Abstract
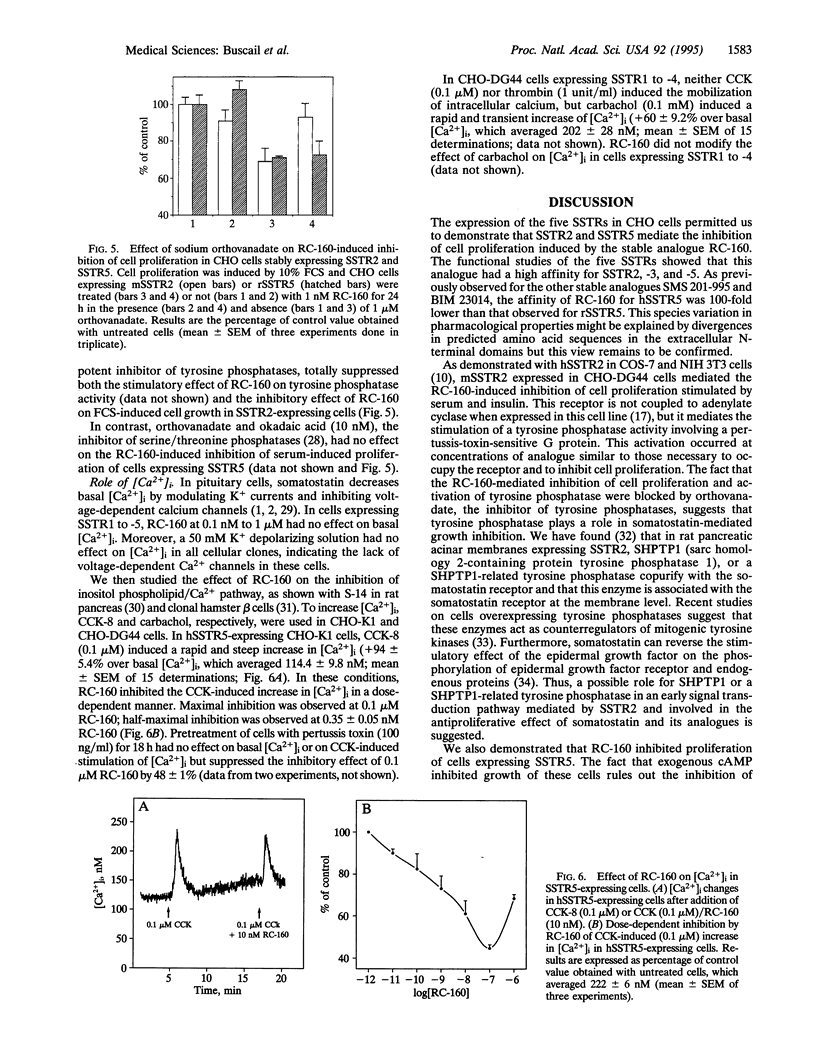
Effects of the stable somatostatin analogue RC-160 on cell proliferation, tyrosine phosphatase activity, and intracellular calcium concentration were investigated in CHO cells expressing the five somatostatin receptor subtypes SSTR1 to -5. Binding experiments were performed on crude membranes by using [125I-labeled Tyr11] somatostatin-14; RC-160 exhibited moderate-to-high affinities for SSTR2, -3, and -5 (IC50, 0.17, 0.1 and 21 nM, respectively) and low affinity for SSTR1 and -4 (IC50, 200 and 620 nM, respectively). Cell proliferation was induced in CHO cells by 10% (vol/vol) fetal calf serum, 1 microM insulin, or 0.1 microM cholecystokinin (CCK)-8; RC-160 inhibited serum-induced proliferation of CHO cells expressing SSTR2 and SSTR5 (EC50, 53 and 150 pM, respectively) but had no effect on growth of cells expressing SSTR1, -3, or -4. In SSTR2-expressing cells, orthovanadate suppressed the growth inhibitory effect of RC-160. This analogue inhibited insulin-induced proliferation and rapidly stimulated the activity of a tyrosine phosphatase in only this cellular clone. This latter effect was observed at doses of RC-160 (EC50, 4.6 pM) similar to those required to inhibit growth (EC50, 53 pM) and binding to the receptor (IC50, 170 pM), implicating tyrosine phosphatase as a transducer of the growth inhibition signal in SSTR2-expressing cells. In SSTR5-expressing cells, the phosphatase pathway was not involved in the inhibitory effect of RC-160 on cell growth, since this action was not influenced by tyrosine and serine/threonine phosphatase inhibitors. In addition, in SSTR5-expressing cells, RC-160 inhibited CCK-stimulated intracellular calcium mobilization at doses (EC50, 0.35 nM) similar to those necessary to inhibit somatostatin-14 binding (IC50, 21 nM) and CCK-induced cell proliferation (EC50, 1.1 nM). This suggests that the inositol phospholipid/calcium pathway could be involved in the antiproliferative effect of RC-160 mediated by SSTR5 in these cells. RC-160 had no effect on the basal or carbachol-stimulated calcium concentration in cells expressing SSTR1 to -4. Thus, we conclude that SSTR2 and SSTR5 bind RC-160 with high affinity and mediate the RC-160-induced inhibition of cell growth by distinct mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Ramachandran J., Capon D. J. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989 Jul 13;340(6229):146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Bertrand V., Bastié M. J., Vaysse N., Pradayrol L. Inhibition of gastrin-induced proliferation of AR4-2J cells by calcium channel antagonists. Int J Cancer. 1994 Feb 1;56(3):427–432. doi: 10.1002/ijc.2910560324. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buscail L., Delesque N., Estève J. P., Saint-Laurent N., Prats H., Clerc P., Robberecht P., Bell G. I., Liebow C., Schally A. V. Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2315–2319. doi: 10.1073/pnas.91.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas B., Cambillau C., Buscail L., Zeggari M., Esteve J. P., Lautre V., Thomas F., Vaysse N., Susini C. Stimulation of a membrane tyrosine phosphatase activity by somatostatin analogues in rat pancreatic acinar cells. Eur J Biochem. 1992 Aug 1;207(3):1017–1024. doi: 10.1111/j.1432-1033.1992.tb17138.x. [DOI] [PubMed] [Google Scholar]
- Eden P. A., Taylor J. E. Somatostatin receptor subtype gene expression in human and rodent tumors. Life Sci. 1993;53(1):85–90. doi: 10.1016/0024-3205(93)90614-9. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L. Mitogenic pathways regulated by G protein oncogenes. Mol Biol Cell. 1992 Jan;3(1):123–128. doi: 10.1091/mbc.3.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuhtsen S., Esteve J. P., Bernadet B., Vaysse N., Susini C. Molecular characterization of the solubilized receptor of somatostatin from rat pancreatic acinar membranes. Biochem J. 1988 Sep 15;254(3):641–647. doi: 10.1042/bj2540641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A., Yamada Y., Kagimoto S., Shimatsu A., Imamura M., Tsuda K., Imura H., Seino S., Seino Y. Identification of somatostatin receptor subtypes and an implication for the efficacy of somatostatin analogue SMS 201-995 in treatment of human endocrine tumors. J Clin Invest. 1994 Mar;93(3):1321–1325. doi: 10.1172/JCI117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. F., Zaina S., Sweet R., Yasuda K., Bell G. I., Stadel J., Reisine T. Gi alpha 1 selectively couples somatostatin receptor subtype 3 to adenylyl cyclase: identification of the functional domains of this alpha subunit necessary for mediating the inhibition by somatostatin of cAMP formation. Mol Pharmacol. 1994 Apr;45(4):587–590. [PubMed] [Google Scholar]
- Lee M. T., Liebow C., Kamer A. R., Schally A. V. Effects of epidermal growth factor and analogues of luteinizing hormone-releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyrosine residues of specific protein substrates in various tumors. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1656–1660. doi: 10.1073/pnas.88.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M. J. The somatostatin receptor in the GI tract. Annu Rev Physiol. 1992;54:455–468. doi: 10.1146/annurev.ph.54.030192.002323. [DOI] [PubMed] [Google Scholar]
- Linard C., Reyl-Desmars F., Lewin M. J. Somatostatin inhibition of phosphoinositides turnover in isolated rat acinar pancreatic cells: interaction with bombesin. Regul Pept. 1992 Oct 13;41(3):219–226. doi: 10.1016/0167-0115(92)90115-b. [DOI] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Schofield G., Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986 Nov;6(11):3128–3132. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas M., Gervin D., Englesberg E. The insulin receptor as a transmitter of a mitogenic signal in Chinese hamster ovary CHO-K1 cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9294–9298. doi: 10.1073/pnas.86.23.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- O'Carroll A. M., Raynor K., Lolait S. J., Reisine T. Characterization of cloned human somatostatin receptor SSTR5. Mol Pharmacol. 1994 Aug;46(2):291–298. [PubMed] [Google Scholar]
- Patel Y. C., Greenwood M. T., Warszynska A., Panetta R., Srikant C. B. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun. 1994 Jan 28;198(2):605–612. doi: 10.1006/bbrc.1994.1088. [DOI] [PubMed] [Google Scholar]
- Pinski J., Halmos G., Yano T., Szepeshazi K., Qin Y., Ertl T., Schally A. V. Inhibition of growth of MKN45 human gastric-carcinoma xenografts in nude mice by treatment with bombesin/gastrin-releasing-peptide antagonist (RC-3095) and somatostatin analogue RC-160. Int J Cancer. 1994 May 15;57(4):574–580. doi: 10.1002/ijc.2910570422. [DOI] [PubMed] [Google Scholar]
- Radulovic S., Miller G., Schally A. V. Inhibition of growth of HT-29 human colon cancer xenografts in nude mice by treatment with bombesin/gastrin releasing peptide antagonist (RC-3095). Cancer Res. 1991 Nov 1;51(21):6006–6009. [PubMed] [Google Scholar]
- Raynor K., O'Carroll A. M., Kong H., Yasuda K., Mahan L. C., Bell G. I., Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993 Aug;44(2):385–392. [PubMed] [Google Scholar]
- Rens-Domiano S., Law S. F., Yamada Y., Seino S., Bell G. I., Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992 Jul;42(1):28–34. [PubMed] [Google Scholar]
- Rens-Domiano S., Reisine T. Biochemical and functional properties of somatostatin receptors. J Neurochem. 1992 Jun;58(6):1987–1996. doi: 10.1111/j.1471-4159.1992.tb10938.x. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Schaer J. C., Waser B., Mengod G. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994 Jul 1;54(13):3455–3459. [PubMed] [Google Scholar]
- Richardson S. B., Laya T., Gibson M., Eyler N., Van Ooy M. Somatostatin inhibits vasopressin-stimulated phosphoinositide hydrolysis and influx of extracellular calcium in clonal hamster beta (HIT) cells. Biochem J. 1992 Dec 15;288(Pt 3):847–851. doi: 10.1042/bj2880847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Wagner A. C., Wishart M. J., Yule D. I., Williams J. A. Effects of okadaic acid indicate a role for dephosphorylation in pancreatic stimulus-secretion coupling. Am J Physiol. 1992 Dec;263(6 Pt 1):C1172–C1180. doi: 10.1152/ajpcell.1992.263.6.C1172. [DOI] [PubMed] [Google Scholar]
- Walton K. M., Dixon J. E. Protein tyrosine phosphatases. Annu Rev Biochem. 1993;62:101–120. doi: 10.1146/annurev.bi.62.070193.000533. [DOI] [PubMed] [Google Scholar]
- Xu Y., Song J., Bruno J. F., Berelowitz M. Molecular cloning and sequencing of a human somatostatin receptor, hSSTR4. Biochem Biophys Res Commun. 1993 Jun 15;193(2):648–652. doi: 10.1006/bbrc.1993.1673. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]
- Zeggari M., Esteve J. P., Rauly I., Cambillau C., Mazarguil H., Dufresne M., Pradayrol L., Chayvialle J. A., Vaysse N., Susini C. Co-purification of a protein tyrosine phosphatase with activated somatostatin receptors from rat pancreatic acinar membranes. Biochem J. 1994 Oct 15;303(Pt 2):441–448. doi: 10.1042/bj3030441. [DOI] [PMC free article] [PubMed] [Google Scholar]


