Abstract
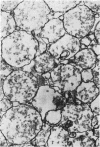
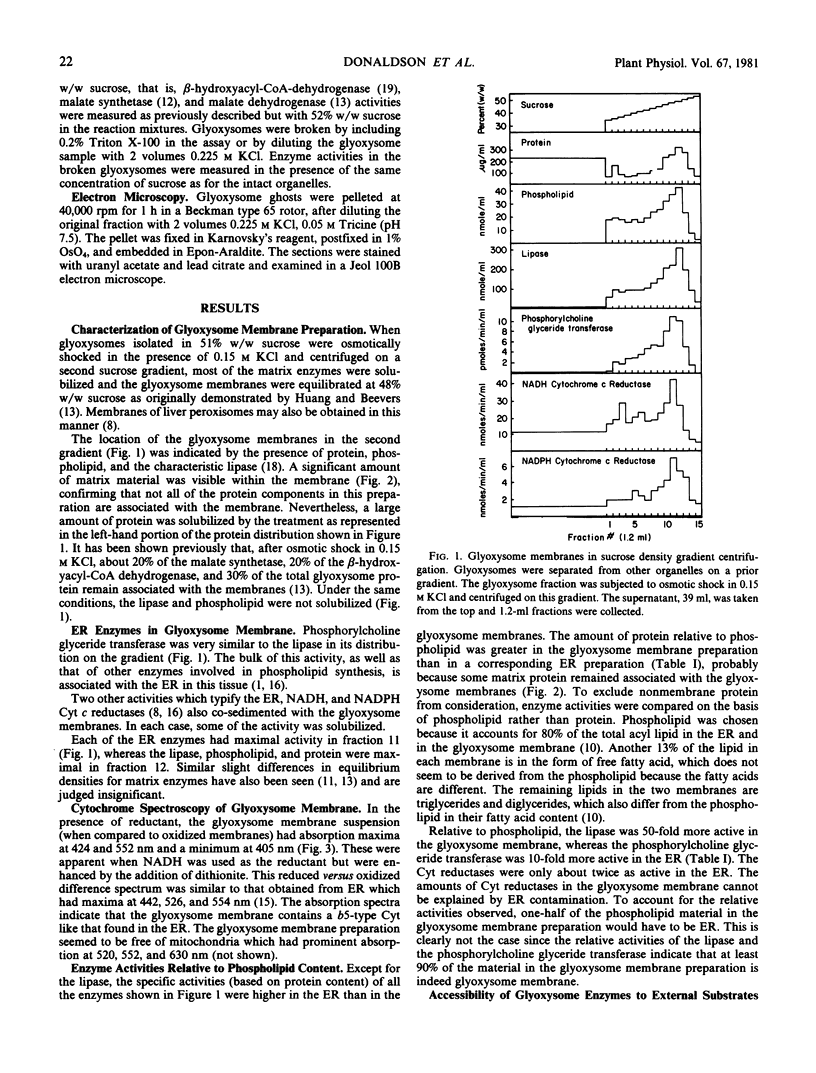
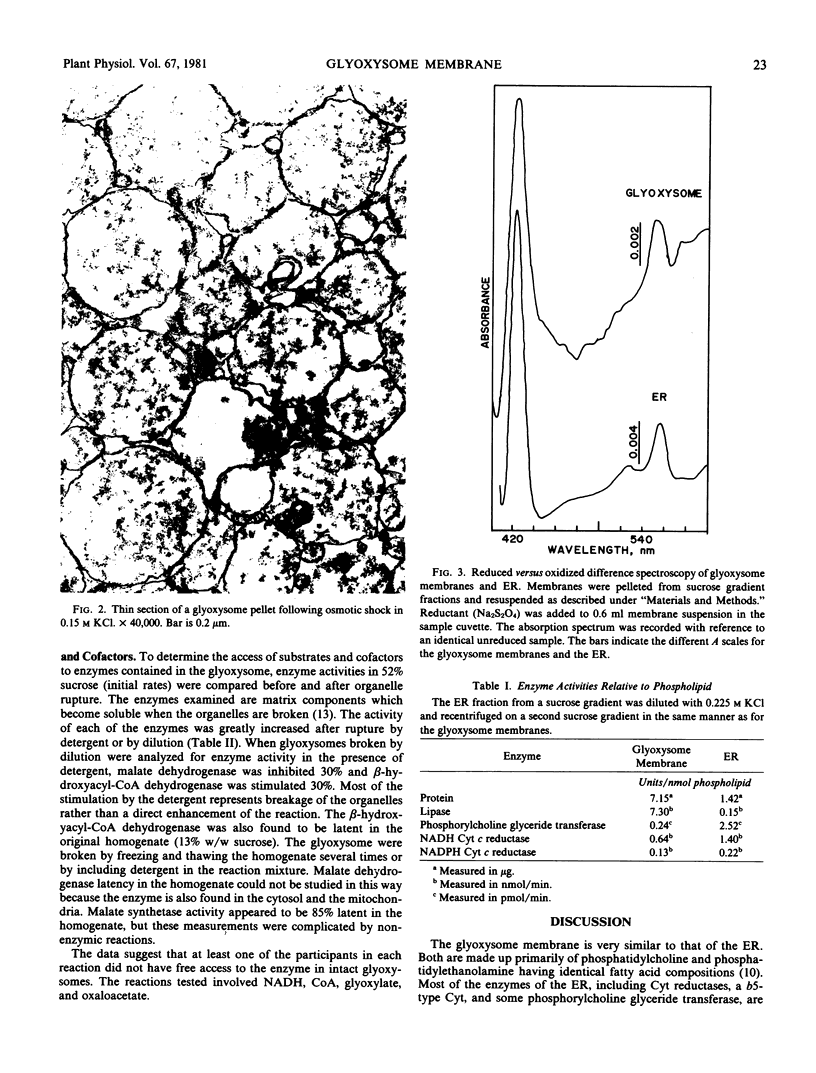
Glyoxysome ghosts were isolated from germinating castor bean endosperms using established methods. Electron microscopic examination showed that some matrix material was retained within the glyoxysomal membrane. Two cytochrome reductases and phosphorylcholine glyceride transferase co-sedimented with the alkaline lipase, a known component of the glyoxysome membrane, in sucrose gradient centrifugation of osmotically shocked glyoxysomes. The activities of these enzymes in the glyoxysome membranes were compared to those in the endoplasmic reticulum relative to phospholipid content. On this basis, the phosphorylcholine glyceride transferase was 10-fold more active in the endoplasmic reticulum, whereas the lipase was 50-fold more active in the glyoxysome membrane. The cytochrome reductases were only 2-fold more active in the endoplasmic reticulum, indicating that they are components of the two membranes. Difference spectroscopy of the glyoxysome membrane suspension revealed the presence of a b5-type cytochrome similar to that found in the endoplasmic reticulum. Since the glyoxysome membrane is apparently derived from the endoplasmic reticulum, components of the endoplasmic reticulum such as these are likely to be incorporated into the glyoxysome membrane during biogenesis.
Enzyme activities involving the cofactors NADH or CoA were measurable in broken, but not in intact, glyoxysomes. Thus, it appears that cofactors for enzymes within the organelle cannot pass through the membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieglmayer C., Nahler G., Ruis H. Membranen von Glyoxysomen aus Rizinus-Endosperm. Weitere Untersuchungen über die membrangebundenden Enzyme des Fettsäureabbaus und des Glyoxylatzyklus. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1121–1128. [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Similarities in the polypeptide composition of glyoxysomal and endoplasmic-reticulum membranes from castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):491–499. doi: 10.1042/bj1540491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. The cellular origin of glyoxysomal proteins in germinating castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):501–506. doi: 10.1042/bj1540501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Donaldson R. P., Beevers H. Lipid composition of organelles from germinating castor bean endosperm. Plant Physiol. 1977 Feb;59(2):259–263. doi: 10.1104/pp.59.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Beevers H. The problem of reduced nicotinamide adenine dinucleotide oxidation in glyoxysomes. Plant Physiol. 1972 Feb;49(2):249–251. doi: 10.1104/pp.49.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Remacle J., Fowler S., Beaufay H., Amarcostesec A., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. VI. Electron microscope examination of microsomes for cytochrome b5 by means of a ferritin-labeled antibody. J Cell Biol. 1976 Nov;71(2):551–564. doi: 10.1083/jcb.71.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. A microsomal cytochrome reductase specific for diphosphopyridine nucleotide. J Biol Chem. 1956 Jul;221(1):277–286. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]