Abstract
The capacity of ligands in xylem fluid to form metal complexes was tested with a series of in vitro experiments using paper electrophoresis and radiographs. The xylem fluid was collected hourly for 8 hours from soybean (Glycine max L. Merr.) and tomato (Lycopersicon esculentum Mill.) plants grown in normal and Zn-phytotoxic nutrient solutions. Metal complexation was assayed by anodic or reduced cathodic movement of radionuclides (63Ni, 65Zn, 109Cd, 54Mn) that were presumed to have formed negatively charged complexes.
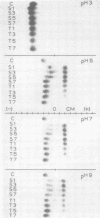
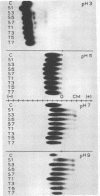
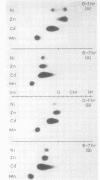
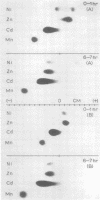
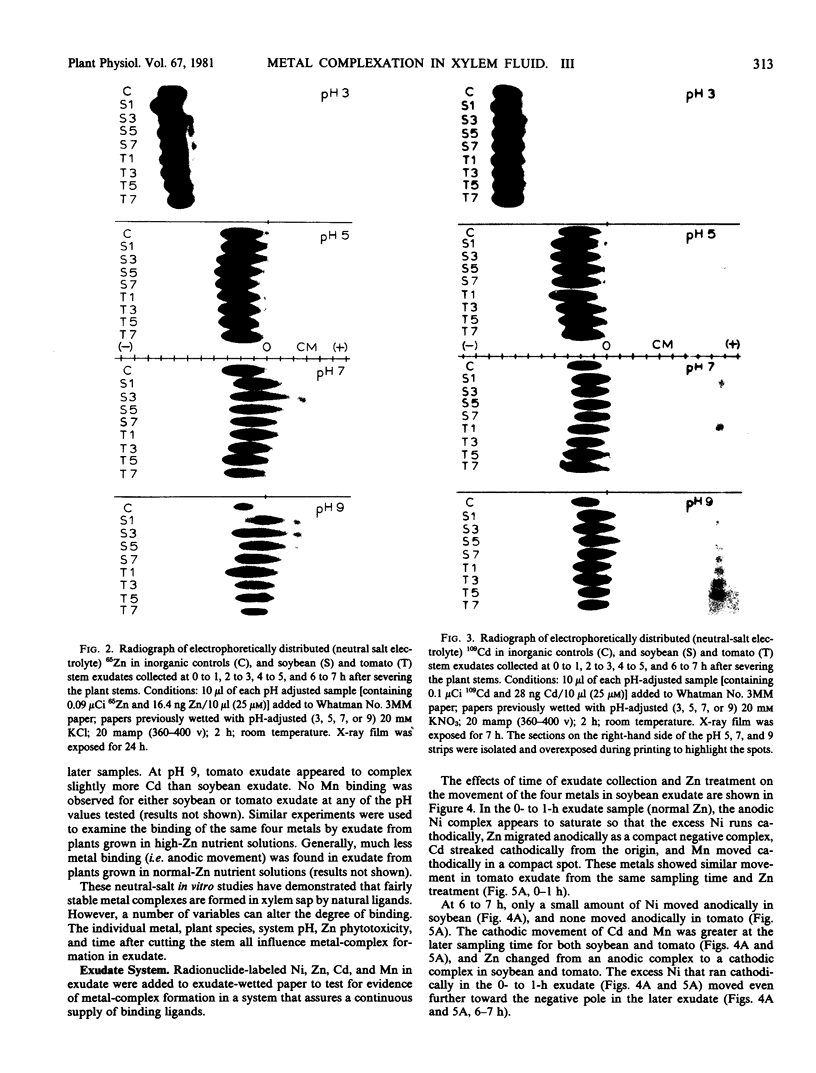
Electrophoretic migration of Ni, Zn, Cd, and Mn added to xylem exudate and spotted on KCl- or KNO3-wetted paper showed that stable Ni, Zn, and Cd metal complexes were formed by exudate ligands. No anodic Mn complexes were observed in this test system. Solution pH, plant species, exudate collection time, and Zn phytotoxicity all affected the amount of metal complex formed in exudate. As the pH increased, there was increased anodic metal movement. Soybean exudate generally bound more of each metal than did tomato exudate. Metal binding usually decreased with increasing exudate collection time, and less metal was bound by the high-Zn exudate.
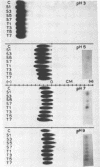
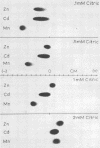
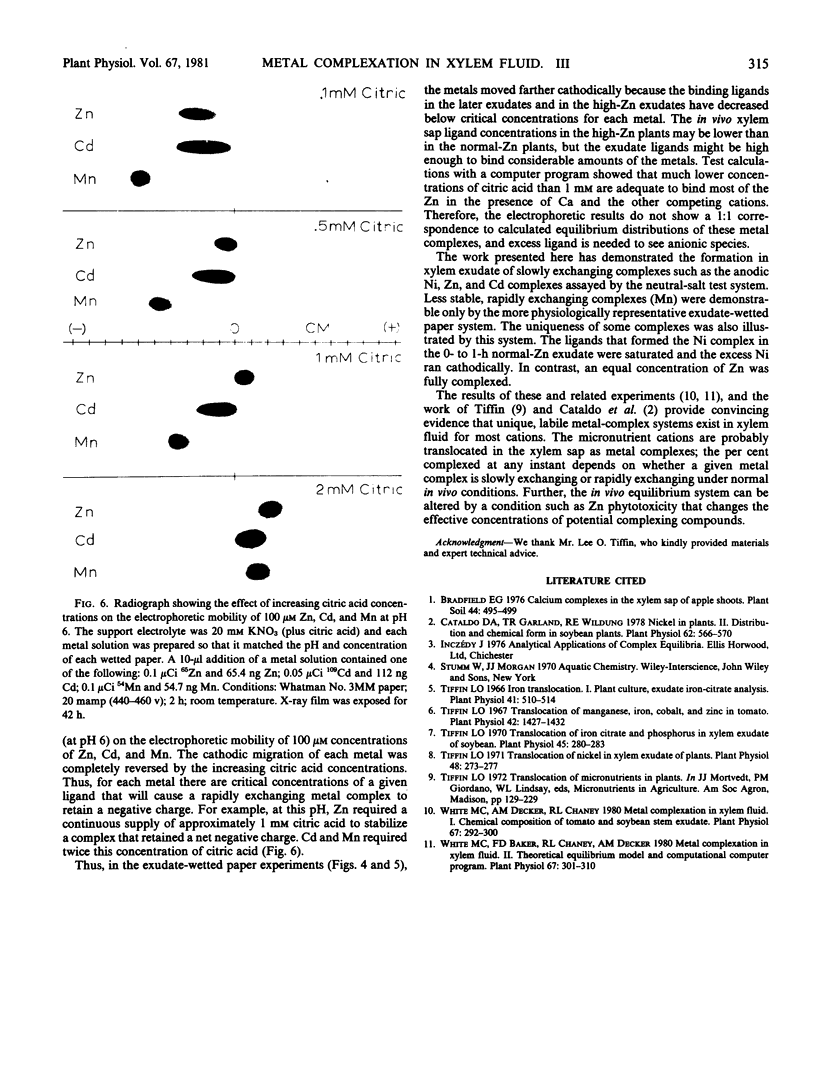
Ni, Zn, Cd, and Mn in exudate added to exudate-wetted paper demonstrated the effect of ligand concentration on stable metal complex formation. Complexes for each metal were demonstratable with this method. Cathodic metal movement increased with time of exudate collection, and it was greater in the high-Zn exudate than in the normal-Zn exudate. A model study illustrated the effect of ligand concentration on metal complex stability in the electrophoretic field. Higher ligand (citric acid) concentrations increased the stability for all metals tested.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A., Garland T. R., Wildung R. E. Nickel in Plants: II. Distribution and Chemical Form in Soybean Plants. Plant Physiol. 1978 Oct;62(4):566–570. doi: 10.1104/pp.62.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Iron translocation I. Plant culture, exudate sampling, iron-citrate analysis. Plant Physiol. 1966 Mar;41(3):510–514. doi: 10.1104/pp.41.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol. 1970 Mar;45(3):280–283. doi: 10.1104/pp.45.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of manganese, iron, cobalt, and zinc in tomato. Plant Physiol. 1967 Oct;42(10):1427–1432. doi: 10.1104/pp.42.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of nickel in xylem exudate of plants. Plant Physiol. 1971 Sep;48(3):273–277. doi: 10.1104/pp.48.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]