Abstract
Cardiac muscle relaxation is an essential step in the cardiac cycle. Even when the contraction of the heart is normal and forceful, a relaxation phase that is too slow will limit proper filling of the ventricles. Relaxation is too often thought of as a mere passive process that follows contraction. However, many decades of advancements in our understanding of cardiac muscle relaxation have shown it is a highly complex and well-regulated process. In this review, we will discuss three distinct events that can limit the rate of cardiac muscle relaxation: the rate of intracellular calcium decline, the rate of thin-filament de-activation, and the rate of cross-bridge cycling. Each of these processes are directly impacted by a plethora of molecular events. In addition, these three processes interact with each other, further complicating our understanding of relaxation. Each of these processes is continuously modulated by the need to couple bodily oxygen demand to cardiac output by the major cardiac physiological regulators. Length-dependent activation, frequency-dependent activation, and beta-adrenergic regulation all directly and indirectly modulate calcium decline, thin-filament deactivation, and cross-bridge kinetics. We hope to convey our conclusion that cardiac muscle relaxation is a process of intricate checks and balances, and should not be thought of as a single rate-limiting step that is regulated at a single protein level. Cardiac muscle relaxation is a system level property that requires fundamental integration of three governing systems: intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics.
Keywords: Cardiac relaxation, Diastole, Myofilaments, Calcium handling, Contraction, Kinetics
Introduction
Proper cardiac muscle relaxation is of paramount importance for a healthy circulation. In between two beats, the heart muscle needs to relax to allow for ventricular filling. If the relaxation of the cardiac muscle tissue is impaired, the ventricle will be incompletely filled upon initiation of the next beat, resulting in a low volume at the beginning of contraction. Thus, even if the contractile function (activation) of the heart muscle is completely normal, slowed relaxation would lead to a lack of diastolic volume, and thus to a reduced cardiac output. As a result, despite having a good ventricular ejection fraction, insufficient amounts of oxygenated blood are circulated, and, when left untreated, this can result in development of heart failure and other cardiac pathology. Clinically, aberrant relaxation is referred to as diastolic dysfunction, diastolic heart failure, or heart failure with preserved ejection fraction (HFpEF) (Borlaug and Kass 2008; Janssen and Periasamy 2007). A better understanding of how cardiac muscle relaxation is regulated is needed in order to strategize and optimize treatments for cardiac diseases where muscle relaxation impairment manifests. Although many parameters, including ventricular geometry, drugs, and pathologies, are known to impact cardiac relaxation, we will specifically focus this review on the basic relaxation properties and regulation in the healthy heart muscle.
The heart is only in equilibrium once
From its first beat in utero until death, the heart is in a dynamically changing contractile state. At no point during a normal lifespan are the governing parameters of contractile strength and kinetics in equilibrium or at steady-state. In fact, heart rate variability is a sign of a healthy heart (Counihan et al. 1993; Torres et al. 2008), and this heart rate variability is diminished with both disease and with aging (Corino et al. 2007). Thus, even under resting conditions, heart rate, wall-stress, cytosolic ion concentrations, and ion channel activities are never in equilibrium. How quickly these governing processes can respond to affect relaxation depends on the dynamic temporal nature of the individual processes. In addition, many of the rate-limiting steps of these processes are adjusted to modulate contraction and relaxation kinetics (ultimately controlling cardiac output) when the body’s demand for oxygen changes (Janssen 2010a). The strength and speed of this dynamic contraction is regulated via several mechanisms in order to adjust cardiac output to meet the bodily demand for oxygen. The three main physiological mechanisms that change cardiac muscle strength are length-dependent activation (ter Keurs et al. 1980), frequency-dependent activation (Bouchard and Bose 1989; Janssen and Periasamy 2007), and adrenergic stimulation (Bers and Ziolo 2001; Kranias and Solaro 1982). These governing mechanisms of cardiac output impact not only individual parameters that feed into cardiac muscle strength and kinetics but they also impact interactions between parameters. Thus, the cross-talk between these mechanisms further complicates the dynamic nature of cardiac inotropy and lusitropy. Most, if not all, these parameters, interactions, and governing mechanisms impact on the relaxation phase of cardiac muscle (Brutsaert et al. 1978).
Governing of relaxation
The governing of cardiac relaxation stems from the complex interaction of a multitude of ion movements and protein interactions, each with their own temporal resolution of function. Furthermore, these processes impact on physical entities such as sarcomere length and tension/stress, that in turn feed back on the interaction between processes, as well as on themselves (Rice et al. 1999; Hofmann and Fuchs 1987; de Tombe 1998). This highly dynamic nature, where none of the interactors are in equilibrium, makes understanding of the governing processes difficult. Three interactive processes are thought to contain potentially rate-limiting properties and have put them at the forefront of investigation into governing of cardiac muscle relaxation: (1) the rate at which the calcium is lowered in the cytosol (Fig. 1); (2) the rate at which the thin filament deactivates (Fig. 2); and (3) the rate at which actin-myosin (cross-bridges) interact (Fig. 3). In the first part of this review, we will address these main mechanisms, and their direct impact of relaxation. In the second part, we discuss how these mechanisms and interactions are regulated and modified when the demand for cardiac output changes.
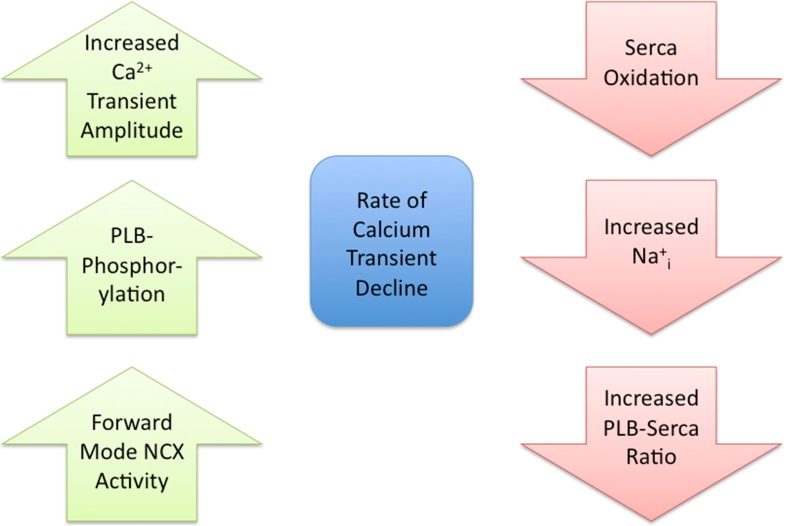
Fig. 1.
Regulation of the rate of intracellular calcium transient decline. Left column 3 examples (no particular order) of molecular processes that fasten the rate of intracellular calcium transient decline. PLB phospholamban; NCX sodium–calcium exchanger. Right column 3 examples of molecular processes that slow down the rate of intracellular calcium transient decline
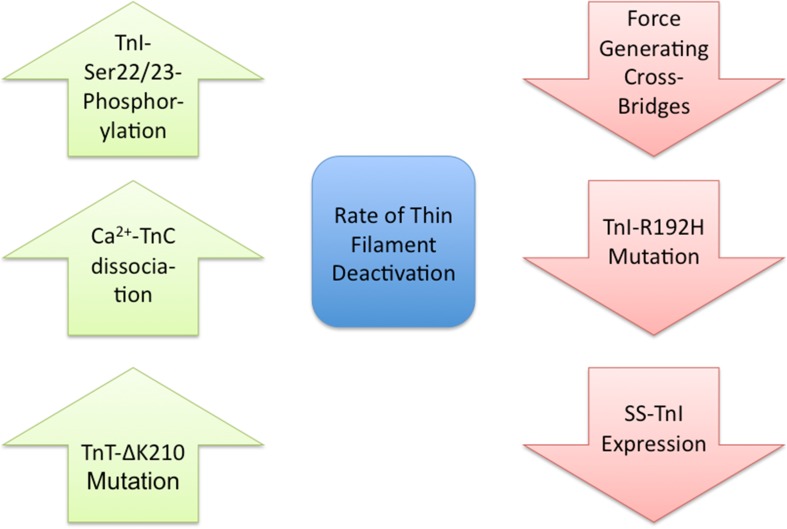
Fig. 2.
Regulation of the rate of thin filament deactivation. Left column 3 examples (no particular order) of molecular processes that fasten the rate of thin filament deactivation. TnI troponin-I; TnC troponin-C; TnT troponin-T; SS slow skeletal. Right column 3 examples of molecular processes that slow down the rate of thin filament deactivation
Fig. 3.
Regulation of the rate of cross-bridge cycling. Left column 3 examples (no particular order) of molecular processes that fasten the rate of cross-bridge cycling. MyBP-C myosin-binding protein C. Right column 3 examples of molecular processes that slow down the rate of cross-bridge cycling. RLC regulatory light chain
Calcium transient decline
An increase in cytosolic Ca2+ (i.e., systolic Ca2+) is required for initiation of contraction. Within ventricular myocytes, this increase in Ca2+ is achieved by Ca2+ influx through L-type Ca2+ channels (LTCC) and Ca2+ expelled from the sarcoplasmic reticulum (SR) by the SR Ca2+ release channel (ryanodine receptor, RyR) in a process termed Ca2+-induced Ca2+-release (CICR) (Bers 2002a). The rate at which systolic Ca2+ (also known as the Ca2+ transient) declines is an important factor that contributes to relaxation, since it is the initiating event for the process, and this decline can be modulated via many molecular events (Fig. 1). While LTCC and RyR do not directly contribute to this decline, the systolic [Ca2+]i is a determinant of the rate of Ca2+ decline (Bers and Berlin 1995; Roof et al. 2011). During systole, cytosolic Ca2+ levels are lowered via four Ca2+ transport systems. These systems remove Ca2+ from the cytosol into the SR via the SR Ca2+ ATPase, the mitochondria via the Ca2+ uniporter and out of the myocyte via the Na+/Ca2+ exchanger (NCX) and sarcolemmal (SL) Ca2+ ATPase.
The SR is the major Ca2+ storage organelle within the myocyte. In order to maintain a normal calcium SR load over time, approximately the same amount of Ca2+ dispensed from the SR during CICR needs to be returned to the SR. This occurs via the cardiac SR Ca2+ ATPase isoform (SERCA2a). Located mostly at the longitudinal tubules of the SR, SERCA2a pumps 2 Ca2+ ions per ATP molecule. SERCA2a activity is regulated by its endogenous inhibitor phospholamban (PLB). PLB binding to SERCA2a decreases Ca2+ transport into the SR by decreasing the Ca2+ affinity of the pump without altering Vmax. In mice, it has been proposed that ~40 % of the pumps are basally regulated by PLB, while it is ~60 % in rabbits (Waggoner et al. 2009).
Ca2+ enters the mitochondria via the uniporter down a large electrochemical gradient (Williams et al. 2013). Mitochondrial Ca2+ uptake plays little role in decreasing cytosolic Ca2+ levels on a beat-to-beat basis (discussed further below) but is important in regulating mitochondrial energy production (Moravec et al. 1997).
The Na+/Ca2+ exchanger, NCX1, is located on the sarcolemma and functions by exchanging one Ca2+ ion for three Na+ ions. NCX1 is able to remove Ca2+ from the myocyte (forward mode) or bring Ca2+ into the myocyte (reverse mode) (Ottolia and Philipson 2013). During normal cardiac function, the forward mode contributes to the decline of the Ca2+ transient.
The SL Ca2+ ATPase pumps 1 Ca2+ ion per ATP molecule out of the myocyte . The SL Ca2+ ATPase plays little role in decreasing cytosolic Ca2+ levels (discussed further below) but is involved in modulating various signaling pathways (Bassani et al. 1995; Oceandy et al. 2007).
Role of each Ca2+ transport system
Each of the above Ca2+ transport systems contributes differently, in both timing and quantity, to the decline of the Ca2+ transient. The majority of the Ca2+ transient decline is due to SERCA2a. There are species differences in the fraction of the Ca2+ transient decline via SERCA2a. For example, in mouse and rat myocytes, >95 % of the decline is due to SERCA2a, while in larger mammals (rabbit, dog, human), only ~70 % of the decline is due to SERCA2a (Milani-Nejad and Janssen 2014). This corresponds to the number of myocyte Ca2+ pumps in the various species (i.e., there are more pumps in mouse/rat than rabbit/dog) (Bers 2001). Another major contributor to the decline of the Ca2+ transient is NCX1. To maintain normal calcium levels inside the myocyte, the Ca2+ that entered the myocyte through the LTCC must be removed. The vast majority of this Ca2 is removed via NCX1. As with SERCA2a, there are species differences in the fraction of the Ca2+ transient decline that is due to NCX1 (Bers 2002b). In mouse and rat myocytes, <4 % of the decline is due to NCX1, while in larger mammals, ~28 % of the decline is due to NCX1. This corresponds nicely to the amount of inward NCX1 current in the various species (i.e., greater NCX1 current in dog/rabbit vs. rat/mouse). The other two systems (mitochondrial Ca2+ uniporter and SL Ca2+ ATPase) together account for ~1 % of the Ca2+ transient decline (Puglisi et al. 1996), and thus have little effect in modulating relaxation, and will not be further discussed in this review.
In addition to species differences of the various Ca2+ transport systems, there are shifts in the contribution of these systems in disease. For example, in human and rabbit heart failure, there is a reduction in SERCA2a expression and an increase in NCX1 expression (Houser et al. 2000). These protein changes result in a reduced contribution of the SR Ca2+ ATPase transport system to ~50 % of the Ca2+ transient decline, and an increase in the contribution of the NCX1 transport system to ~50 % of the Ca2+ transient decline (Piacentino et al. 2003). Since SERCA2a has a much greater Ca2+ transport rate than NCX1, these protein expression changes result in a general slowing of Ca2+ transient decline (Ziolo et al. 2004). There is also a slowing of the Ca2+ transient decline with acidosis and an associated increase in diastolic [Ca2+]i (Westerblad and Allen 1993; DeSantiago et al. 2004). The molecular mechanisms responsible for the slowed [Ca2+]i decline are a reduction in SR Ca2+ uptake (SERCa2a activity) and decreased forward mode of NCX. These data demonstrate that, in addition to protein expression levels, Ca2+ transient decline can also be acutely modulated.
Regulation of Ca2+ transient decline by post-translational modifications
The prototypic post-translational modification (PTM) to acutely modulate protein function is phosphorylation. In addition to being an endogenous inhibitor of SERCA2a, PLB is also a key phosphoprotein in the heart (MacLennan and Kranias 2003). PLB can be phosphorylated at Serine16 by the cAMP-dependent protein kinase (PKA) and at Threonine17 by the Ca2+/CaM-dependent protein kinase (CaMKII). Phosphorylation at either site relieves PLB inhibition of SERCA2a leading to a substantial increase in the Ca2+ pump rate to greatly hasten Ca2+ decline and relaxation. The role of phosphorylation to modulate NCX activity is controversial. While a study did show that PKA can upregulate NCX activity (Perchenet et al. 2000), this effect is not universally observed (Ginsburg and Bers 2005). Nevertheless, we believe if PKA can upregulate NCX activity that this will have little effect in hastening Ca2+ decline under physiological conditions. PKA is activated via β-adrenergic stimulation (discussed below) and with the PKA-mediated phosphorylation of PLB, the Ca2+ decline rate even in large mammals is almost entirely (>90 %) due to SERCA2a activity (Waggoner et al. 2009).
Besides direct phosphorylation of Ca2+ handling proteins by PKA, an emerging area is demonstrating that other signaling pathways (in addition to β-AR stimulation) can also directly regulate PKA. For example, we have shown that neuronal nitric oxide synthase (NOS1) signaling results in increased PLB Serine16 phosphorylation to hasten Ca2+ transient decline (Wang et al. 2008). We further demonstrated that PKA activation was a direct, cAMP-independent effect of peroxynitrite, the signaling molecule of NOS1 (Kohr et al. 2010). In fact, we observed that NOS1 modulation of PLB phosphorylation is a key determinant of the basal (non-stimulated) Ca2+ transient decline rate (Roof et al. 2012). Protein phosphorylation levels are not only determined by kinase activity but also by the removal of the phosphate by phosphatases (PP), in which their activity can also be modulated. Interestingly, while low levels of peroxynitrite activate PKA, high levels of peroxynitrite activate PP resulting in the dephosphorylation of PLB and slowed Ca2+ decline (Kohr et al. 2008). In addition to modulating kinase and PP activity, peroxynitrite, and other reactive oxygen and nitrogen species (ROS, RNS) can also directly (i.e., oxidation, S-glutathionlyation, S-nitrosylation) modulate Ca2+ handling proteins to alter the Ca2+ transient decline rate. For example, SERCA2a can be S-nitrosylated/S-glutationlated to increase activity (Bencsik et al. 2008; Lancel et al. 2009) or oxidized to decrease activity (Lancel et al. 2010; Morris and Sulakhe 1997; Scherer and Deamer 1986; Xu et al. 1997) (Fig. 1). These changes in SERCA2a activity will result in a corresponding change in the Ca2+ transient decline rate. It has also been demonstrated that SERCA2a can undergo SUMOylation to increase activity. In HF patients, decreased SERCA2a SUMOylation contributes to the decreased SERCA2a activity in this syndrome (Kho et al. 2011).
In addition to SERCA2a, PLB is also modulated by RNS. For example, we have shown that RNS (specifically nitroxyl) modifies PLB cysteines to increase PLB pentamer formation, decreasing monomeric PLB (the form that inhibits SERCA2a) and resulting in a faster Ca2+ transient decline (Sivakumaran et al. 2013; Kohr et al. 2010).
While NCX phosphorylation is debatable, it has been clearly demonstrated that NCX can be oxidized leading to increased activity (Goldhaber 1996; Kuster et al. 2010; Reeves et al. 1986). While an increase in NCX activity would enhance the rate of [Ca2+]i decline, one must remember that total myocyte oxidation will also result in SERCA2a oxidation to actually slow Ca2+ transient decline. However, the enhanced NCX activity with oxidation will, however, limit the increase in diastolic Ca2+ levels.
Since precise Ca2+ decline is so vital for proper relaxation, not only are protein expression levels important, the heart has constructed ways to acutely modulate Ca2+ transient decline via various post-translational modification (discussed further below).
The calcium–troponin interaction
While the rate of cytosolic Ca2+ decline is an important and initiating event in the process of relaxation, it is ultimately the removal of Ca2+ from troponin and subsequent deactivation of the thin filament that inhibits the interaction of myosin with actin allowing myocardial relaxation and diastolic ventricular filling. To understand how the thin filament modulates myocardial relaxation one must first understand how Ca2+ interacts with troponin to regulate the thin filament between the active and inactive states.
The interaction of Ca2+ with troponin is essential to the regulation of the thin filament. While intracellular Ca2+ is the signal initiating the interaction of myosin with actin, and thus contraction, Ca2+ itself does not directly regulate this interaction. Instead, the signaling molecule Ca2+ functions through the thin filament regulatory proteins: tropomyosin (Tm) and the troponin complex (troponin C (TnC), troponin I (TnI) and troponin T (TnT)) (for detailed reviews, see Kobayashi and Solaro 2005; Solaro and Westfall 2005; Tardiff 2011; Davis and Tikunova 2008).
During elevated systolic levels of intracellular free Ca2+, Ca2+ is bound to TnC of the troponin complex to maintain the activated state of the thin filament. In this activated state, TnI is bound to TnC stabilizing TnC-Ca2+ binding and Tm occupies a position on actin-exposing myosin binding sites. As long as the thin filament is in this active state, myosin cross-bridges will freely cycle with actin and the muscle will contract.
For cardiac muscle relaxation to ensue, the thin filament must be inactivated to block the interaction of myosin with actin. As the result of intracellular Ca2+ decline by the mechanisms discussed above, free cytosolic Ca2+ eventually drops below the threshold for binding to TnC and Ca2+ disassociates. This disassociation of Ca2+ weakens TnI-TnC binding to favor TnI inhibitory binding to actin. Additional changes in TnT and Tm contribute to the movement of Tm into the “closed” position on actin causing deactivation of the thin filament. In the inactive state, these regulatory proteins sterically block the interaction of myosin with actin to promote relaxation of the cardiac muscle.
Influences on the rate of thin filament deactivation
While cardiac muscle relaxation is clearly initiated by the Ca2+ transient decline, the significant lack of Ca2+ transient and mechanical relaxation coupling in time (e.g., the Ca2+ transient decays prior to force dissipation) indicates a role of the myofilament in the regulation of relaxation (Monasky et al. 2008; Gordon et al. 2000). The dynamics of relaxation are therefore not solely determined by the rate of the cytosolic Ca2+ transient decline but are therefore also determined by the rate of thin filament return to the deactivated state (Fig. 2).
Force generating cross-bridges are critical to the thin filament transition from the active to the inactive state. For the thin filament to enter the inactive state, cross-bridges must release from actin such that Tm and TnI can block myosin rebinding. While the rate of cross-bridge release from actin directly contributes to the rate of relaxation (discussed below), cross-bridge detachment also indirectly decreases the affinity of TnC for Ca2+ and accelerates deactivation of the thin filament (Pan and Solaro 1987; Davis et al. 2007). Although the release of force producing cross-bridges is critical to deactivation, the rate of Ca2+ dissociation from TnC is essential for thin filament deactivation and therefore imparts a significant influence on relaxation (Davis and Tikunova 2008).
The rate of Ca2+ dissociation from the thin filament can be increased or decreased by troponin and Tm isoform switching, mutations and PTMs (Ertz-Berger et al. 2005; Kentish et al. 2001; Nixon et al. 2012, 2013; Liu et al. 2012, 2014; Davis et al. 2007; Rice et al. 2010). As discussed above, transition from the active to inactive state of the thin filament and relaxation involves not only the disassociation of Ca2+ from TnC but also the rebinding of TnI to actin, structural changes in TnT, and Tm movement. Therefore, the alteration in any of these protein–protein transitions could modulate the rate of thin filament deactivation. With the exception of Ca2+ disassociation from TnC, the rates for the majority of these protein transitions have not been elucidated. In the future, a detailed understanding of these protein transition rates will be important to fully understand the complex nature of thin filament deactivation. It is clear, however, that the PTM of the regulatory proteins represents a significant mechanism to affect the rate of thin filament inactivation by altering the protein–protein interactions that modulate the rate of Ca2+ disassociation form TnC.
Regulation of thin filament deactivation by PTM
The PTM of myocardial proteins has evolved as a significant mechanism to rapidly modulate myocardial muscle mechanics on a beat-to-beat basis (Solaro 2001). The majority of regulatory thin filament proteins undergo PTM to rapidly alter Ca2+ binding to, and/or disassociation from, TnC to modulate the rate of relaxation (Nixon et al. 2012, 2013; Liu et al. 2014). Within the thin filament, a number of PTMs can occur; however, phosphorylation represents the most prevalent myofilament signaling PTM to date. Phosphorylation occurs in all thin filament proteins except TnC and actin.
To discuss the role of thin-filament PTM on myocardial relaxation regulation, we present the prototypical effects of cardiac TnI Ser-23 and Ser-24 phosphorylation via PKA (Fig. 2). The dual phosphorylation of TnI at Ser-23/24 is a well-defined thin filament phosphorylation regulatory response (Kranias and Solaro 1982; Kentish et al. 2001; Pena and Wolska 2004; Kooij et al. 2010; Biesiadecki et al. 2007a). The phosphorylation of cardiac TnI at Ser-23/24 exists at approximately a 40 % basal baseline state (Ayaz-Guner et al. 2009). Changes from this baseline state represent a critical mechanism to modulate beat-to-beat relaxation alterations in response to both physiological and pathological cardiac stress. Increases in TnI Ser-23/24 phosphorylation result in decreased Ca2+ binding to TnC evident experimentally as decreased TnC Ca2+ affinity and an acceleration in the rate of Ca2+ dissociation from TnC. These changes in TnC Ca2+ binding result in accelerated thin filament deactivation (Kentish et al. 2001; Nixon et al. 2012, 2014; Walker et al. 2011). Accordingly, TnI Ser-23/24 phosphorylation and the transgenic expression of pseudo-phosphorylated TnI (Ser-23/24 mutated to Asp) in the mouse heart results in accelerated muscle contraction and heart relaxation (accelerated -dP/dt) (Kentish et al. 2001; Zhang et al. 1995; Takimoto et al. 2004; Pena and Wolska 2004; Yasuda et al. 2007). Thus, TnI Ser-23/24 phosphorylation-induced decreased TnC-Ca2+ affinity results in in vivo acceleration of diastolic cardiac relaxation. In addition to altering relaxation through TnC Ca2+ affinity, TnI Ser-23/24 has also been implicated in downstream modulation of the myosin–actin cross-bridge, while others question such an effect (Turnbull et al. 2002; Strang et al. 1994; Biesiadecki et al. 2007b; Bilchick et al. 2007; Stelzer et al. 2007; Tong et al. 2008).
A number of thin filament protein residues besides TnI Ser-23/24 undergo phosphorylation. TnI can be phosphorylated at varied residues, while TnT and Tm are also phosphorylated (Buscemi et al. 2002; Perry 1999; Zhang et al. 2012; Jideama et al. 1996; Sumandea et al. 2003; Mak et al. 1978). The functional effects of the majority of these phosphorylation events have been demonstrated in isolation; however, the question of their physiological integration largely remains. In response to altered myocardial demand, multiple thin filament phosphorylation events occur simultaneously in the heart. The functional effects of these integrated phosphorylation events are largely unknown.
Recent evidence has demonstrated the function of TnI Ser-23/24 phosphorylation in myocardial regulation is dependant upon other myofilament protein mutations, PTMs and TnI phosphorylations (Biesiadecki et al. 2007a; Boontje et al. 2011; Nixon et al. 2012, 2014; Deng et al. 2001). Recently, we demonstrated that, while TnI Ser-150 phosphorylation slowed TnC Ca2+ disassociation, when TnI Ser-150 and Ser-23/24 phosphorylation occurred together Ca2+ dissociation kinetics were accelerated (Nixon et al. 2012, 2014). The effect of TnI Ser-150 on TnC Ca2+ disassociation is therefore dependent upon its integration with TnI Ser-23/24 phosphorylation. These findings begin to demonstrate the importance of investigating thin filament phosphorylation as the integrated events that occur in response to increased myocardial demand.
Thin filament proteins can also undergo a number of other non-phosphorylation PTMs. These non-phosphorylation thin filament PTMs include modifications such as proteolysis, glycosylation, oxidation, and nitrosylation. Again, as a model, we discuss TnI. In addition to phosphorylation, TnI can also undergo proteolysis and O-glycosylation. In isolation, these PTMs have been shown to directly modulate thin filament deactivation. During myocardial ischemia, TnI undergoes selective proteolysis removing the 17 C-terminal amino acids that result in increased TnC Ca2+ affinity and slowed myofibril relaxation kinetics (Narolska et al. 2006; McDonough et al. 1999; Foster et al. 2003). On the other hand, the modification of TnI Ser-150 by O-glycosylation decreases TnC Ca2+ affinity (Ramirez-Correa et al. 2008). Little work has been done to investigate the integrated effects of non-phosphorylation thin filament PTMs with phosphorylation on thin filament deactivation and myocardial relaxation.
Overall, it is becoming clear from our data as well as that of others that the effects of multiple thin filament protein PTMs cannot be deduced from their individual effects alone. The lack of the Ca2+ transient and mechanical relaxation coupling in time, and our recent findings indicating the significance of TnI phosphorlyation to myocardial relaxation (Monasky et al. 2010, 2013), necessitates further investigation into the role of both isolated and integrated thin filament PTMs on the regulation of myocardial relaxation.
Cross-bridge cycling
It is necessary to keep in mind what precisely cardiac muscle is relaxing from: pressure caused by high wall stress. The stress and strains within cardiac muscle tissue is caused by forces and motions generated within the myocytes that make up the bulk of the cardiac walls. These forces and motions are generated by energy demanding interactions between the numerous interdigitating thin and thick filament proteins actin-Tm-Tn and myosin. This process, cross-bridge cycling, is modulated by many factors (Fig. 3). While the removal of cytosolic Ca2+ and deactivation of the thin filament are necessary to inhibit cross-bridge formation, it is ultimately the structural changes specifically within the motor protein myosin that are responsible for relaxation of the myocytes (for review, see Gordon et al. 2000).
The kinetic behavior of myosin has been proposed to possess within its cycle the rate-limiting step of cardiac isolated myofibrils relaxation (Stehle and Iorga 2010). There is ample evidence that altering (either increasing or decreasing) the rate at which cardiac muscle myosin utilizes its fuel source, ATP, can have profound effects on influencing the rates of both cardiac muscle contraction and relaxation (Gordon et al. 2000). Changes in the expression pattern between the two cardiac myosin isozymes may be the best example where a change in myosin kinetics alters both cardiac muscle contraction and relaxation and therefore the overall function of the heart (Fitzsimons et al. 1998). Not unique to cardiac muscle, classic experiments have demonstrated that different muscles (striated, smooth, fast-twitch, slow-twitch, and cardiac) found across the animal kingdom exhibit an extremely wide-ranging and linear relationship between the maximal speeds of muscle shortening and myosin’s ATPase rate (a mechanical event coupled to a biochemical reaction) (Barany 1967). Across numerous species and experimental conditions, there is also a strong correlation between the speeds at which a cardiac muscle contracts and relaxes (Janssen 2010b). Considering myosin plays a fundamental role in both phenomena, it would appear that myosin is at minimum tuned kinetically, if not rate-limiting, for the required mechanical performance of the heart.
Six separate polypeptide chains (two heavy, ~200-kD, chains and four light, ~17-kD, chains) form the myosin molecule (for review, see Gordon et al. 2000). The two heavy chains coil around one another at their C-termini forming a long double helix. This long helix packs tail to tail to form a large part of each ~1-um-long thick filament. Protruding from the helical core of the thick filaments are the remaining heavy chains that eventually split into individual lever arms attached to separate globular heads. Each lever arm is stabilized by the binding of a single essential and regulatory light chain. The globular head contains all the necessary machinery to transfer the chemical energy of ATP into mechanical power when coupled with actin. Each myosin head can only generate ~5–10 pN of force (comparable to the weight of a single E. coli bacterium or the force generated by a HeNe laser pin shown on a surface) or move ~10 nm (slightly longer than the distance across the plasma membrane) (Takagi et al. 2006). However, packed within each cardiac myocyte are approximately 2 billion myosin heads, while there are approximately 6 billion myocytes in the human left ventricle (Beltrami et al. 2001). This is more than enough myosin to build and sustain the ventricular pressure required to pump the blood throughout the body.
At any given moment toward the end of systole, there are a large number of cycling myosin strongly bound to actin (although not known, this number is probably less than 20 % of the total number of myosin available within the muscle). These myosins are also tightly bound by adenosine diphosphate (ADP). Before the myosin can detach from the thin filament, allowing the muscle to relax and relinquish the pressure within the chamber, ADP must dissociate from the acto–myosin complex (for review, see Gordon et al. 2000). Once this happens, one of the ample supply of cellular ATP (~10 mM) nearly instantaneously binds the nucleotide free rigor head causing it to relinquish its grip on actin. Very quickly after myosin dissociates from actin, the head hydrolyzes the bound ATP into tightly bound inorganic phosphate (Pi) and ADP. The high energy stored in the terminal phosphate bond of ATP is used to swing the lever arm (and the head with it) into a pre-power stroke position - akin to cocking the hammer back on a pistol. In essence, the myosin is now energized and ready to go, a state in which the majority of cardiac myosin is typically found, especially during late diastole. Due to the close proximity of the thin filament to the myosin heads (essentially a very high effective concentration of actin), myosin can quickly search for and find another actin if the thin filament is still activated. Once myosin finds another actin, it re-attaches weakly at first. Soon after rebinding to actin, the myosin head releases its bound Pi. Simultaneously with the release of Pi, myosin performs its powerstroke, swinging the lever arm back to its original position. This generates a force on the actin filament that if not resisted will move the thin filament and shorten the myocyte. Once again, myosin is tightly bound to actin and cannot dissipate the force until ADP dissociates. This cross-bridge cycle will continuously occur as long as a myosin can find an available actin. Both Ca2+-bound thin filaments and strongly bound cross-bridges increase the probability that a detached myosin head will find an available actin (for review, see Gordon et al. 2000). In these regards, the biochemical reaction of ADP dissociation may be the rate-limiting step of the mechanical events of cardiac muscle relaxation (Siemankowski et al. 1985; Little et al. 2012)
It is not surprising then that there are numerous biochemical and mechanical influences on the rate of cardiac muscle relaxation that likewise affect ADP dissociation. Forces that resist the motion of the powerstroke (preload) slow the rate of ADP dissociation and relaxation, while forces that lower the burden on any one particular myosin help to accelerate ADP dissociation and relaxation (especially movements in the same direction of the powerstroke, i.e. other myosin powerstrokes) (Nyitrai and Geeves 2004). Intracellular environmental changes, such as elevated [ADP] and acidosis also slow the rate of ADP dissociation, whereas an increase in [Pi] accelerates relaxation in several skinned cardiac preparations (Simnett et al. 1998; Herzig et al. 1981), and in human atrial and ventricular myofibrils (Piroddi et al. 2007). As noted above, different isozymes of myosin have intrinsically different rates of ADP dissociation and rates of relaxation (Fitzsimons et al. 1998; Palmiter et al. 1999; Suzuki et al. 2009). The rate of cardiac muscle relaxation inversely scales with body size (Janssen 2010a). Similarly, the smaller the mammal, the faster the cardiac myosin releases ADP (predominately the alpha MHC lineage in small rodents and the beta MHC lineage in larger mammals) (Palmiter et al. 1999; Suzuki et al. 2009). Within a species, alpha myosin has a two–five times faster rate of ADP dissociation than does beta myosin. Across species, the smaller the mammal, the faster both classes of myosin release ADP. Thus, there is a clear correlation between the rates at which cardiac muscle relax to the biochemical rate of ADP dissociation.
In addition to the regulation of relaxation through the effects of Ca2+ concentration and thin filament deactivation, the actin–myosin cross-bridge cycling itself can be regulated to modulate cardiac muscle relaxation through thick filament-associated regulatory proteins. The most significant of these include the myosin light chains, myosin binding protein C, and titin. The effects of these thick filament regulatory proteins on cardiac muscle function in general have been previously reviewed (Kamm and Stull 2011; Szczesna 2003; Hernandez et al. 2007; Barefield and Sadayappan 2010; Granzier et al. 2005; LeWinter and Granzier 2010; Kruger and Linke 2011); here, we focus on their contribution to the regulation of myosin cross-bridge cycling and their effects on cardiac muscle relaxation.
Directly associated with the lever arm domain of each myosin motor are two constitutively bound myosin light chains: the essential light chain (ELC) and the regulatory (phosphorylatable) light chain (RLC). Both these myosin light chains interact with myosin in the myosin neck region and provide mechanical stability. This location is significant as the neck region is critical to myosin cross-bridge cycling and thus positions the light chains in an optimal position to directly modulate myosin cycling. Expression of the ELC in cardiac muscle consists of atrial and ventricular isoforms (Hernandez et al. 2007). The expression of these isoforms differentially modulates myosin cross-bridge kinetics with the atrial isoform exhibiting increased myosin cross-bridge cycling compared to that of the slower ventricular isoform (Hernandez et al. 2007). In normal adult ventricular myocardium, the ventricular ELC is the solely expressed isoform. During cardiac disease, the atrial isoform can become expressed, resulting in increased actin–myosin cross-bridge cycling and altered myocardial relaxation. The modulation of cardiac muscle relaxation through the ELC is largely limited to the effects of these isoform alterations and therefore imparts functional effects on a long-term basis. Similar to the ELC, the RLC is expressed as ventricular and atrial chamber-specific isoforms that demonstrate different effects on cross-bridge regulation. The RLC is a phospho-protein maintained at a basal level of phosphorylation in the normal heart (Metzger et al. 1989; van der Velden et al. 2003; High and Stull 1980). Alteration of this basal phosphorylation level can modulate the actin–myosin cross-bridge on a beat-to-beat basis. Phosphorylation of the RLC by the myosin light chain kinase family (Kamm and Stull 2011) to induce direct alteration of the myosin structure results in its displacement away from the thick filament (Levine et al. 1996, 1998; Colson et al. 2010). This displacement moves the myosin head closer to actin and increases the likelihood of forming the actin–myosin cross-bridge. While this increased cross-bridge formation increases the rate of force development (Olsson et al. 2004), it results in a slowed rate of relaxation (Patel et al. 1998) as the result of the increased likelihood of cross-bridge rebinding to actin at an unaffected detachment rate. Thus, through either isoform switching or specific phosphorylation, the myosin light chains are critical to the modulation of cross-bridge kinetics and cardiac relaxation.
Similar to the myosin light chains, myosin binding protein-C (MyBP-C) directly associates with the myosin head; however, MyBP-C binding does not occur on every myosin head. In the ventricle, MyBP-C is arranged in 7–9 “stripes” perpendicular to the long axis of the myosin filaments (Luther et al. 2008; Tong et al. 2008). While MyBP-C is expressed as three isoforms in various striated muscles, only the cardiac isoform is expressed in ventricular muscle. The location of MyBP-C both contributes to the structural organization of the myofilament and invokes a modulatory effect on actin–myosin cross-bridge. Similar to the RLC, the cardiac MyBP-C isoform is a phospho-protein able to modulate myosin cycling on a beat-to-beat basis (Gautel et al. 1995; Yuan et al. 2006, 2008). The cardiac-specific phosphorylation of MyBP-C can become altered in response to cardiac stress and in cardiac disease through various signaling pathways (Kuster et al. 2012). The significance of MyBP-C phosphorylation to modulate the actin–myosin interaction has been demonstrated following PKA phosphorylation (Gautel et al. 1995). Phosphorylation of the MyBP-C PKA sites induces a structural change in the myosin head, moving the myosin cross-bridge away from the thin filament backbone and increasing its proximity to actin (Weisberg and Winegrad 1996; Sadayappan et al. 2006). This increased proximity of the myosin to actin has been proposed as the mechanism of MyBP-C phosphorylation to increase actin–myosin cross-bridge cycling and impact on relaxation (Stelzer et al. 2007; Tong et al. 2008). Thus, the dynamic phosphorylation of MyBP-C functions as a significant mechanism to modulate the actin–myosin interaction. Indeed, a truncated, non-functional form of MyBP-C in the mouse (Palmer et al. 2004) showed a significant impact on the balance between speed of contraction and speed of relaxation in isometric muscles under a variety of conditions (Janssen 2010a, b), likely indicating MyBP-C as a critical and structural component in governing cardiac kinetics.
The titin molecule functions as a myofibrilar scaffolding protein spanning from the Z-disk to the M-band (Granzier and Irving 1995). In addition to this scaffolding role, titin also functions as a molecular spring contributing to the rate of cardiac relaxation. Titin contains a number of various elements that are altered during sarcomere re-lengthening to generate an elastic recoil, thus altering the passive tension of the muscle and aiding in muscle re-lengthening (LeWinter and Granzier 2010; Kruger and Linke 2011). In the normal heart, titin is co-expressed as 2 isoforms that exhibit different spring properties and therefore elastic recoil. The expression ratio of these isoforms can become altered in various cardiac diseases to affect titin stiffness and cardiac relaxation (Wu et al. 2002). In addition to isoform switching titin is also a phospho-protein (Anderson and Granzier 2012; James and Robbins 2011). While a number of titin phosphorylation sites are proposed, not all function to affect relaxation (for review, see LeWinter and Granzier 2010). Two kinase specific phosphorylations of titin have, however, been demonstrated to specifically modulate cardiomyocyte passive tension and myocardial relaxation, PKA and protein kinase C (PKC). In humans, titin phosphorylation of the elastic N2B-Bus region by PKA increases the persistence length of this region, reducing passive tension resulting in decreased myocardial stiffness and improved diastolic filling (Yamasaki et al. 2002; Kruger et al. 2006, 2009). Titin is also phosphorylated by PKCα in the PVEK region. Opposite to PKA phosphorylation, PKC phosphorylation reduces persistence length of the PEVK region to elevate titin-based passive tension which may also contribute to impaired myocardial relaxation (Hidalgo et al. 2009). Thus, through isoform switching and the phosphorylation of varied regions, titin functions to modulate cardiac myocyte shortening and myocardial relaxation.
Length-dependent activation
Length-dependent activation is the muscle-equivalent of the Frank–Starling mechanism (de Tombe et al. 2010; Frank 1895; Starling 1918). As the ventricular volume increases (i.e. longer muscle/sarcomere length), intrinsic pressure (i.e. force) development is increased. In addition to the impact on force, the kinetics of muscle contraction and relaxation are also impacted by muscle length (Edman 1979; Allen and Kurihara 1979; Janssen and Hunter 1995). At a longer muscle length, the time needed to reach peak isometric force is longer, and the time required for the muscle to relax is likewise significantly prolonged (Kentish et al. 1986; Janssen and Hunter 1995). Often, the first derivative of pressure (dP/dt) is taken as an indicator of contractile performance. Due to the increased pressure (or tension/stress in case of a linear muscle), maximal dP/dt increases, and this is often mistakenly interpreted as faster contractile kinetics. Similarly, the maximal fall of pressure/tension, the minimal value of dP/dt, is often erroneously interpreted as accelerated relaxation. When dP/dt is corrected for pressure development (i.e. divided by P), then the true kinetic timing parameter (s−1) typically shows that, with increased muscle length, kinetics of the contraction and relaxation are slower (Janssen 2010b). After a step-change in muscle length, the changes in kinetics of force are much more pronounced than changes in the Ca2+ transient decline rate (Allen and Kurihara 1982). In fact, at steady state, taken as a pure rate (s−1), Ca2+ transient decline in isolated muscles at physiological temperature accelerates only slightly at higher length, while the decline in force is significantly decelerated (Monasky et al. 2008). In addition to these kinetic changes in the dynamic state, myofilament Ca2+ sensitivity is increased at longer muscle lengths (Allen and Kentish 1985; Kentish et al. 1986), largely explaining the molecular mechanism responsible for the slowed kinetics at longer sarcomere length.
Another part of length-dependence occurs via the Anrep effect. After an increase in sarcomere/muscle length, force is changed in a near-immediate step (Mateja and de Tombe 2012), followed by a much slower process that takes minutes. This latter process involves a change in the calcium transient (Alvarez et al. 1999; Luers et al. 2005), as well as changes in myofilament phosphorylation events (Monasky et al. 2010, 2013; Wijnker et al. 2014). Combined, these changes further fine-tune the level of contractile activation, and prolong the relaxation at increased sarcomere/muscle length.
Frequency-dependent activation
As the heart rate increases, two distinct contractile parameters change: the force of contraction is generally increased, while the kinetics of the contraction are faster (Janssen and Periasamy 2007). Within the physiologically relevant heart rate, mammals increase force of contraction with frequency. This is most pronounced in larger mammals: humans can more than double their contractile force over their normal in vivo frequency range, while in rats this is only ~30 %, and in mice often no more than 10 % (Janssen and Periasamy 2007). For all species, the rate of contraction accelerates when heart rate goes up. The amplitude of contraction is mainly enhanced with frequency by enhanced Ca2+ cycling: with an increased number of beats, a net influx of Ca2+ “loads” the SR until a new “steady state” is reached, at which the cytosolic Ca2+ amplitude is greater. Although the amount of Ca2+ that enters the cell per beat is not drastically different, the number of beats per time unit is mainly causing the net increase in Ca2+ at higher frequency. The acceleration of contractile kinetics is much less understood, and involves changes in the kinetics of the Ca2+ transient, but likely also changes in the myofilament responsiveness (Varian and Janssen 2007; Varian et al. 2009; Tong et al. 2004), while changes in cross-bridge kinetics are potentially also a contributing factor. Combined, with increasing frequency of stimulation, the heart muscle accelerates, allowing for enhanced relaxation that is needed in order to allow sufficient filling prior to the initiation of the next beat. Again, acceleration is typically observed in multiple processes, Ca2+ transient decline, thin-filament activation, and cross-bridge kinetics, working in very close regulation to provide adequate cardiac muscle relaxation.
Adrenergic stimulation
The third main physiological regulator of cardiac pump function is beta-adrenergic stimulation. Independent of the typical increase in heart rate, covered above, β-adrenergic stimulation impacts several processes that in turn can impact on the rate of cardiac muscle relaxation. Upon adrenergic stimulation, PKA is the main enzyme responsible for phosphorylation of several proteins that then change their properties. LTCC phosphorylation will increase the amount of Ca2+ allowed to enter the cell each beat, and this increase in Ca2+ can indirectly impact the relaxation rate via Ca2+-dependent Serca activity. Phosphorylation of phospholamban is a major governor of the rate of Ca2+ transient decline (Roof et al. 2011; Kranias and Solaro 1982). Combined, this results in a very large increase in the intracellular Ca2+ transient. At the myofilament level, several proteins are impacted by PKA. Phosphorylation of TnI typically desensitizes the myofilaments, but is quantitatively less than the increase in the Ca2+ transient (i.e. the increase in Ca2+ outweighs the decrease in Ca2+ sensitivity), resulting in a net increase force production. Specifically on relaxation, the impact of phospholamban (increase speed of Ca2+ decline) and TnI phosphorylation (decrease in Ca2+ sensitivity) are synergetic: they both promote increased relaxation kinetics. Again, quantitatively, the impact on Ca2+ transient decline and thin filament (de-)activation are rather similar, and none could be truly labeled a rate-limiting step.
Integration of relaxation
The length- and frequency-dependent dissociation of the Ca2+ and force decline rates in the dynamic state strongly suggests that length-induced changes in contractile kinetics are not solely, nor directly, governed by altered Ca2+ transient decline, but rather at either the thin filament deactivation level or alternatively at the level of cross-bridge kinetics. The rate of force redevelopment in intact cardiac muscle is close to the rate of thin filament Ca2+ dissociation, a critical step in contractile de-activation. At the peak of contraction, which occurs at or near the systolic length in isometric contractions, cross-bridge cycling was estimated using the rate of tension redevelopment (k tr). This k tr was ~46 s−1 in rat muscles at 37 °C (Milani-Nejad et al. 2013), virtually identical to the rate of Ca2+ dissociation from isolated cardiac myofibrils (~50 s−1) at 37 °C as recently reported (Little et al. 2012). Moreover, the rates of these two processes are also similar to the rate at which intracellular Ca2+ declines in multicellular preparations (25–55 s−1) at 37 °C, when fluorescent ratios are calibrated and deconvoluted for speed of the dye under similar temperature and preparations (Janssen et al. 2002; Slabaugh et al. 2012). Thus, these three critical rates/processes have nearly identical rates at physiological temperature. This implies that in vivo there may not be a single step in kinetic governance which is rate-limiting in the sense that acceleration of that step will produce a comparable increase in the rate of the force development (Davis et al. 2007). Thus, accelerating pressure development and decay in a beating heart may require changes in multiple kinetic steps. In contrast to acceleration, the rate could be decelerated by slowing down any single one of the contributing steps. This view would be consistent with previous finding of a tight coupling between the rate of force development and rate of relaxation, and that the ratio of these rates is unaffected by load, frequency, or β-adrenergic stimulation (Janssen 2010a, b).
On a cellular level, the pumping capability of the heart is co-determined by the number of active cross-bridges and the speed at which they operate. Therefore, any condition that increases the rate of cross-bridge cycling can potentially enhance cardiac output. Based on our recent report demonstrating the acceleration of k tr in intact muscles under near physiological conditions (Milani-Nejad et al. 2013), and experiments by others (Adhikari et al. 2004; Stelzer and Moss 2006; Korte and McDonald 2007), a decrease in muscle length is a candidate for such condition. Physiologically, a decrease in muscle length is encountered as the heart ejects towards the end of systole. Therefore, as the heart ejects, the rate of cross-bridge cycling is continuously accelerated which aids in increasing the amount of blood that is being pumped. Normally, cardiac output is reduced at lower preloads as dictated by the classical Frank–Starling relationship, since the dramatic decline in force production outweighs an increase in cross-bridge cycling rate. It is probable that this length-dependent acceleration of cross-bridge cycling can potentially act as a system of checks and balances that acts to prevent a substantial drop in cardiac output and to partially restore the amount of blood that is being pumped by the heart at lower sarcomere lengths. This idea has been proposed by others (Hanft et al. 2008; McDonald 2011), and we believe it is a credible proposition to explain the role of accelerated cross-bridge kinetics at lower muscle lengths.
Limitation for the past and future
Quantification of the processes that impact overall cardiac muscle relaxation are unavoidably limited by the complexity of the system. The more reductionistic the method of assessment, the more accurate one can quantify a specific molecular process. Calcium transient decline, thin filament deactivation, and cross-bridge kinetics are all interrelated. Thus, the further a molecular step in any of these three processes is removed from integration with the other two, regulatory information is often lost, and the more ambiguous its interpretation for physiological relevance becomes. A careful consideration of each assessment system is warranted in light of the assessed parameter and its contribution to the overall process. Future studies should carefully weigh the impact of the degrees of reduction of the method and the experimental milieu (temperature, mechanical load, pH, etc.) of a single assessed parameter into the interpretation of its overall importance. Likewise, functional assessments in a complex system require cautions interpretation of the outcome parameter to specific underlying molecular events. To bridge the gap between individual molecular steps and complex integrative regulation, further developments of techniques that can probe specific molecular events in a complex system are needed. Enabling the investigation of a specific molecular interaction while preserving the complex integrative regulatory structure of the myocardium will ultimately unravel the regulatory contribution of each interaction in the complex nature of myocardial relaxation.
Relaxation, it is complicated: a summarizing conclusion
The relaxation of cardiac muscle is a multifaceted process (Fig. 4). Although definitive quantification of separate processes can be technically achieved, the synergistic and antagonistic properties of each of the three main players in relaxation, intracellular Ca2+ decline, thin filament deactivation, and cross-bridge cycling kinetics, prevents the outcome of a single parameter measured in isolation to be an unambiguous quantitator. We believe that the quantitative impact of these three systems, when integrated, is so close that they each provide a nearly inseparable rate-limiting step in the overall process; if one of the three processes is sped-up by only a small amount, the other two would directly become rate-limiting. Conversely, slowing relaxation can be directly achieved by the impact of any of these three parameters, which then becomes (more) rate-limiting. Relaxation is a process of intricate checks and balances, and should not be thought of as a single rate-limiting step that is regulated at a single protein level, but as a system-level property that requires a fundamental integration of three governing systems: intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics.
Fig. 4.
Cardiac muscle relaxation is a multifaceted process. Blue squares three main processes that heavily impact on the rate of cardiac muscle relaxation. Orange ovals three main physiological processes that modulate the rate of cardiac muscle relaxation. Major direct interaction/impact between two processes is depicted by two-headed solid blue arrows, unresolved or minor interactions are depicted by two-headed gray dashed arrows. Green dashed arrows indicate the impact of a process on a rate that results in acceleration of the process. Red dashed arrows indicate the impact of a process on a rate that results in the slowing of the process. One-headed dashed gray arrows indicate unresolved, disputed, or minor impact. Red pentagons post-translational modifications can impact each regulatory component of cardiac muscle relaxation
Acknowledgments
This effort was supported by NIH HL grants R01 HL114904 (B.J.B.), R01 HL091986 (J.P.D.), K02 HL094692 (M.T.Z.), and R01113084 (P.M.L.J.). This article does not contain any studies with human or animal subjects performed by the any of the authors.
Compliance with Ethical Standards
ᅟ
Conflict of interest
Brandon J. Biesiadecki declares that he has not conflict of interest. Jonathan P. Davis declares that he has no conflict of interest. Mark T. Ziolo declares that he has no conflict of interest. Paul M.L. Janssen declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J. 2004;87(3):1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. Calcium transients at different muscle lengths in rat ventricular muscle [proceedings] J Physiol Lond. 1979;292:68P–69P. [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol Lond. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca(2+) transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. 1999;85(8):716–722. doi: 10.1161/01.res.85.8.716. [DOI] [PubMed] [Google Scholar]
- Anderson BR, Granzier HL. Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations. Prog Biophys Mol Biol. 2012;110(2–3):204–217. doi: 10.1016/j.pbiomolbio.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In vivo phosphorylation site mapping in mouse cardiac troponin I by high resolution top-down electron capture dissociation mass spectrometry: Ser22/23 are the only sites basally phosphorylated. Biochemistry. 2009;48(34):8161–8170. doi: 10.1021/bi900739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50((6):Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48(5):866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Bassani JW, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflugers Arch. 1995;430(4):573–578. doi: 10.1007/BF00373894. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- Bencsik P, Kupai K, Giricz Z, Gorbe A, Huliak I, Furst S, Dux L, Csont T, Jancso G, Ferdinandy P. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: role of peroxynitrite. Br J Pharmacol. 2008;153(3):488–496. doi: 10.1038/sj.bjp.0707599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer; 2001. [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac Na/Ca Exchange Function in Rabbit, Mouse and Man: What’s the Difference? J Mol Cell Cardiol. 2002;34(4):369–373. doi: 10.1006/jmcc.2002.1530. [DOI] [PubMed] [Google Scholar]
- Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol. 1995;268(1 Pt 1):C271–277. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
- Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001;89(5):373–375. [PubMed] [Google Scholar]
- Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100(10):1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100(10):1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007;292(1):H318–325. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- Boontje NM, Merkus D, Zaremba R, Versteilen A, de Waard MC, Mearini G, de Beer VJ, Carrier L, Walker LA, Niessen HW, Dobrev D, Stienen GJ, Duncker DJ, van der Velden J. Enhanced myofilament responsiveness upon beta-adrenergic stimulation in post-infarct remodeled myocardium. J Mol Cell Cardiol. 2011;50(3):487–499. doi: 10.1016/j.yjmcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4(1):23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard RA, Bose D. Analysis of the interval-force relationship in rat and canine ventricular myocardium. Am J Physiol. 1989;257(6 Pt 2):H2036–2047. doi: 10.1152/ajpheart.1989.257.6.H2036. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, de Clerck NM, Goethals MA, Housmans PR. Relaxation of ventricular cardiac muscle. J Physiol Lond. 1978;283(17):469–480. doi: 10.1113/jphysiol.1978.sp012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res. 2002;91(6):509–516. doi: 10.1161/01.res.0000035246.27856.53. [DOI] [PubMed] [Google Scholar]
- Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588(Pt 6):981–993. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corino VD, Matteucci M, Mainardi LT. Analysis of heart rate variability to predict patient age in a healthy population. Methods Inf Med. 2007;46(2):191–195. [PubMed] [Google Scholar]
- Counihan PJ, Fei L, Bashir Y, Farrell TG, Haywood GA, McKenna WJ. Assessment of heart rate variability in hypertrophic cardiomyopathy. Association with clinical and prognostic features. Circulation. 1993;88(4 Pt 1):1682–1690. doi: 10.1161/01.cir.88.4.1682. [DOI] [PubMed] [Google Scholar]
- Davis JP, Tikunova SB. Ca(2+) exchange with troponin C and cardiac muscle dynamics. Cardiovasc Res. 2008;77(4):619–626. doi: 10.1093/cvr/cvm098. [DOI] [PubMed] [Google Scholar]
- Davis J, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of Thin and Thick Filament Proteins on Calcium Binding and Exchange with Cardiac Troponin C. Biophys J. 2007;92(9):3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe PP. Congestive heart failure: role of cross-bridge cycle kinetics [editorial] Cardiovasc Res. 1998;40(3):440–443. doi: 10.1016/s0008-6363(98)00247-8. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, Mateja RD, Tachampa K, Mou YA, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Schmidtmann A, Redlich A, Westerdorf B, Jaquet K, Thieleczek R. Effects of phosphorylation and mutation R145G on human cardiac troponin I function. Biochemistry. 2001;40(48):14593–14602. doi: 10.1021/bi0115232. [DOI] [PubMed] [Google Scholar]
- DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca transients and SR Ca reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol. 2004;36(1):67–74. doi: 10.1016/j.yjmcc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol Lond. 1979;291(7):143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertz-Berger BR, He H, Dowell C, Factor SM, Haim TE, Nunez S, Schwartz SD, Ingwall JS, Tardiff JC. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc Natl Acad Sci USA. 2005;102(50):18219–18224. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol. 1998;513(Pt 1):171–183. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DB, Noguchi T, VanBuren P, Murphy AM, Van Eyk JE. C-terminal truncation of cardiac troponin I causes divergent effects on ATPase and force: implications for the pathophysiology of myocardial stunning. Circ Res. 2003;93(10):917–924. doi: 10.1161/01.RES.0000099889.35340.6F. [DOI] [PubMed] [Google Scholar]
- Frank O. Zur Dynamik des Herzmuskels. Z Biol. 1895;32:370–447. [Google Scholar]
- Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? EMBO J. 1995;14(9):1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg KS, Bers DM. Isoproterenol does not enhance Ca-dependent Na/Ca exchange current in intact rabbit ventricular myocytes. J Mol Cell Cardiol. 2005;39(6):972–981. doi: 10.1016/j.yjmcc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271(3 Pt 2):H823–833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10(3):211–223. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- Hanft LM, Korte FS, McDonald KS. Cardiac function and modulation of sarcomeric function by length. Cardiovasc Res. 2008;77(4):627–636. doi: 10.1093/cvr/cvm099. [DOI] [PubMed] [Google Scholar]
- Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292(4):H1643–1654. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- Herzig JW, Peterson JW, Ruegg JC, Solaro RJ. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H (2009) PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res 105 (7):631–638, 17 pp following 638. [DOI] [PMC free article] [PubMed]
- High CW, Stull JT. Phosphorylation of myosin in perfused rabbit and rat hearts. Am J Physiol. 1980;239(6):H756–764. doi: 10.1152/ajpheart.1980.239.6.H756. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987;253(4 Pt 1):C541–546. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32(9):1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- James J, Robbins J. Signaling and myosin-binding protein C. J Biol Chem. 2011;286(12):9913–9919. doi: 10.1074/jbc.R110.171801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML. 54th Bowditch Lecture: Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol. 2010;299(6):H1741–1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML. Kinetics of Cardiac Muscle Contraction and Relaxation are Linked and Determined by Properties of the Cardiac Sarcomere. Am J Physiol Heart Circ Physiol. 2010;299:H1092–1099. doi: 10.1152/ajpheart.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML, Hunter WC. Force, not sarcomere length, correlates with prolongation of isosarcometric contraction. Am J Physiol Heart Circ Physiol. 1995;269:H676–685. doi: 10.1152/ajpheart.1995.269.2.H676. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43(5):523–531. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–H507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- Jideama NM, Noland TA, Jr, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem. 1996;271(38):23277–23283. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem. 2011;286(12):9941–9947. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88(10):1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477(7366):601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77(2):353–361. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr MJ, Traynham CJ, Roof SR, Davis JP, Ziolo MT. cAMP-independent activation of protein kinase A by the peroxynitrite generator SIN-1 elicits positive inotropic effects in cardiomyocytes. J Mol Cell Cardiol. 2010;48(4):645–648. doi: 10.1016/j.yjmcc.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, Dos Remedios C, van der Velden J, Stienen GJ. Effect of troponin I Ser23/24 phosphorylation on Ca2 + −sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol. 2010;48(5):954–963. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte FS, McDonald KS. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J Physiol. 2007;581(Pt 2):725–739. doi: 10.1113/jphysiol.2007.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298(5870):182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- Kruger M, Linke WA. The giant protein titin: a regulatory node that integrates myocyte signaling pathways. J Biol Chem. 2011;286(12):9905–9912. doi: 10.1074/jbc.R110.173260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Kohl T, Linke WA. Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol Heart Circ Physiol. 2006;291(2):H496–506. doi: 10.1152/ajpheart.00114.2006. [DOI] [PubMed] [Google Scholar]
- Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104(1):87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na + −Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48(9):1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster DW, Bawazeer AC, Zaremba R, Goebel M, Boontje NM, van der Velden J. Cardiac myosin binding protein C phosphorylation in cardiac disease. J Muscle Res Cell Motil. 2012;33(1):43–52. doi: 10.1007/s10974-011-9280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104(6):720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res. 2010;107(2):228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J. 1996;71(2):898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Yang Z, Epstein ND, Fananapazir L, Stull JT, Sweeney HL. Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J Struct Biol. 1998;122(1–2):149–161. doi: 10.1006/jsbi.1998.3980. [DOI] [PubMed] [Google Scholar]
- LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121(19):2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Biesiadecki BJ, Kilic A, Higgins RS, Janssen PM, Davis JP. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C. J Biol Chem. 2012;287(33):27930–27940. doi: 10.1074/jbc.M111.337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tikunova SB, Kline KP, Siddiqui JK, Davis JP. Disease-related cardiac troponins alter thin filament Ca2+ association and dissociation rates. PLoS ONE. 2012;7(6):e38259. doi: 10.1371/journal.pone.0038259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Lopez JJ, Biesiadecki BJ, Davis JP. Protein kinase C phosphomimetics alter thin filament ca(2+) binding properties. PLoS ONE. 2014;9(1):e86279. doi: 10.1371/journal.pone.0086279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luers C, Fialka F, Elgner A, Zhu D, Kockskamper J, Von Lewinski D, Pieske B. Stretch-dependent modulation of [Na+]i, [Ca2+]i, and pHi in rabbit myocardium–a mechanism for the slow force response. Cardiovasc Res. 2005;68:454–463. doi: 10.1016/j.cardiores.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384(1):60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4(7):566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Mak A, Smillie LB, Barany M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1978;75(8):3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateja RD, de Tombe PP. Myofilament length-dependent activation develops within 5 ms in guinea-pig myocardium. Biophys J. 2012;103(1):L13–15. doi: 10.1016/j.bpj.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS. The interdependence of Ca2+ activation, sarcomere length, and power output in the heart. Pflugers Arch. 2011;462(1):61–67. doi: 10.1007/s00424-011-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough JL, Arrell DK, Van Eyk JE. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res. 1999;84(1):9–20. doi: 10.1161/01.res.84.1.9. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. 2014;141(3):235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM. Effect of muscle length on cross-bridge kinetics in intact cardiac trabeculae at body temperature. J Gen Physiol. 2013;141(1):133–139. doi: 10.1085/jgp.201210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasky MM, Varian KD, Davis JP, Janssen PML. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium. Pflugers Arch. 2008;456(2):267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- Monasky MM, Biesiadecki BJ, Janssen PM. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J Mol Cell Cardiol. 2010;48(5):1023–1028. doi: 10.1016/j.yjmcc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasky MM, Taglieri DM, Jacobson AK, Haizlip KM, Solaro RJ, Janssen PM. Post-translational modifications of myofilament proteins involved in length-dependent prolongation of relaxation in rabbit right ventricular myocardium. Arch Biochem Biophys. 2013;535(1):22–29. doi: 10.1016/j.abb.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravec CS, Desnoyer RW, Milovanovic M, Schluchter MD, Bond M. Mitochondrial calcium content in isolated perfused heart: effects of inotropic stimulation. Am J Physiol. 1997;273(3 Pt 2):H1432–1439. doi: 10.1152/ajpheart.1997.273.3.H1432. [DOI] [PubMed] [Google Scholar]
- Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca(2+)-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med. 1997;22(1–2):37–47. doi: 10.1016/s0891-5849(96)00238-9. [DOI] [PubMed] [Google Scholar]
- Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, dos Remedios C, Jaquet K, Foster DB, Murphy AM, van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJ. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99(9):1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- Nixon BR, Thawornkaiwong A, Jin J, Brundage EA, Little SC, Davis JP, Solaro RJ, Biesiadecki BJ. AMP-activated protein kinase phosphorylates cardiac troponin I at Ser-150 to increase myofilament calcium sensitivity and blunt PKA-dependent function. J Biol Chem. 2012;287(23):19136–19147. doi: 10.1074/jbc.M111.323048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon BR, Liu B, Scellini B, Tesi C, Piroddi N, Ogut O, Solaro RJ, Ziolo MT, Janssen PM, Davis JP, Poggesi C, Biesiadecki BJ. Tropomyosin Ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch Biochem Biophys. 2013;535(1):30–38. doi: 10.1016/j.abb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ (2014) Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca2+ sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol (in press) [DOI] [PMC free article] [PubMed]
- Nyitrai M, Geeves MA. Adenosine diphosphate and strain sensitivity in myosin motors. Philos Trans R Soc Lond B. 2004;359(1452):1867–1877. doi: 10.1098/rstb.2004.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oceandy D, Stanley PJ, Cartwright EJ, Neyses L. The regulatory function of plasma-membrane Ca(2+)-ATPase (PMCA) in the heart. Biochem Soc Trans. 2007;35(Pt 5):927–930. doi: 10.1042/BST0350927. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287(6):H2712–2718. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- Ottolia M, Philipson KD. NCX1: mechanism of transport. Adv Exp Med Biol. 2013;961:49–54. doi: 10.1007/978-1-4614-4756-6_5. [DOI] [PubMed] [Google Scholar]
- Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res. 2004;94(9):1249–1255. doi: 10.1161/01.RES.0000126898.95550.31. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J Physiol. 1999;519(Pt 3):669–678. doi: 10.1111/j.1469-7793.1999.0669n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BS, Solaro RJ. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987;262(16):7839–7849. [PubMed] [Google Scholar]
- Patel JR, Diffee GM, Huang XP, Moss RL. Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophys J. 1998;74(1):360–368. doi: 10.1016/S0006-3495(98)77793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JR, Wolska BM. Troponin I phosphorylation plays an important role in the relaxant effect of beta-adrenergic stimulation in mouse hearts. Cardiovasc Res. 2004;61(4):756–763. doi: 10.1016/j.cardiores.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Perchenet L, Hinde AK, Patel KC, Hancox JC, Levi AJ. Stimulation of Na/Ca exchange by the beta-adrenergic/protein kinase A pathway in guinea-pig ventricular myocytes at 37 degrees C. Pflugers Arch. 2000;439(6):822–828. doi: 10.1007/s004249900218. [DOI] [PubMed] [Google Scholar]
- Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem. 1999;190(1–2):9–32. [PubMed] [Google Scholar]
- Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- Piroddi N, Belus A, Scellini B, Tesi C, Giunti G, Cerbai E, Mugelli A, Poggesi C. Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflugers Arch. 2007;454(1):63–73. doi: 10.1007/s00424-006-0181-3. [DOI] [PubMed] [Google Scholar]
- Puglisi JL, Bassani RA, Bassani JW, Amin JN, Bers DM. Temperature and relative contributions of Ca transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270(5 Pt 2):H1772–1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, Vecoli C, Hart GW, Murphy AM. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res. 2008;103(12):1354–1358. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JP, Bailey CA, Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261(11):4948–4955. [PubMed] [Google Scholar]
- Rice JJ, Winslow RL, Hunter WC. Comparison of putative cooperative mechanisms in cardiac muscle: length dependence and dynamic responses. Am J Physiol. 1999;276(5 Pt 2):H1734–1754. doi: 10.1152/ajpheart.1999.276.5.H1734. [DOI] [PubMed] [Google Scholar]
- Rice R, Guinto P, Dowell-Martino C, He H, Hoyer K, Krenz M, Robbins J, Ingwall JS, Tardiff JC. Cardiac myosin heavy chain isoform exchange alters the phenotype of cTnT-related cardiomyopathies in mouse hearts. J Mol Cell Cardiol. 2010;48(5):979–988. doi: 10.1016/j.yjmcc.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof SR, Shannon TR, Janssen PM, Ziolo MT. Effects of increased systolic Ca2+ and phospholamban phosphorylation during {beta}-adrenergic stimulation on Ca2+ transient kinetics in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301(4):H1570–1578. doi: 10.1152/ajpheart.00402.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof SR, Biesiadecki BJ, Davis JP, Janssen PM, Ziolo MT. Effects of increased systolic Ca(2+) and beta-adrenergic stimulation on Ca(2+) transient decline in NOS1 knockout cardiac myocytes. Nitric Oxide. 2012;27(4):242–247. doi: 10.1016/j.niox.2012.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 2006;103(45):16918–16923. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer NM, Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2 + −ATPase. Arch Biochem Biophys. 1986;246(2):589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA. 1985;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simnett SJ, Johns EC, Lipscomb S, Mulligan IP, Ashley CC. Effect of pH, phosphate, and ADP on relaxation of myocardium after photolysis of diazo 2. Am J Physiol. 1998;275(3 Pt 2):H951–960. doi: 10.1152/ajpheart.1998.275.3.H951. [DOI] [PubMed] [Google Scholar]
- Sivakumaran V, Stanley BA, Tocchetti CG, Ballin JD, Caceres V, Zhou L, Keceli G, Rainer PP, Lee DI, Huke S, Ziolo MT, Kranias EG, Toscano JP, Wilson GM, O’Rourke B, Kass DA, Mahaney JE, Paolocci N. HNO Enhances SERCA2a Activity and Cardiomyocyte Function by Promoting Redox-Dependent Phospholamban Oligomerization. Antioxid Redox Signal. 2013;19(11):1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh JL, Brunello L, Gyorke S, Janssen PM. Contractile parameters and occurrence of alternans in isolated rat myocardium at supra-physiological stimulation frequency. Am J Physiol Heart Circ Physiol. 2012;302(11):H2267–2275. doi: 10.1152/ajpheart.01004.2011. [DOI] [PubMed] [Google Scholar]
- Solaro RJ. Modulation of cardiac Myofilament activity by protein phosphorylation. In: Page E, Fozzard H, Solaro RJ, editors. Handbook of Physiology: Section 2. New York: Oxford University Press; 2001. pp. 264–300. [Google Scholar]
- Solaro RJ, Westfall M. Physiology of the myocardium. In: Sellke FW, editor. Surgery of the Chest. Philadelphia: Elsevier Sanders; 2005. pp. 767–779. [Google Scholar]
- Starling EH. The Lineacre lecture on the law of the heart. London: Longmans Green; 1918. [Google Scholar]
- Stehle R, Iorga B. Kinetics of cardiac sarcomeric processes and rate-limiting steps in contraction and relaxation. J Mol Cell Cardiol. 2010;48(5):843–850. doi: 10.1016/j.yjmcc.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Moss RL. Contributions of stretch activation to length-dependent contraction in murine myocardium. J Gen Physiol. 2006;128(4):461–471. doi: 10.1085/jgp.200609634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101(5):503–511. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994;74(3):542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278(37):35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Palmer BM, James J, Wang Y, Chen Z, VanBuren P, Maughan DW, Robbins J, LeWinter MM. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail. 2009;2(4):334–341. doi: 10.1161/CIRCHEARTFAILURE.108.802298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3(2):187–197. doi: 10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Homsher EE, Goldman YE, Shuman H. Force generation in single conventional actomyosin complexes under high dynamic load. Biophys J. 2006;90(4):1295–1307. doi: 10.1529/biophysj.105.068429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94(4):496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108(6):765–782. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae, Evidence of length-dependent activation. Circ Res. 1980;46(5):703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M (2004) Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol 558 (0022–3751 (Print)):927–941. [DOI] [PMC free article] [PubMed]
- Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res. 2008;103(9):974–982. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CAA, Varian KD, Janssen PML. Variability in interbeat duration influences myocardial contractility in rat cardaic trabeculae. Open Cardiovasc Med J. 2008;2:96–100. doi: 10.2174/1874192400802010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull L, Hoh JF, Ludowyke RI, Rossmanith GH. Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue. J Physiol. 2002;542(Pt 3):911–920. doi: 10.1113/jphysiol.2002.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJ. The effect of myosin light chain 2 dephosphorylation on Ca2 + − sensitivity of force is enhanced in failing human hearts. Cardiovasc Res. 2003;57(2):505–514. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- Varian KD, Janssen PML. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol. 2007;292(5):H2212–2219. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- Varian KD, Kijtawornrat A, Gupta SC, Torres CA, Monasky MM, Hiranandani N, Delfin DA, Rafael-Fortney JA, Periasamy M, Hamlin RL, Janssen PML. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization rabbit right ventricular hypertrophy. Circ Heart Fail. 2009;2(5):472–481. doi: 10.1161/CIRCHEARTFAILURE.109.853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JR, Ginsburg KS, Mitton B, Haghighi K, Robbins J, Bers DM, Kranias EG. Phospholamban overexpression in rabbit ventricular myocytes does not alter sarcoplasmic reticulum Ca transport. Am J Physiol Heart Circ Physiol. 2009;296(3):H698–703. doi: 10.1152/ajpheart.00272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LA, Medway AM, Walker JS, Cleveland JC, Jr, Buttrick PM. Tissue procurement strategies affect the protein biochemistry of human heart samples. J Muscle Res Cell Motil. 2011;31(5–6):309–314. doi: 10.1007/s10974-010-9233-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294(6):C1566–1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci USA. 1996;93(17):8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Wijnker PJ, Sequeira V, Foster DB, Li Y, Dos Remedios CG, Murphy AM, Stienen GJ, van der Velden J. Length-dependent activation is modulated by cardiac troponin-I biphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol Heart Circ Physiol. 2014;306:H1171–H1181. doi: 10.1152/ajpheart.00580.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA. 2013;110(26):10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106(11):1384–1389. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80(1):76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90(11):1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101(4):377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts– evidence for novel phosphorylation sites. Proteomics. 2006;6(14):4176–4186. doi: 10.1002/pmic.200500894. [DOI] [PubMed] [Google Scholar]
- Yuan C, Sheng Q, Tang H, Li Y, Zeng R, Solaro RJ. Quantitative comparison of sarcomeric phosphoproteomes of neonatal and adult rat hearts. Am J Physiol Heart Circ Physiol. 2008;295(2):H647–656. doi: 10.1152/ajpheart.00357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76(6):1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation. 2012;126(15):1828–1837. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation. 2004;109(15):1886–1891. doi: 10.1161/01.CIR.0000124231.98250.A8. [DOI] [PubMed] [Google Scholar]