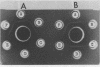
Abstract
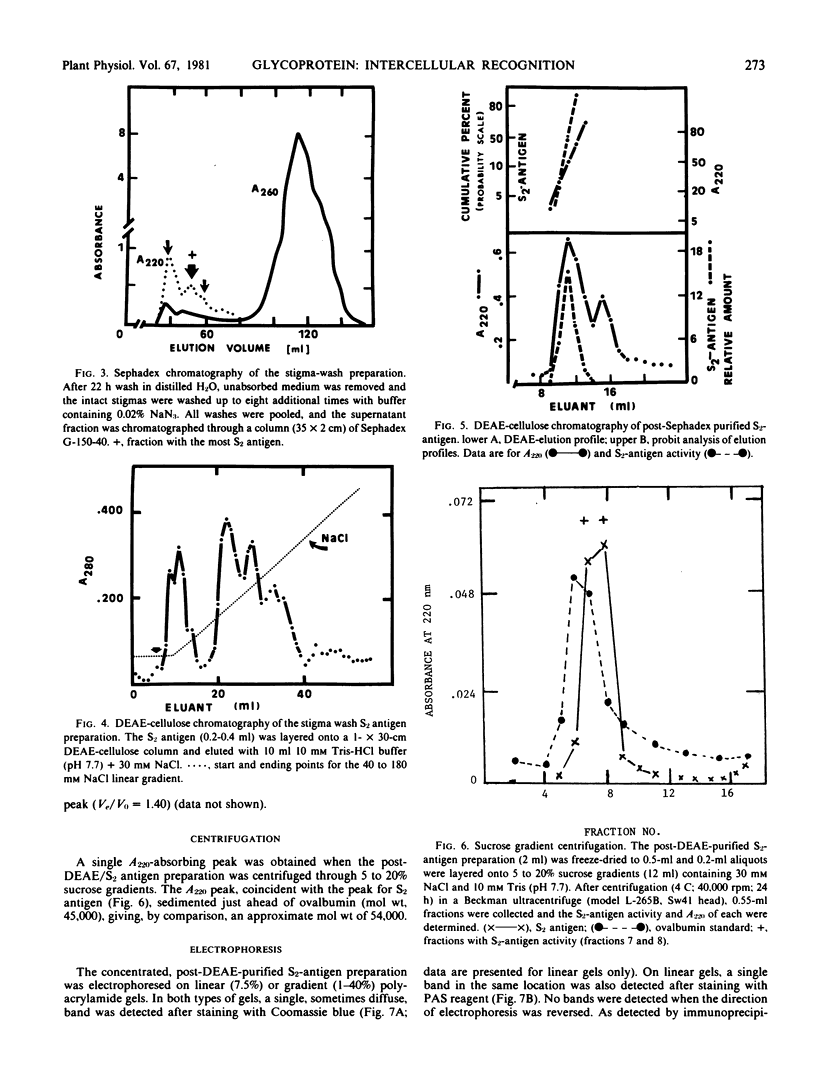
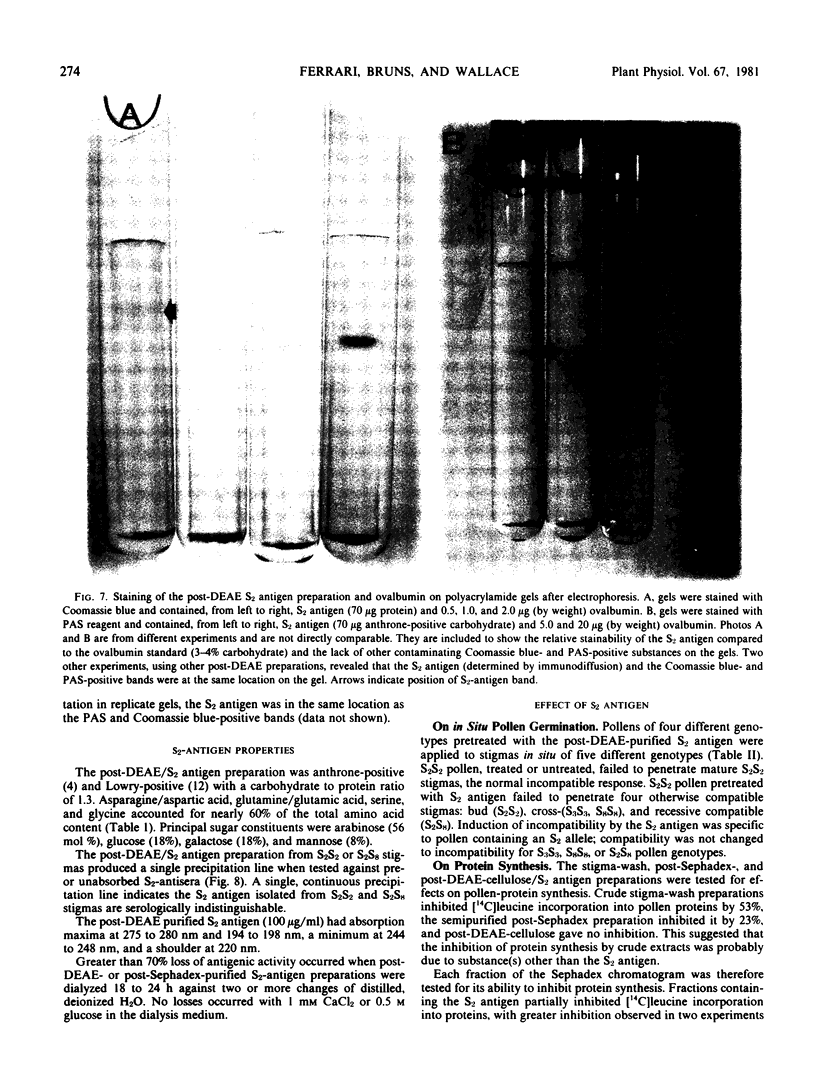
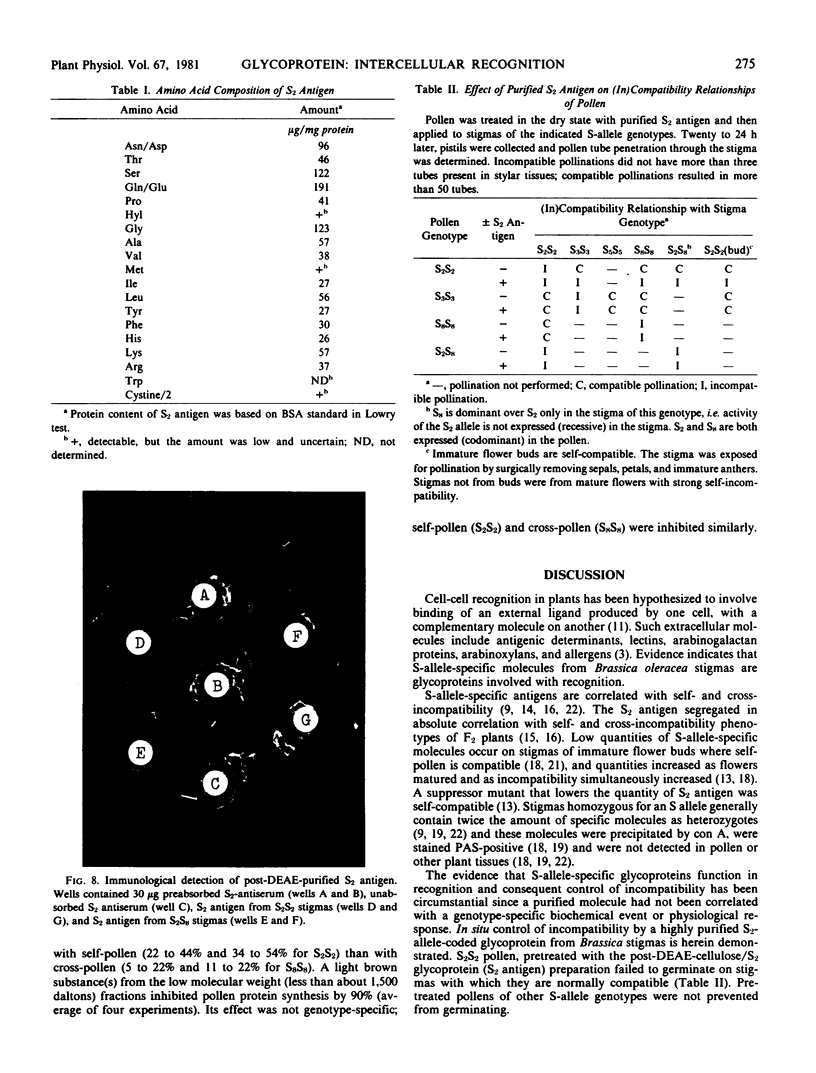
A recognition molecule was isolated from stigmas of S-allele genotype S2S2 of Brassica oleracea var. capitata L. After Sephadex chromatography, it eluted as a single symmetrical peak during diethylaminoethane-cellulose chromatography. A high degree of purity was affirmed by: sedimentation as a single peak during ultracentrifugation through 5 to 20% sucrose gradients; elution as a single peak from Sephadex G-100; visualization as a single band which stains with Coomassie blue and periodic acid Schiff reagent after electrophoresis on polyacrylamide gels. Other criteria supporting the conclusion that it is a glycoprotein are: (a) the highly purified preparation is anthrone-positive and has a Lowry protein to anthrone-positive carbohydrate ratio of 1.3; (b) the preparation contains arabinose, galactose, glucose, and mannose, although it is not precipitated by concanavalin A; (c) the immunological properties of the molecule are lost following protease treatment, and it has a molecular weight of 90,000 by Sephadex gel-filtration analysis and 54,500 by velocity sedimentation analysis.
In vitro pretreatment of S2S2 pollen with the post-diethylaminoethane-purified S2 glycoprotein prevented the S2S2 pollen from germinating on three classes of compatible stigmas: (a) mature stigmas of genotypes S3S3 and S8S8, which are non-self genotypes; (b) immature stigmas of genotype S2S2, where incompatibility is not expressed; and (c) mature stigmas with a recessive S2 allele. Pretreatment of S3S3 and S8S8 pollen with the S2 glycoprotein did not interfere with their germination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Ferrari T. E., Wallace D. H. Incompatibility on brassica stigmas is overcome by treating pollen with cycloheximide. Science. 1977 Apr 22;196(4288):436–438. doi: 10.1126/science.196.4288.436. [DOI] [PubMed] [Google Scholar]
- Kozulić B., Ries B., Mildner P. N-acetylation of amino sugar methyl glycosides for gas-liquid chromatographic analysis. Anal Biochem. 1979 Apr 1;94(1):36–39. doi: 10.1016/0003-2697(79)90786-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nasrallah M. E. Genetic control of quantitative variation in self-incompatibility proteins detected by immunodiffusion. Genetics. 1974 Jan;76(1):45–50. doi: 10.1093/genetics/76.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Studies and critique of Amido Black 10B, Coomassie Blue R, and Fast Green FCF as stains for proteins after polyacrylamide gel electrophoresis. Anal Biochem. 1979 Jul 15;96(2):263–278. doi: 10.1016/0003-2697(79)90581-5. [DOI] [PubMed] [Google Scholar]