Abstract
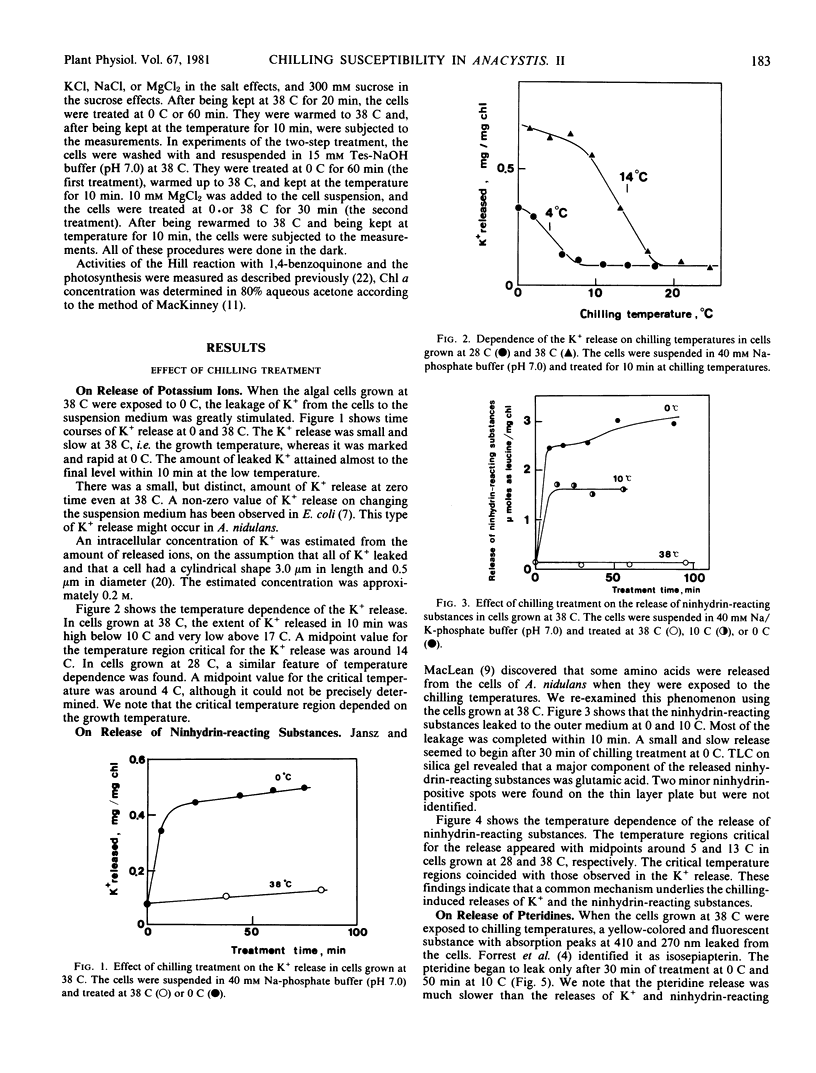
Potassium ions and amino acids were found to leak from the cytoplasm to the outer medium when the blue-green alga, Anacystis nidulans, was exposed to the chilling temperatures. The leakage was marked below the critical temperature regions, the midpoint values for which were around 5 and 14 C in cells grown at 28 and 38 C, respectively. These temperature regions coincided with those critical for the susceptibility of the photosynthetic activities and the carotenoid absorption spectrum previously studied (Ono TA, N Murata 1981 Plant Physiol 67: 176-181).
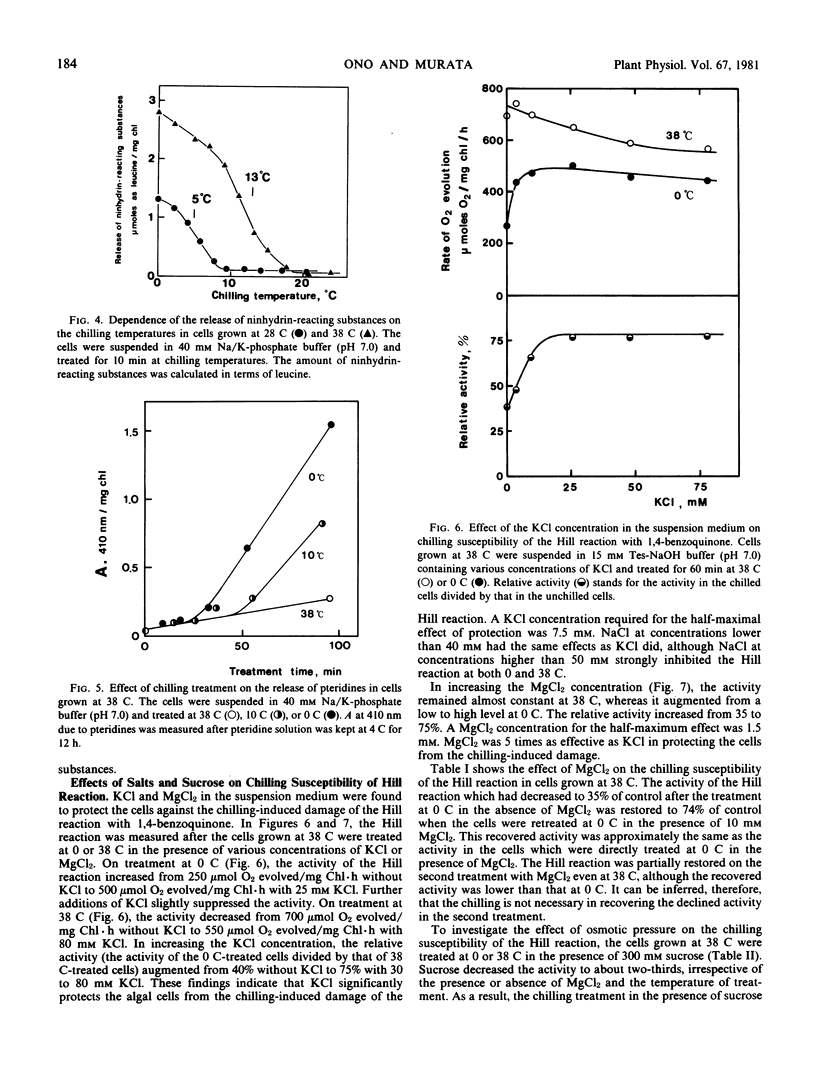
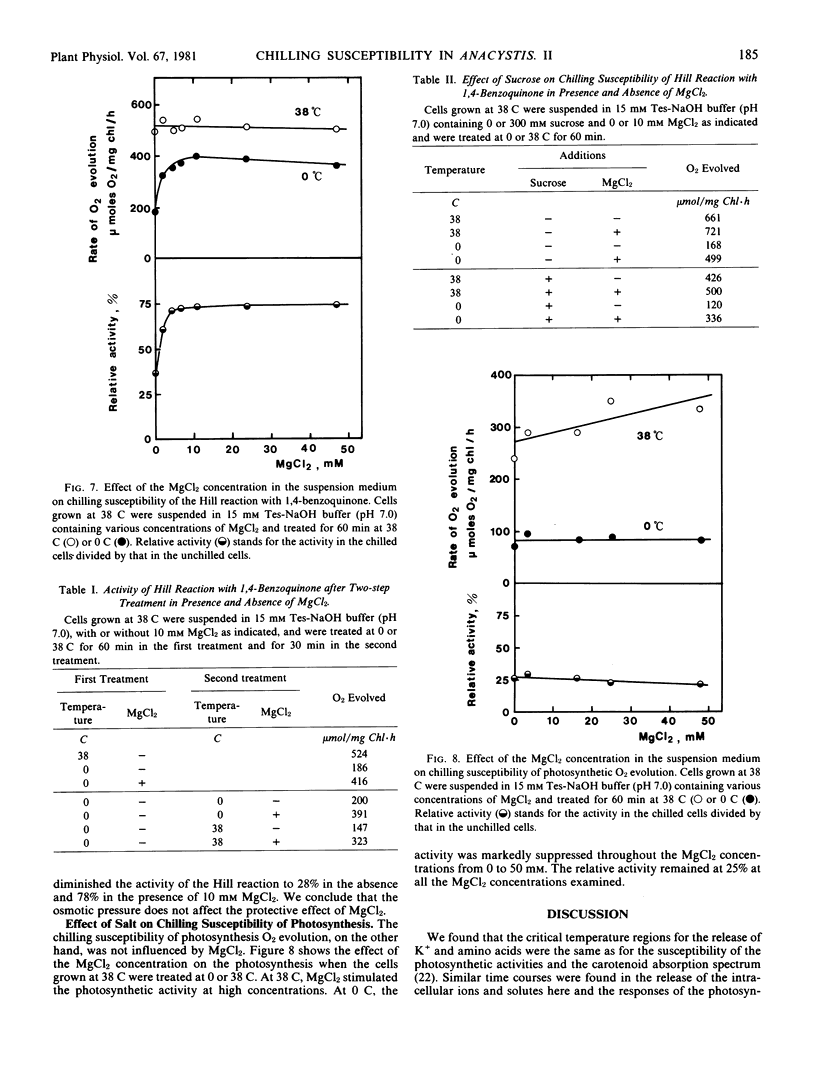
Potassium and magnesium ions in the cell suspension medium protected the algal cells from the chilling-induced damage of the Hill reaction with 1,4-benzoquinone. The activity of the Hill reaction which had been diminished by the first chilling treatment in a low salt medium was restored by the second chilling treatment of a high salt medium. The chilling susceptibility of the Hill reaction could be attributed to the leakage of cations from the cytoplasm due to increased permeability of the cytoplasmic membrane at the chilling temperatures.
A mechanism is proposed to interpret the chilling susceptibility of A. nidulans: (a) at chilling temperatures, the bilayer lipids of the cytoplasmic membrane are in the phase separation state; (b) ions and solutes having low molecular weights leak from the cytoplasm to the outer medium when the lipids of the cytoplasmic membrane are in the phase separation state; (c) decreases in the intracellular concentrations of ions and solutes degrade the physiological activities of the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Staehelin L. A. Lateral and vertical displacement of integral membrane proteins during lipid phase transition in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1901–1905. doi: 10.1073/pnas.76.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- Furtado D., Williams W. P., Brain A. P., Quinn P. J. Phase separations in membranes of Anacystis nidulans grown at different temperatures. Biochim Biophys Acta. 1979 Aug 7;555(2):352–357. doi: 10.1016/0005-2736(79)90174-3. [DOI] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Es G. A., Verkleij A. J., van Deenen L. L. Fragility of the permeability barrier of Escherichia coli. Biochim Biophys Acta. 1972 Oct 23;288(1):43–53. doi: 10.1016/0005-2736(72)90221-0. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of salts and electron transport on the conformation of isolated chloroplasts. I. Light-scattering and volume changes. Plant Physiol. 1966 Mar;41(3):533–543. doi: 10.1104/pp.41.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansz E. R., Maclean F. I. The effect of cold shock on the blue-green alga Anacystis nidulans. Can J Microbiol. 1973 Mar;19(3):381–387. doi: 10.1139/m73-062. [DOI] [PubMed] [Google Scholar]
- Leder I. G. Interrelated effects of cold shock and osmotic pressure on the permeability of the Escherichia coli membrane to permease accumulated substrates. J Bacteriol. 1972 Jul;111(1):211–219. doi: 10.1128/jb.111.1.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Effects of monovalent cations on light energy distribution between two pigment systems of photosynthesis in isolated spinach chloroplasts. Biochim Biophys Acta. 1971 Mar 2;226(2):422–432. doi: 10.1016/0005-2728(71)90109-5. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Tashiro H., Takamiya A. Effects of divalent metal ions on chlorophyll a fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1970 Mar 3;197(2):250–256. doi: 10.1016/0005-2728(70)90035-6. [DOI] [PubMed] [Google Scholar]
- Murata N. Temperature dependence of chlorophyll a fluorescence in relation to the physical phase of membrane lipids algae and higher plants. Plant Physiol. 1975 Dec;56(6):791–796. doi: 10.1104/pp.56.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Yamato I., Anraku Y. Quantitative analysis of potassium ion pool in Escherichia coli K-12. J Biochem. 1979 Jan;85(1):303–310. doi: 10.1093/oxfordjournals.jbchem.a132325. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Chilling Susceptibility of the Blue-green Alga Anacystis nidulans: I. EFFECT OF GROWTH TEMPERATURE. Plant Physiol. 1981 Jan;67(1):176–181. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Temperature dependence of the photosynthetic activities in the thylakoid membranes from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1979 Jan 11;545(1):69–76. doi: 10.1016/0005-2728(79)90114-2. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Temperature dependence on the delayed fluorescence of chlorophyll a in blue-green algae. Biochim Biophys Acta. 1977 May 11;460(2):220–229. doi: 10.1016/0005-2728(77)90208-0. [DOI] [PubMed] [Google Scholar]
- Ono T., Murata N. Photosynthetic electron transport and phosphorylation reactions in thylakoid membranes from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1978 Jun 8;502(3):477–485. doi: 10.1016/0005-2728(78)90080-4. [DOI] [PubMed] [Google Scholar]
- RING K. DER EINFLUSS DER ADAPTATIONSSTEMPERATUR AUF DIE KAELTESTABILITAET DER ZELLMEMBRAN VON STREPTOMYCES HYDROGENANS. Biochim Biophys Acta. 1965 Mar 29;94:598–600. [PubMed] [Google Scholar]
- Verwer W., Ververgaert P. H., Leunissen-Bijvelt J., Verkleij A. J. Particle aggregation in photosynthetic membranes of the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1978 Oct 11;504(1):231–234. doi: 10.1016/0005-2728(78)90021-x. [DOI] [PubMed] [Google Scholar]
- de Gier J., Blok M. C., van Dijck P. W., Mombers C., Verkley A. J., van der Neut-Kok E. C., van Deenen L. L. Relations between liposomes and biomembranes. Ann N Y Acad Sci. 1978;308:85–100. doi: 10.1111/j.1749-6632.1978.tb22015.x. [DOI] [PubMed] [Google Scholar]


