrast outline of the left pulmonary veins (asterisks). Contrast opacification defines an interatrial communication (arrowheads) that arises from the superomedial left atrial wall and anastomoses with the superior vena cava (arrows) and right atrium.
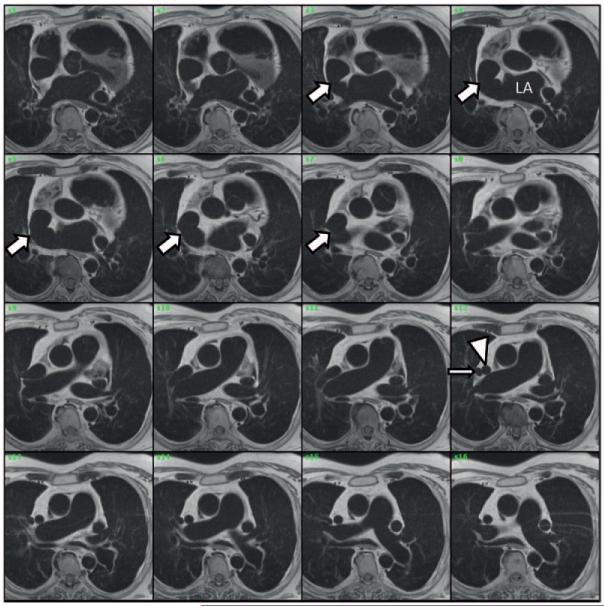
Patient presentation (continued): A multimodality imaging strategy was used to characterize further the patient’s suspected superior SVD. TTE confirmed a markedly dilated RV with diffuse RV hypokinesis (Figure 4A and Movie II in the online-only Data Supplement). Agitated saline contrast injection from the right upper extremity resulted in nearly simultaneous opacification of the LA and RV, without filling of the coronary sinus (Movie III in the online-only Data Supplement). When agitated saline contrast was injected via the left antecubital vein during TEE, however, the coronary sinus was opacified before the appreciation of contrast filling of the right-sided cardiac chambers (Movie IV in the online-only Data Supplement). Defects in the interatrial septum proper (eg, primum or secundum ASD) were not visualized by either TTE or TEE. However, TEE confirmed a communication between the LA and the SVC-RA junction (Figures 4B–4D and Movies V and VI in the online only Data Supplement) and an anomalous right superior pulmonary vein (RSPV) anastomosed to the SVC (Movie VII in the online-only Data Supplement).
Figure 4.
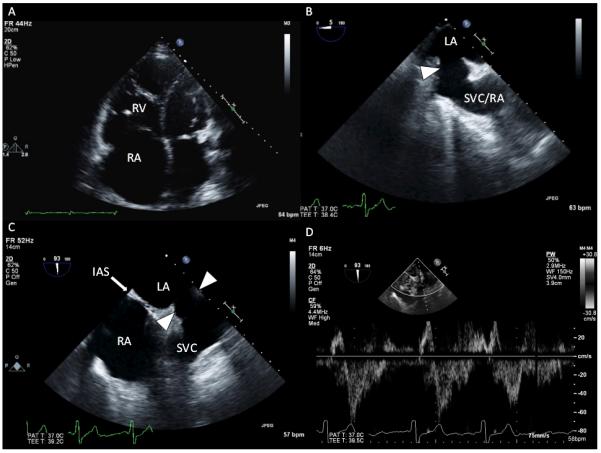
Representative images from transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE). A, TTE demonstrates marked right atrial (RA) and right ventricular (RV) dilatation. B, TEE image at a 5° omniplane angle and above the level of the aortic valve demonstrates an abnormal interatrial communication from the superior vena cava (SVC)–RA to the left atrium (LA; arrow). C, TEE image in a plane orthogonal to B, at a 93° omniplane angle, demonstrates a modified bicaval view showing structures superior to the interatrial septum (IAS). There is a communication from the LA to the SVC that occurs as a result of the absence of tissue separating the SVC from the LA (arrows). D, Pulsed-wave Doppler through the interatrial communication shows bidirectional but predominantly left-to-right flows.
Dr Jayashri R. Aragam: Results from the agitated saline contrast injections, performed from both the right and left upper extremities, are pathognomonic for aberrant extracardiac vascular connections creating an abnormal pattern of blood circulation. Injection from the right arm confirms that the right-sided SVC is in communication with both atria. This finding is consistent with a superior SVD. Injection via the left antecubital vein results in opacification of the coronary sinus antecedent to opacification of the RA or RV. This contrast pattern is consistent with a left-sided SVC that drains to the RA via the coronary sinus; lack of LA opacification excludes an unroofed coronary sinus defect. Contrast echocardiography is not able to define pulmonary venous anatomy. Indeed, the precise documentation of a superior SVD on echocardiography is technically difficult because the defect lies in the far field of routine TTE views and detection in most cases requires dedicated views.11,12 When performing a TTE to evaluate for ASD, the sonographer should interrogate the interatrial septum from multiple windows, including the apical 4-chamber, subcostal 4-chamber, and right parasternal views. Moreover, the sonographer must achieve additional superior angulation in the subcostal bicaval view to expose the location of a superior SVD. This is challenging, and the false-negative rate of TTE for superior SVD detection is predictably high. When SVD is suspected, additional advanced imaging such as TEE, cardiac computed tomography, or cardiac magnetic resonance imaging is often required. Even routine TEE midesophageal bicaval views depicting the interatrial septum are generally not sensitive enough to detect the superior SVD. When a superior SVD is suspected, the echocardiographer must map the interatrial septum from a more superior station in the esophagus, as in Figure 4C.12 In addition, complete mapping of pulmonary venous anatomy by TEE is essential because anomalous pulmonary venous return is very common with SVD, as in this case.
Advanced imaging with cardiac computed tomography or cardiac magnetic resonance imaging is advised in adults with congenital heart disease as another means by which to rigorously define defect anatomy, given the associated important therapeutic implications. In this case, cardiac magnetic resonance imaging also delineated the course of the anomalous RSPV and defined the superior SVD (Figure 5). The RSPV anastomoses with the SVC slightly superior to the level of the RA-SVC junction. On axial plane images located inferior to the RSPV-SVC anastomosis, there is communication from the RSPV-SVC to the LA (Figure 5). Thus, in the setting of an intact atrial septum, the superior SVD creates an interatrial shunt and is frequently associated with anomalous pulmonary venous return.
Figure 5.
Anomalous right superior pulmonary vein (RSPV) and superior sinus venosus defect are identified by cardiac magnetic resonance imaging (cMR). Serial axial cMR slices are shown, with the first image at the level of the aortic valve and sequential images obtained by moving superiorly. These images demonstrate both the anastomosis of the superior vena cava (SVC; arrowhead) and RSPV (thin arrow) and the presence of an abnormal communication between the left atrium (LA) and the SVC–right atrial junction (arrows). The morphology and geometry of the interatrial communication mirror the anatomy shown by transesophageal echocardiography. In addition, the pulmonary artery is dilated, and the persistent left SVC is visible.
Patient presentation (continued): Correction of the superior SVD was recommended because of progressive heart failure, adverse remodeling of the right side of the heart, New York Heart Association class III functional status, and the absence of elevated PVR. Intraoperative TEE again demonstrated the communication between the LA and SVC (Figure 6). After surgical right atriotomy in this patient, the SVD was visible as a defect in the posterosuperior RA (Figure IA in the online-only Data Supplement). Repair was accommodated with a Dacron patch deployed over the SVD to close the interatrial shunt (Figure IB in the online-only Data Supplement) that was also intended to baffle flow from the RSPV to the LA. The patient also underwent concurrent single-vessel saphenous vein–to–left anterior descending coronary artery bypass grafting.
Figure 6.
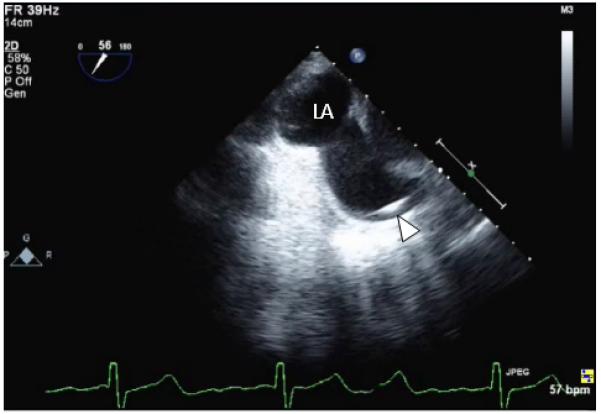
Intraoperative transesophageal echocardiography (TEE) image before patch repair. A Swan-Ganz catheter is visible within the superior vena cava (SVC; arrowhead), and this TEE image at a 56° omniplane angle shows that the SVC communicates with the left atrium.
Dr Maron: Treatment options include medical therapy to attenuate symptoms of heart failure, surgical repair of the congenital abnormality, or a hybrid strategy in which optimal medical therapy is required in advance of surgery to improve cardiopulmonary hemodynamics and thus operative candidacy. Overall, success of surgical repair hinges on defect anatomy, pulmonary vascular disease severity and reversibility, and access to an experienced surgeon.1 The physiology of the superior SVD is similar to that of other ASDs, so clinical features that favor surgical intervention include enlargement of the right side of the heart, an indicator of hemodynamically significant left-to-right shunt. On the other hand, advanced or irreversible pulmonary hypertension may serve as a contraindication to repair (Table 2).1 Although few data are available to systematically characterize the outcome of congenital heart repair in late adulthood, advanced age alone is not a contraindication to surgery. Nevertheless, residual RV dilation and dysfunction and increased risk for atrial arrhythmia in the postoperative phase are reported for patients of older compared with younger age.
Table 2. Indications for ASD Closure.
| Indication for ASD Closure | AHA/ACC Recommendation |
|---|---|
| RA and RV enlargement, with or without symptoms |
Class I |
| Paradoxical embolism | Class IIa |
| Platypnea-orthodeoxia | Class IIa |
| Net left-to-right shunt (Qp/Qs >1) | Class IIb |
| Pulmonary artery pressure <2/3 systemic pressure |
Class IIb |
| PVR <2/3 systemic vascular resistance | Class IIb |
| Elevated pulmonary pressures or pulmonary vascular resistance but reversible with pulmonary vasodilator or transient percutaneous test occlusion (percutaneous test occlusion may not be possible for superior sinus venosus defect) |
Class IIb |
| Severe, irreversible pulmonary hypertension |
Class III |
| No evidence of left-to-right shunt | Class III |
ACC indicates American College of Cardiology; AHA, American Heart Association; ASD, atrial septal defect; PVR, pulmonary vascular resistance; RA, right atrial; and RV, right ventricular. Adapted from Warnes et al.1
Although catheter-based strategies are available for closure of patent foramen ovale and ostium secundum ASDs, the superior SVD lacks the necessary surrounding rim of tissue to accommodate closure by a percutaneous device. Accordingly, surgical rather than percutaneous repair of the superior SVD is required.1
Patient presentation (continued): At 6 days postoperatively, a TTE was repeated, showing decreased RV size with the Dacron patch visible in the posterior RA (Figure 7). The patient was discharged on warfarin, aspirin, furosemide, digoxin, metoprolol tartrate, and simvastatin. At 4 months postoperatively, the patient reported substantial improvement in functional class, including participation in moderate-level aerobic exercise. At 7 months after surgery, the patient reported less fatigue and dyspnea and that he was participating in vigorous exercise, including heavy yard work, despite persistent atrial fibrillation.
Figure 7.
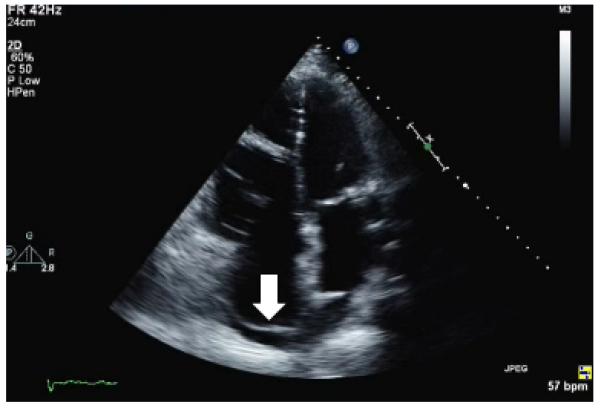
Postoperative transthoracic echocardiography demonstrates a patch in the posterior right atrium (arrow) and reduced right atrial and ventricular dimensions.
Discussion
Embryology of SVD
Approximately 10% of adult congenital heart defects involve an ASD, of which ≈95% are ostium secundum or ostium primum defects (Figure 8). In contrast, the superior SVD comprises ≈5% of the ASD spectrum pathophysiology, and the inferior SVD is even rarer (Table 3). The underlying defect in superior SVD is in the sinus venarum, which is derived from the sinus venosus and during embryogenesis becomes incorporated as the smooth-walled portion of the posterior RA wall that would normally serve to separate the RSPV and LA from the SVC and RA. Incomplete formation of the sinus venarum creates the SVD, which is a “sino-septal” type of interatrial communication that is posterosuperior to an intact fossa ovalis (embryology reviewed elsewhere14-16). Abnormal pulmonary venous return is commonly associated with SVD, and it may result from anomalous pulmonary vein courses or unroofing of ≥1 right sided pulmonary veins by failure of sinus venarum involution.14
Figure 8.
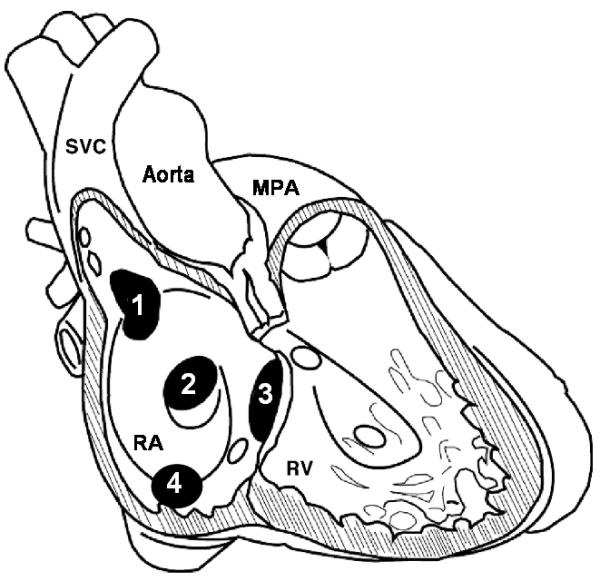
Types and locations of interatrial defects. In this schematic, right atrial (RA) and right ventricular (RV) free walls are removed to reveal the interatrial septum as viewed from the right side. Four interatrial defect types are presented in the characteristic location of each: 1, superior sinus venosus defect; 2, ostium secundum atrial septal defect; 3, ostium primum defect; and 4, inferior sinus venosus defect. With the superior sinus venosus defect, the superior vena cava (SVC) communicates with both the RA and left atrium. MPA indicates main pulmonary artery. Modified from Sommer RJ et al.13
Table 3. Summary of Types of ASDs and Their Characteristics.
| ASD Type | Defect Location | ASD-Type Defects, % | Male:Female Ratio | Associated PAPVR, % | Ability to Detect on TTE, % |
Amenable to Percutaneous Treatment |
|---|---|---|---|---|---|---|
| Ostium secundum | Fossa ovalis | ≈75–80 | 1:2–3 | ≈10 | 89–99 | Yes |
| Ostium primum | Endocardial cushion; often associated with atrioventricular valve dysfunction |
≈15–20 | ≈1:1 | Usually cleft mitral valve or subaortic stenosis |
100 | No |
| Superior SVD | Anastomosis of SVC and RA | ≈5 | ≈1:1 | ≈95–97 | 12 | No |
| Inferior SVD | Anastomosis of IVC and RA | <1 | ? | ? | Low | No |
Pathophysiology and Symptoms of SVD Intracardiac Shunt
The (patho)physiological consequences of the SVD depend on defect size, resistance of the pulmonary and systemic vascular beds, and compliance of cardiac chambers, which collectively determine shunt direction and volume. In turn, net directionality is dynamic and may be influenced by chronic processes that alter cardiopulmonary and systemic hemodynamics, including, for example, systemic hypertension (favoring left-to-right flow) or impaired RV compliance (favoring right-to-left flow), as well as acute perturbations such as respiration and the Valsalva maneuver.17 The degree to which left-to-right shunt promotes RV dilation and pulmonary vascular remodeling correlates with Qp/Qs magnitude, particularly at levels >2:1. Nevertheless, the development of clinically important pulmonary vascular disease is idiosyncratic, and many patients with large ASDs may never develop elevated PVR. However, specifically investigating for the presence of an intracardiac shunt in patients with otherwise unexplained RA or RV dilatation is an American Heart Association/American College of Cardiology expert consensus guideline Class IC recommendation.1
Dyspnea, a common symptom in the natural history of intracardiac shunt, is reported to affect up to ≈75% of patients with an uncorrected ASD by the fifth decade of life.18 Consequences of RV volume overload are common and ascribed to increased pulmonary blood flow that induces a congestive pulmonary vasculopathy, increased PVR, and possibly the development of Eisenmenger physiology (ie, irreversible right-to-left flow with clinically important increases in PVR and RV thickness). Stroke resulting from paradoxical thromboembolism is an established sequela of SVD. These patients also have increased rates of sinus node dysfunction and ectopic atrial rhythms owing to the close anatomic proximity of the sinoatrial node to the sinus venarum, and atrial arrhythmias are commonly caused by sinus node dysfunction and atrial and pulmonary vein dilatation.
SVD Therapies
Therapy for any ASD is aimed at attenuating symptoms and preventing progressive right-sided heart dysfunction and associated arrhythmias. Enlargement of the RA and RV is an American Heart Association/American College of Cardiology Class I indication for surgical SVD correction, regardless of symptom burden. Alternative indications for SVD closure are provided in Table 2 and include hemodynamic evidence of important shunting and the absence of severe irreversible pulmonary hypertension, whereas shunt closure is contraindicated in the absence of a left-to-right shunt or irreversible pulmonary hypertension.
Definitive correction of the superior SVD requires surgery. Percutaneous closure of SVD lesions is not possible owing to inadequate tissue rims required to fasten the closure device and the risk for obstruction to pulmonary and systemic venous flow. Surgical strategies include single patch repair, double patch repair (eg, SVD patch repair with atriocavalplasty patch enlargement), and caval translocation.19,20 The superior SVD repair typically requires a right atriotomy, which has traditionally been performed via a median sternotomy, although right posterior thoracotomy has recently evolved as an acceptable approach option.21 The goals of patch repair approaches are to close the interatrial communication and to isolate anomalous pulmonary veins from the right-sided chambers; a second patch can be used to augment the proximal SVC to minimize the likelihood of SVC obstruction.22 Given the high incidence of sinus node dysfunction, many patients will require transvenous pacemaker leads to be placed, which increases the propensity to develop SVC obstruction. To minimize rates of venous obstruction and atrial arrhythmias after SVD repair, the Warden procedure was proposed in the 1980s. This surgery involves division of the SVC above the level of anomalous pulmonary veins with a neocavoatrial anastomosis made at the RA appendage and a patch closure over the entire SVC orifice to separate it from the RA.23 This arrangement leaves the SVD intact and preserves anomalous pulmonary venous return to the SVC remnant, but the patch directs pulmonary venous return to the LA. Upper thoracic systemic venous return is rerouted to the RA via the neocavoatrial anastomosis created at the RA appendage. Because it spares intricate instrumentation of the posterosuperior RA, the aim was that this approach would be associated with lower rates of pulmonary vein and SVC obstruction and of sinus node dysfunction. The Warden procedure has become preferred for addressing anomalous pulmonary veins that anastomose more cephalad in the SVC, with patch repairs acceptable for anomalous pulmonary veins that anastomose directly into the RA or low SVC.1,24
Follow-Up
Periodic follow-up in the postoperative phase for adult patients after SVD repair is required to monitor for the development of pulmonary vascular disease, sinus node dysfunction, atrial arrhythmias, ventricular dysfunction, and changes in coexisting valvular lesions or other cardiac lesions.1 Clinicians should be mindful of symptoms suggestive of right-sided congestive heart failure, which may indicate residual intracardiac shunt resulting from incomplete repair. Likewise, patients with substantial pulmonary hypertension, persistent arrhythmias, unresolved hypoxemia, or other high-risk clinical features require routine long-term follow-up.1
Prognosis
The indications for SVD closure are outlined in Table 2. Repair should be performed soon after diagnosis.16,25 Patients with SVD repaired successfully in youth demonstrate a life expectancy similar to that of age-matched control subjects. The prognosis of superior SVD repair in adults has been assessed in 2 single-center retrospective series involving long-term follow-up. In 1 study spanning 38 years, outcome was reported for 104 consecutive SVD patients (mean age, 29±23 years; range, 1–70 years) undergoing surgical repair (91 single patch repairs, 7 Warden procedures, and 6 double patch repairs). In that study, 10- and 30-year survival rates were 97% and 79%, respectively,24 which were lower than expected compared with age- and sex-matched control subjects. Older age at time of repair was the only predictor of decreased survival, and older age was also associated with increased rates of major adverse cardiac events. In another series of 109 SVD patients (mean age, 34±23 years; range, 1–80 years) corrected by single patch (n=76), double patch (n=27), or Warden (n=6) procedures,26 observed survival did not differ significantly from expected survival. In that study, older age at repair correlated with postoperative dyspnea and new atrial fibrillation (14% rate overall). About three quarters of patients had improvement in functional class, although 16% had a long-term worsening in symptoms.
Conclusions
This case illustrates that latent congenital heart disease is an important cardiovascular cause of unexplained dyspnea, even in older adults. The pathophysiological consequences of SVD hinge, in part, on the effect of intracardiac shunting on the development of right-sided heart failure. Whenever unexplained RA or RV dilatation is observed, further evaluation by a cardiologist or other appropriate specialist is necessary. The diagnosis of SVD and other similar cardiac lesions often requires a multidimensional approach involving clinical awareness and acumen, cardiopulmonary hemodynamic assessment, and advanced cardiac imaging. Although there are only limited data to guide clinical management of SVD when diagnosed initially in adults, the indications for surgical repair in this patient population align with those for younger cohorts but must be considered in the context of comorbidities unique to this age group that increase perioperative risk, including access to a surgical center with expertise in congenital
Supplementary Material
Acknowledgments
We graciously acknowledge the contributions to the care of the patient by Dr Miguel Haime and the expert editorial assistance and advice from Dr Alexander Opotowsky.
Sources of Funding
This work was supported in part by the National Institutes of Health (1K08HL111207-01A1), Pulmonary Hypertension Association, Pulmonary Vascular Research Institute, and Klarman Foundations at Brigham and Women’s Hospital to Dr Maron.
Footnotes
Disclosures
Dr Maron receives funding to study pulmonary hypertension from Gilead Sciences Inc. Dr Dudzinski received payment from Lippincott for editorial work and consulting fees from Sanofi and AdvantageHealthCare. Dr Bhatt discloses the following: advisory board: Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; Board of Directors: Boston VA Research Institute and Society of Cardiovascular Patient Care; chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committee: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; honoraria: American College of Cardiology (editor, Clinical Trials, Cardiosource), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (editor in chief, Journal of Invasive Cardiology), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today’s Intervention), and WebMD (CME steering committees); other: Clinical Cardiology (associate editor) and Journal of the American College of Cardiology (section editor, Pharmacology); research grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, and The Medicines Company; and unfunded research: FlowCo, PLx Pharma, and Takeda. The contents of this scientific manuscript are the work of the listed authors and do not represent the views of the Department of Veterans Affairs or the US Government. Dr Aragam reports no conflicts.
References
- 1.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e714–e833. [Google Scholar]
- 2.Clarke JC, Aragam JR, Bhatt DL, Brown JD, Ferrazzani S, Pietro DA, Maron BA. An unusual cause of dyspnea diagnosed late in life: severe pulmonary hypertension resulting from isolated anomalous pulmonary venous connection. Circ Cardiovasc Imaging. 2013;6:349–351. doi: 10.1161/CIRCIMAGING.112.000145. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud E, Raisinghani A, Keramati S, Auger W, Blanchard DG, DeMaria AN. Dilation of the coronary sinus on echocardiogram: prevalence and significance in patients with chronic pulmonary hypertension. J Am Soc Echocardiogr. 2001;14:44–49. doi: 10.1067/mje.2001.108538. [DOI] [PubMed] [Google Scholar]
- 4.Kong PK, Ahmad F. Unroofed coronary sinus and persistent left superior vena cava. Eur J Echocardiogr. 2007;8:398–401. doi: 10.1016/j.euje.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Alonso A, Downey BC, Kuvin JT. Uncorrected tetralogy of Fallot in an 86-year-old patient. Am J Geriatr Cardiol. 2007;16:38–41. doi: 10.1111/j.1076-7460.2007.05425.x. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Bhatt DL. Right heart catheterization, coronary angiography, and percutaneous coronary intervention. Circulation. 2011;124:e428–e433. doi: 10.1161/CIRCULATIONAHA.111.065219. [DOI] [PubMed] [Google Scholar]
- 7.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 8.Hillis LD, Firth BG, Winniford MD. Variability of right-sided cardiac oxygen saturations in adults with and without left-to-right intracardiac shunting. Am J Cardiol. 1986;58:129–132. doi: 10.1016/0002-9149(86)90255-9. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson JD. Hemodynamic calculations in the catheter laboratory. Heart. 2001;85:113–20. doi: 10.1136/heart.85.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman W. Shunt detection and quantification. In: Baim DS, editor. Grossman’s Cardiac Catheterization, Angiography, and Intervention. 7th ed Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 163–172. [Google Scholar]
- 11.Johri AM, Rojas CA, El-Sherief A, Witzke CF, Chitty DW, Palacios IF, Passeri JJ, King ME, Abbara S. Imaging of atrial septal defects: echocardiography and CT correlation. Heart. 2011;97:1441–1453. doi: 10.1136/hrt.2010.205732. [DOI] [PubMed] [Google Scholar]
- 12.Pascoe RD, Oh JK, Warnes CA, Danielson GK, Tajik AJ, Seward JB. Diagnosis of sinus venosus atrial septal defect with transesophageal echocardiography. Circulation. 1996;94:1049–1055. doi: 10.1161/01.cir.94.5.1049. [DOI] [PubMed] [Google Scholar]
- 13.Sommer RJ, Hiyazi ZM, Rhodes JF., Jr Pathophysiology of congenital heart disease in the adult: part I: shunt lesions. Circulation. 2008;117:1090–1099. doi: 10.1161/CIRCULATIONAHA.107.714402. [DOI] [PubMed] [Google Scholar]
- 14.Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus venosus defects: unroofing of the right pulmonary veins: anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128:365–379. doi: 10.1016/0002-8703(94)90491-x. [DOI] [PubMed] [Google Scholar]
- 15.Sadler TS. Langman’s Medical Embryology. 11th ed Lippincott Williams & Wilkins; Philadelphia, PA: 2010. Cardiovascular system; pp. 168–172. [Google Scholar]
- 16.Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH. Larsen’s Human Embryology. 4th ed. Churchill Livingstone/Elsevier; Philadelphia, PA: 2009. Development of the heart; pp. 355–359. [Google Scholar]
- 17.Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645–1653. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 18.Webb GD, Smallhorn JF, Therrien J, Redington A. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed Elsevier/Saunders; Philadelphia, PA: 2008. Congenital heart disease; pp. 1411–1467. [Google Scholar]
- 19.Stewart RD, Bailliard F, Kelle AM, Backer CL, Young L, Mavroudis C. Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstruction. Ann Thorac Surg. 2007;84:1651–1655. doi: 10.1016/j.athoracsur.2007.04.130. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber C, Hörer J, Vogt M, Kühn A, Libera P, Lange R, Anderson RH. The surgical anatomy and treatment of interatrial communications. Multimed Man Cardiothorac Surg. 2007;2007 doi: 10.1510/mmcts.2006.002386. mmcts.2006.002386. [DOI] [PubMed] [Google Scholar]
- 21.Nassar M, Fouilloux V, Macé L, Kreitmann B, Metras D. Transcaval correction of partial anomalous pulmonary venous drainage into the superior vena cava. Ann Thorac Surg. 2012;93:193–196. doi: 10.1016/j.athoracsur.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Iyer AP, Somanrema K, Pathak S, Manjunath PY, Pradhan S, Krishnan S. Comparative study of single- and double-patch techniques for sinus venosus atrial septal defect with partial anomalous pulmonary venous connection. J Thorac Cardiovasc Surg. 2007;133:656–659. doi: 10.1016/j.jtcvs.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 23.Warden HE, Gustafson RA, Tarnay TJ, Neal WA. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg. 1984;38:601–605. doi: 10.1016/s0003-4975(10)62317-x. [DOI] [PubMed] [Google Scholar]
- 24.Luciani GB, Viscardi F, Pilati M, Crepaz R, Faggian G, Mazzucco A. Age at repair affects the very long-term outcome of sinus venosus defect. Ann Thorac Surg. 2008;86:153–159. doi: 10.1016/j.athoracsur.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, McGoon DC, Puga FJ, Kirklin JW, Danielson GK. Long-term outcome after surgical repair of isolated atrial septal defect: follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645–1650. doi: 10.1056/NEJM199012133232401. [DOI] [PubMed] [Google Scholar]
- 26.Attenhofer Jost CH, Connolly HM, Danielson GK, Bailey KR, Schaff HV, Shen WK, Warnes CA, Seward JB, Puga FJ, Tajik AJ. Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation. 2005;112:1953–1958. doi: 10.1161/CIRCULATIONAHA.104.493775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.