Abstract
Polyribosomal RNA isolated from pea cotyledons at various developmental stages programmed the cell-free synthesis of polypeptides which were recognized by antibodies specific for pea storage proteins. There were quantitative and qualitative changes in the template activity during seed maturation. Most of the polysomal RNA was associated with the membrane fraction, and all of the template for storage protein occurred in this fraction. Using RNA from a stage of seed maturation at which the synthesis of the high-molecular weight vicilin polypeptides predominate, it was found that the major translation products, although antigenically recognizable as storage protein, did not coincide with the authentic vicillin polypeptides on denaturing polyacrylamide gels. The addition during translation of microsomal membranes from dog pancreas or pea cotyledons resulted in the appearance of new polypeptides which did coincide with some of the authentic vicilin polypeptides (in the apparent molecular weight regions of 75,000 and 50,000) and were antigenically recognizable as storage protein. Other translation products related to storage protein were not visibly altered in their electrophoretic mobility by the addition of membranes. Microsomal membranes treated with Triton X-100 were not effective in modifying the cell-free products. The modified vicilin polypeptides and at least two other translation products were protected from proteolytic degradation, suggesting that they were sequestered within microsomal vesicles. Thus, these storage protein components may be synthesized by a mechanism analogous to that described for membrane and secretory proteins (Blobel G, B Dobberstein 1975 J Cell Biol 67: 835-851).
Full text
PDF

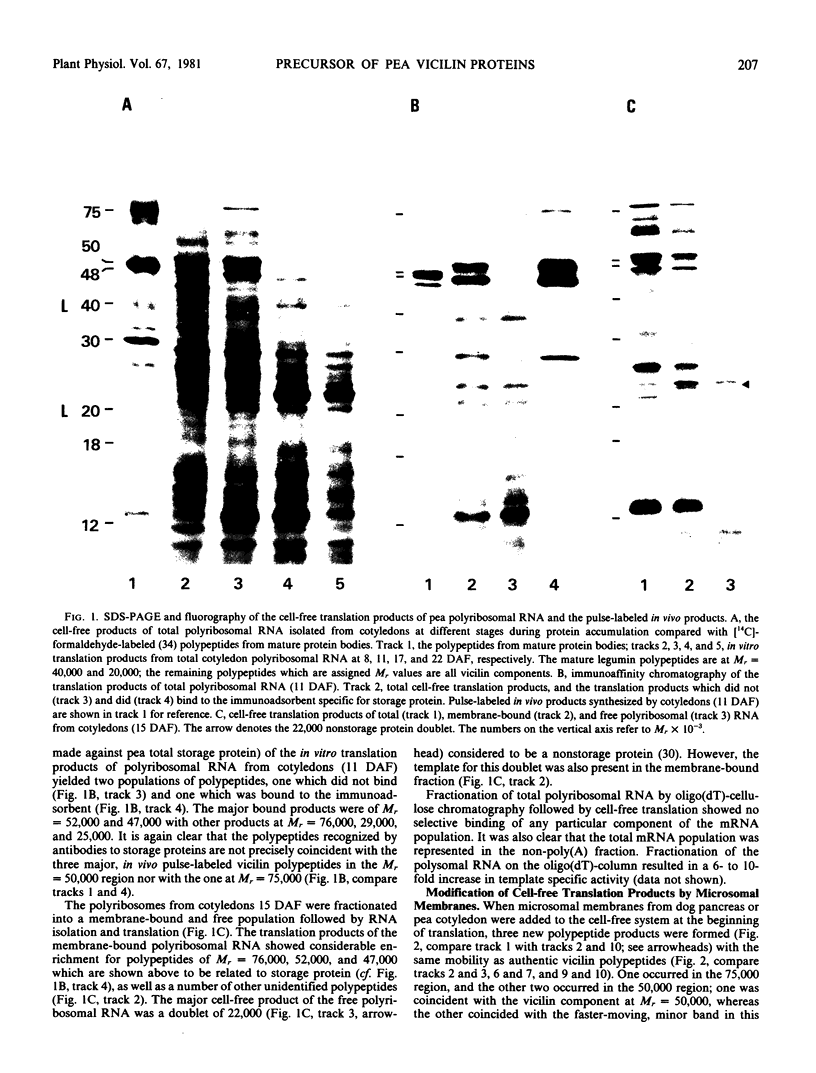

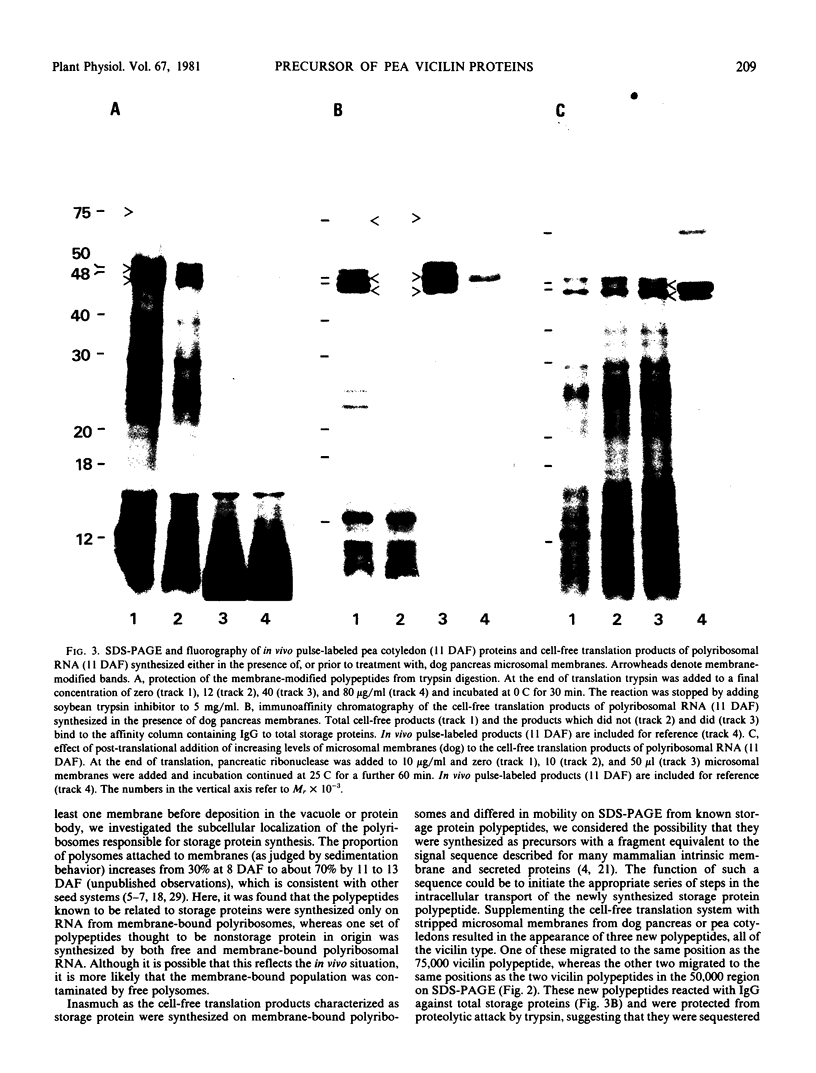


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N. In Vitro Synthesis of the alpha and alpha' Subunits of the 7S Storage Proteins (Conglycinin) of Soybean Seeds. Plant Physiol. 1980 May;65(5):990–994. doi: 10.1104/pp.65.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A., Rubenstein I., Simon M. N. Purification and translation of zein messenger RNA from maize endosperm protein bodies. Proc Natl Acad Sci U S A. 1978 Feb;75(2):696–700. doi: 10.1073/pnas.75.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Ma Y., Buchbinder B. U., Pyne J. W., Sun S. M., Bliss F. A. Messenger RNA for G1 protein of French bean seeds: Cell-free translation and product characterization. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Spencer D. Cell-free Synthesis of Pea Seed Proteins. Plant Physiol. 1977 Nov;60(5):655–661. doi: 10.1104/pp.60.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher U. In Vitro Synthesis of a Precursor to the Methionine-rich Polypeptide of the Zein Fraction of Corn. Plant Physiol. 1979 Feb;63(2):354–358. doi: 10.1104/pp.63.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Püchel M., Müntz K., Parthier B., Aurich O., Bassüner R., Manteuffel R., Schmidt P. RNA metabolism and membrane-bound polysomes in relation to globulin biosynthesis in cotyledons of developing field beans (Vicia faba L.). Eur J Biochem. 1979 May 15;96(2):321–329. doi: 10.1111/j.1432-1033.1979.tb13043.x. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienand U., Feix G. Electrophoretic fractionation and translation in vitro of poly(rA)-containing RNA from maize endosperm. Evidence of two mRNAs coding for zein protein. Eur J Biochem. 1978 Dec;92(2):605–611. doi: 10.1111/j.1432-1033.1978.tb12783.x. [DOI] [PubMed] [Google Scholar]