Abstract
The parabrachial nucleus (PBN) is a key nucleus for the regulation of feeding behavior. Inhibitory inputs from the hypothalamus to the PBN play a crucial role in the normal maintenance of feeding behavior, because their loss leads to starvation. Viscerosensory stimuli result in neuronal activation of the PBN. However, the origin and neurochemical identity of the excitatory neuronal input to the PBN remain largely unexplored. Here, we hypothesize that hindbrain glucagon-like peptide 1 (GLP-1) neurons provide excitatory inputs to the PBN, activation of which may lead to a reduction in feeding behavior. Our data, obtained from mice expressing the yellow fluorescent protein in GLP-1-producing neurons, revealed that hindbrain GLP-1-producing neurons project to the lateral PBN (lPBN). Stimulation of lPBN GLP-1 receptors (GLP-1Rs) reduced the intake of chow and palatable food and decreased body weight in rats. It also activated lPBN neurons, reflected by an increase in the number of c-Fos-positive cells in this region. Further support for an excitatory role of GLP-1 in the PBN is provided by electrophysiological studies showing a remarkable increase in firing of lPBN neurons after Exendin-4 application. We show that within the PBN, GLP-1R activation increased gene expression of 2 energy balance regulating peptides, calcitonin gene-related peptide (CGRP) and IL-6. Moreover, nearly 70% of the lPBN GLP-1 fibers innervated lPBN CGRP neurons. Direct intra-lPBN CGRP application resulted in anorexia. Collectively, our molecular, anatomical, electrophysiological, pharmacological, and behavioral data provide evidence for a functional role of the GLP-1R for feeding control in the PBN.
Glucagon-like peptide 1 (GLP-1), produced in intestinal L-cells and the nucleus of the solitary tract (NTS) in the hindbrain, regulates blood glucose and reduces feeding behavior (1). Much is known about the mechanisms underlying the glucoregulatory function of GLP-1, and this ability of GLP-1 has already been used in the clinic. Genetic and pharmacological data have established that GLP-1 receptor (GLP-1R) activation reduces food intake and, conversely, that a reduction of activity at the GLP-1R increases food intake. Although GLP-1 is a key player in energy balance control, the mechanisms and neural substrates engaged by GLP-1 to regulate food intake are only beginning to be identified. GLP-1 neurons innervate many brain areas relevant for energy balance control (2, 3). Initially, the literature has emphasized the hypothalamus as the primary target for the feeding inhibition by GLP-1 (4, 5). However, the energy balance control system extends beyond the hypothalamus. Subsequent studies indicate that both local GLP-1 neuronal projections within the NTS and far reaching projections to the mesolimbic ventral tegmental area and the nucleus accumbens are important for the normal regulation of feeding (6–9). In addition, GLP-1R and its mRNA have been identified in the pontine parabrachial nucleus (PBN) (10). Here, we investigate the functional role of GLP-1 in this nucleus for feeding control and the mechanisms involved.
The PBN integrates neural and possibly hormonal signals associated with gustatory properties of food as well as visceral satiety and illness signals. Many neuropeptides central to feeding regulation act on PBN neurons to modulate feeding behavior. Melanocortin and prostaglandin receptor ligands applied directly to the PBN decrease feeding behavior (11, 21), whereas cannabinoid and μ-opioid receptor ligands increase feeding (12, 13). The PBN is a critical nucleus for the creation of taste associations but only in rodents; in primates, and presumably also humans, the PBN mainly functions as a relay and integrator for viscerosensory inputs (14). Recently, interest in the PBN has been rejuvenated by data showing that γ-aminobutyric acid (GABA)ergic and glutamatergic inputs to the PBN are pivotal in the regulation of feeding behavior. When the PBN glutamate/GABA input balance is disturbed, mice stop eating and die of starvation (15–18), a finding that underscores the critical role of the PBN in the regulation of feeding behavior.
The PBN receives direct projections from NTS neurons relaying taste and viscerosensory information in rodents (19), but the neuropeptides that these fibers carry have yet to be elucidated. It is well known that GLP-1 is produced by cell bodies of the NTS and that projections from these cells reach many parts of the brain. Here, using a unique mouse model that expresses a fluorescent protein in GLP-1-producing neurons, we investigate whether these GLP-1 neurons also project to the PBN, thereby providing a source of the endogenous ligand to the PBN GLP-1R. We further evaluated whether the PBN GLP-1R plays a role in feeding behavior control. Because treatments that induce hypophagia drastically increase activity in the lateral PBN (lPBN) neurons, we evaluated whether central GLP-1R stimulation can induce c-Fos protein expression in the PBN and whether GLP-1R activation in the PBN changes the electrical activity of the PBN neurons. Lastly, we identify potential downstream mediators of GLP-1R activation in the PBN, which may include calcitonin gene-related peptide (CGRP) and IL-6. Collectively, our molecular, electrophysiological, pharmacological, and behavioral data provide evidence for a functional role of GLP-1R in the PBN in the control of feeding behavior and identify the neurochemical mechanisms involved.
Materials and Methods
Animals
Adult female and male mGLU-124 Venus yellow fluorescent protein (YFP) transgenic mice (YFP-preproglucagon [PPG] mice; University of Cambridge) (20) were housed in plastic cages with water and standard chow available ad libitum. Male Sprague-Dawley rats (180–250 g at arrival and 450 g during the drug administration tests; Charles River) were housed in a 12-hour light, 12-hour dark cycle, in individual cages with free access to chow and water, except during the period of chocolate and saccharine consumption. All studies were carried out with ethical permissions from the Animal Welfare Committee of the Institute of Experimental Medicine and University of Gothenburg, in accordance with legal requirements of the European Community (decree 86/609/EEC).
Surgery
Rats were implanted with a guide cannula targeting the lPBN or the lateral ventricle (26 gauge; Plastics One) under isofluorane anesthesia as described previously (11, 21). The next coordinates were chosen: lateral ventricle, ±1.6/−0.9/−2.5 mm (midline/bregma/skull, respectively), with injector aimed 4.5 mm ventral to skull; and lPBN 2.0/−9.5/4.5 mm, with injector aimed 6.5 mm ventral to skull. PBN cannula placement was verified histologically postmortem by injection of India ink (0.2-μL volume matched drug delivery in the experiments). Only rats whose dye injection site was found within the lPBN were included in the data analysis.
GLP-1 fiber detection
Mice were anesthetized with ketamine/xylazine solution and perfused transcardially with heparinized saline followed by fresh fixative solution (paraformaldehyde 4%) in 0.1M phosphate buffer. The brains were collected, coronal 25-μm sections were cut using a cryostat, then collected in tubes containing tissue storage solution consisting of 50-mL glycerin, 50-mL ethylene glycol, and 100-mL 0.1M phosphate buffer (pH 7.5), and stored until use in 4°C. The sections were washed (3 × 15 min) in TRIS NaCl Tween 20-buffer (TNT-buffer) with Triton X-100 (0.1%) (Sigma-Aldrich). For CGRP visualization, the sections were incubated for 2 days in TRIS NaCl Boehringer Milk Powder-buffer (TNB-buffer) blocking solution (PerkinElmer) with 1:2000 goat polyclonal antibody to CGRP (ab36001; Abcam). The sections were then washed in TNT with Triton X-100 (0.1%) and incubated in TNB blocking solution with 1:1000 Donkey antigoat Alexa Fluor 568 (ab36001; Abcam). The cell nuclei were stained with diamidino-2-phenylindole (DAPI) (1:5000; Life Technologies). The sections were then washed in TNT (2 × 15 min), submerged in 0.1M phosphate buffer (PB), and mounted on microscope slides (Superfrost Plus; Menzel) together with ProLong Gold Antifade (Life Technologies). The GLP-1 fibers were visualized with a confocal microscope (LSM 700; Carl Zeiss). LPBN and medial PBN (mPBN) DAPI-labeled cells and lPBN CGRP-positive cells receiving GLP-1 innervation were quantified from at least 4 25-μm sections per brain. Triple channel confocal images (to cover the entire PBN) were generated with a Plan Fluor ×20/0.75 lens and a solid-state laser. A tile scan of 3 × 3 tiles was obtained from the center of the lPBN and mPBN, respectively. Neurons were considered CGRP-labeled when their staining was clearly above background and their cell nucleus was in the plane of image. Innervation of cells in the PBN by GLP-1 fibers was determined by switching between green- and blue-channel images (for quantification of GLP-1 innervated cells in mPBN and lPBN) and red-, green-, and blue-channel images (for colocalization of GLP-1 fibers with CGRP-labeled neurons).
Food intake and saccharine-drinking measurements after lPBN GLP-1R activation
Consumption of 1) chocolate pellets (n = 11), 2) 0.1% saccharine (n = 12), and 3) chow (n = 11) was measured in 3 groups of rats unilaterally infused (0.2-μL) with a selective and potent GLP-1R agonist Exendin-4 (Ex-4) (0.1- and 1-μg; Tocris) or vehicle (artificial cerebrospinal fluid, aCSF; Tocris) into the lPBN. All injections were performed early in the light cycle. Rats were exposed to both saccharine solution and chocolate pellets on at least 6 occasions before the test to achieve a stable intake and reduce the novelty of the food. Additionally, 24-hour body weight change was measured (n = 11) in the third group of rats. Rats had free access to water at all times and to chow at all times except during the period of chocolate and saccharine intake measurement.
Food intake and body weight measurements after lPBN GLP-1R blockade
Consumption of chow was measured in rats (n = 12) unilaterally infused (0.3 μL) with a selective GLP-1R antagonist Ex-9 (20 μg; Tocris) or vehicle (aCSF; Tocris) into the lPBN. Body weight change was measured overnight, 16 hours after drug injections. Injections were performed 60 minutes before dark onset. Rats had free access to water at all times and to chow at all times except 5 hours before injections (during the light cycle); this was done to ensure equal levels of satiety at the start of the experiment. A similar experimental design was used when testing the effects of intra-lPBN-injected CGRP (3.8 μg; Tocris) or vehicle (aCSF).
Loose-patch clamp electrophysiology
Rats were anesthetized using Isoflurane inhalation. The brain was removed rapidly and immersed in ice-cold sodium-free solution (22). Acute 300-μm-thick coronal slices containing the lPBN were prepared with a VT-1000S vibratome (Leica GmBH) in the sodium-free solution and then equilibrated in normal aCSF (naCSF) (135mM NaCl, 3.5mM KCl, 26mM NaHCO3, 1.2mM MgSO4, 1.25mM NaH2PO4, 2.5mM CaCl2, and 10mM glucose, bubbled with O2/CO2). Loose-patch clamp measurements to record action currents were carried out as described earlier (23) with slight modifications. Briefly, pipette potential was held at 0 mV, pipette resistance 1–2 MΩ, and resistance of loose-patch seal 7–40 MΩ. The pipette solution contained: 123mM NaCl, 3.5mM KCl, 2.5mM CaCl2, 1.3mM MgCl2, 10mM HEPES, and 10mM glucose (pH 7.3; with NaOH). lPBN was identified under microscopic control, and large ovoid cells of this area (24) were chosen for recordings. Measurements were carried out with an initial control recording (4 min); then in the first experimental group of neurons, Ex-4 (1μM) (25) was added to the naCSF by a single bolus into the recording chamber, and the recording continued for a subsequent 11 minutes. In a second experimental group of neurons, the GLP-1R antagonist Ex-9 (1μM; Tocris) (25) was applied after the initial recording of basal firing. Ex-9 was then present in the naCSF continuously. Ten minutes after starting Ex-9 application, firing was recorded, then Ex-4 was added and the recording continued. Each neuron served as its own control when drug effects were evaluated.
c-Fos expression
Treatment
On the day of experiment, rats were injected with Ex-4 (0.3 μg in 1 μL) or aCSF (1 μL) into the lateral ventricle (n = 3–4 per treatment group). Rats were treated and killed during the light cycle. Rats had ad libitum access to food throughout the study. Ninety minutes after the injections, all of the rats were anesthetized with ketamine-xylazine solution and transcardially perfused with heparinized saline solution, followed by 4% paraformaldehyde in 0.1M PB.
Immunocytochemistry for detection of c-Fos-protein in brain sections
Immunohistochemical detection of c-Fos protein was performed as described previously (26). Briefly, coronal sections (40 μm) were cut on a cryostat through the lPBN, and every third section was collected into PB. Endogenous peroxidases were deactivated, and sections were incubated with a rabbit polyclonal anti-Fos antibody (Ab-5, PC-38 Calbiochem; CN Biosciences UK). Bound antibody was detected with peroxidase-labeled goat antirabbit IgG (Vector Laboratories Ltd) and visualized using a nickel-intensified diaminobenzidine reaction giving a purple-black precipitate within cell nuclei. PBN-containing brainstem sections were viewed under a microscope, and all c-Fos-positive cells were counted in the lPBN by an experimenter blinded to the conditions. Data were expressed as average of total c-Fos counts of all rats; total number per rat was calculated by adding total number of c-Fos-positive cells from the left and right lPBN.
RNA isolation and mRNA expression
PBN gene expression levels were measured after lateral ventricle injection of Ex-4 or vehicle (aCSF) in 2 separate groups of rats. One group was restricted to 10 g (∼50% of average overnight intake) of chow overnight, and the second was allowed to eat ad libitum (n = 6–9 per treatment group). The following genes were examined: Calca, Gabr, Gad1, Grin2b, Il1b, Il6, and Mc4r. They were selected because of their reported role in feeding regulation in PBN or their connection to GLP-1. Ninety minutes after Ex-4 or aCSF injection, the brains were rapidly removed, and the PBN was dissected using a brain matrix, frozen in liquid nitrogen, and stored at −80°C. Individual brain samples were homogenized in Qiazol (QIAGEN) using a TissueLyzer (QIAGEN). Total RNA was extracted using RNeasy Lipid Tissue Mini kit (QIAGEN) with additional deoxyribonuclease treatment (QIAGEN). RNA quality and quantity were assessed by spectrophotometric measurements (Nanodrop 1000; NanoDrop Technologies). For cDNA synthesis, iScript cDNA Synthesis kit (Bio-Rad) was used. Real-time RT-PCR was performed using TaqMan probe, and primer sets for target genes were chosen from an on-line catalogue (reference numbers were as follows: Actb-Rn00667869_m1, Mc4r-Rn01491866_s1, Calca-Rn01511353_g1, Grin2b-Rn00680474_m1, Gabrd-Rn01517017_g1, Gad1-Rn00690300_m1, IL1b-Rn00580432_m1, and IL6-Rn01410330_m1) (Applied Biosystems). Gene expression values were calculated based on the ΔΔCt method (27), where the vehicle-injected group was designated as the calibrator (results shown in figure 5 below). β-Actin was used as reference gene.
Statistical analysis
All the data are presented as mean ± SEM. For electrophysiology group, data were expressed as mean ± SEM, and percentage change in the frequency of the firing rate due to the application of the Ex-4 or the Ex-9 was calculated. Each electrophysiological experimental group contained 10 recorded cells from 6–7 animals. Patch clamp recordings were stored and analyzed off-line. Event detection in the recordings was performed using the Clampfit module of PClamp 9.2 software (Molecular Devices Co). For electrophysiology, c-Fos results and feeding data statistical significance was analyzed using Student's t test or one- or two-way ANOVA when appropriate (GraphPad Software, Inc). P < .05 was considered statistically significant.
Results
GLP-1 fibers in the PBN
Fluorescent YFP-PPG neurons were detected at the caudal region of the NTS of YFP-PPG mice (Figure 1, A and B). Green YFP-immunoreactive axons were found to closely appose blue DAPI-labeled cell bodies in the lPBN (Figure 1, C and D). Over half (55 ± 1.2%) of the lPBN cell bodies were found to receive fibers from the hindbrain GLP-1 neurons. The medial region of the PBN was also found to receive YFP-immunoreactive fibers, however to a lesser extent than the lPBN region (31 ± 2.3% of the DAPI-positive mPBN cells were innervated by GLP-1 fibers) (Figure 1, E and F).
Figure 1.

GLP-1 innervation of the PBN. Fluorescent YFP-PPG neurons (green) and DAPI (nuclear stain, blue) in coronal sections through the PBN and the NTS of YFP-PPG mice. Micrographs showing the cell bodies of green YFP-immunoreactive PPG neurons (yellow arrows) in the NTS (A and B). Micrographs showing the lPBN (C and D), and the region of the mPBN just below the superior cerebellar peduncles (scp) (E and F). Many green YFP-immunoreactive axons closely appose blue DAPI-labeled cell bodies in the lPBN. White arrows indicate PBN cell bodies closely apposed by the GLP-1 fibers, whereas red arrows indicate cell bodies in this region that were not apposed by the GLP-1 fibers. Insets in B, D, and F show the interaction at a single cell level. cc, central canal. B, D, and F show higher magnification of areas in A, C, and D, respectively.
Food intake, saccharine drinking, and body weight after intra-lPBN GLP-1R stimulation
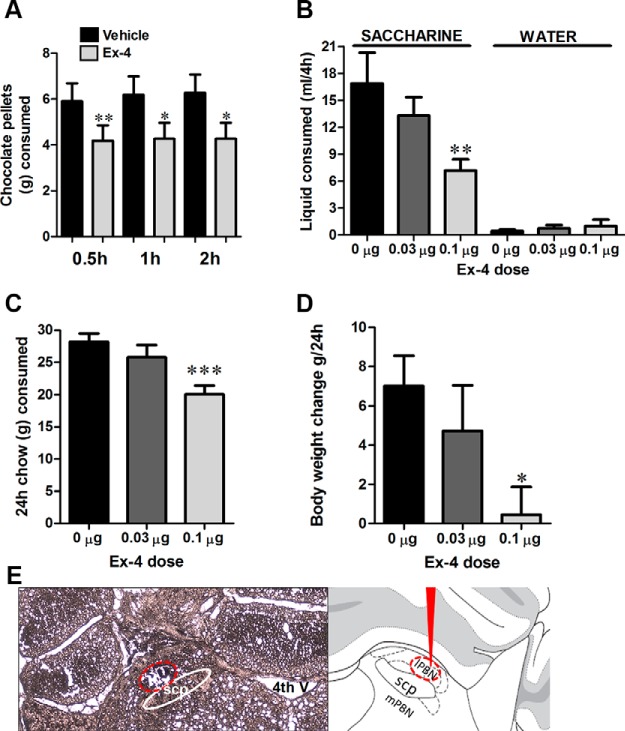
The goal of the in vivo experiments was to determine whether GLP-1R activation in the PBN can contribute to food intake reduction across a variety of caloric, palatable and less palatable, as well as noncaloric sweet liquid foods. PBN GLP-1R stimulation via microinjection of a selective GLP-1R agonist Ex-4 significantly reduced chocolate pellet consumption over the 2-hour period of measurement (Figure 2A). Intra-PBN Ex-4 microinjection also reduced the amount of saccharine drunk by a separate group of rats (one-way ANOVA, F(2,35) = 5.96; P < .008) (Figure 2B) without affecting water drinking (offered in parallel with saccharine). Moreover, the intake of normal chow (one-way ANOVA, F(2,38) = 9.19; P < .001) (Figure 2C) and body weight (one-way ANOVA, F(2,35) = 10.5; P < .001) (Figure 2D) were also reduced when measured over a 24-hour period.
Figure 2.
GLP-1R stimulation by Ex-4 in the lPBN reduces food intake and body weight. Intra-lPBN delivery of Ex-4 reduced the consumption of chocolate pellets over the 2-hour period of data collection (A), the amount of saccharine drank (but not water consumption) over 4 hours of data collection (B), the 24-hour chow intake (C), and 24-hour body weight change (D). Data are expressed as mean ± SEM. *, P < .05; **, P < .01; ***, P < .005. E, Representative photomicrograph of a coronal section of rat brain at the level of the lPBN illustrating the microinjection site (encircled area) for the behavioral experiments (left panel) and a schematic representation of the PBN (right panel). scp, superior cerebellar peduncles; 4th V, 4th ventricle.
Food intake and body weight after intra-lPBN GLP-1R blockade
Blockade of lPBN GLP-1Rs resulted in a significant increase in chow intake at 2 and 3 hours after Ex-9 injections and a significant increase in body weight measured overnight 16 hours after Ex-9 injection (Figure 3, A–C).
Figure 3.
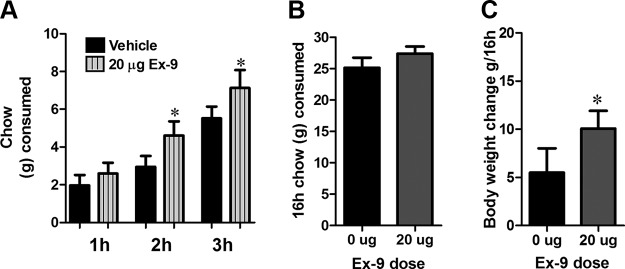
GLP-1R blockade in the lPBN increases food intake and body weight. Intra-lPBN delivery of Ex-9 increased chow intake at 2 and 3 hours after injection (A). Overnight chow intake measured at 16 hours after Ex-9 injection was not altered (B). Ex-9 increased 16-hour body weight gain. C, Data are expressed as mean ± SEM. *, P < .05.
PBN GLP-1R stimulation results in increased firing rate of lPBN neurons
To test the hypothesis that Ex-4 influences function of large ovoid neurons in the lPBN, we examined the electrophysiological response of these neurons to Ex-4. In the first experimental group, Ex-4 (1μM) was applied and increased the firing rate significantly (330 ± 60% of the control) (Figure 4, A and B). The basal firing rate (without any drugs) was 1.02 ± 0.44 Hz (Figure 4A). In a second experimental group, Ex-4 was administered in the presence of the GLP-1 antagonist, Ex-9 (1μM), and the firing rate remained unaltered (135 ± 52% from the firing rate obtained with Ex-9) (Figure 4, C and D). This value was, however, significantly different from that achieved with Ex-4 alone (Figure 4E). Application of Ex-9 alone did not affect the firing rate of the recorded neuron (115 ± 34% of the basal firing rate).
Figure 4.
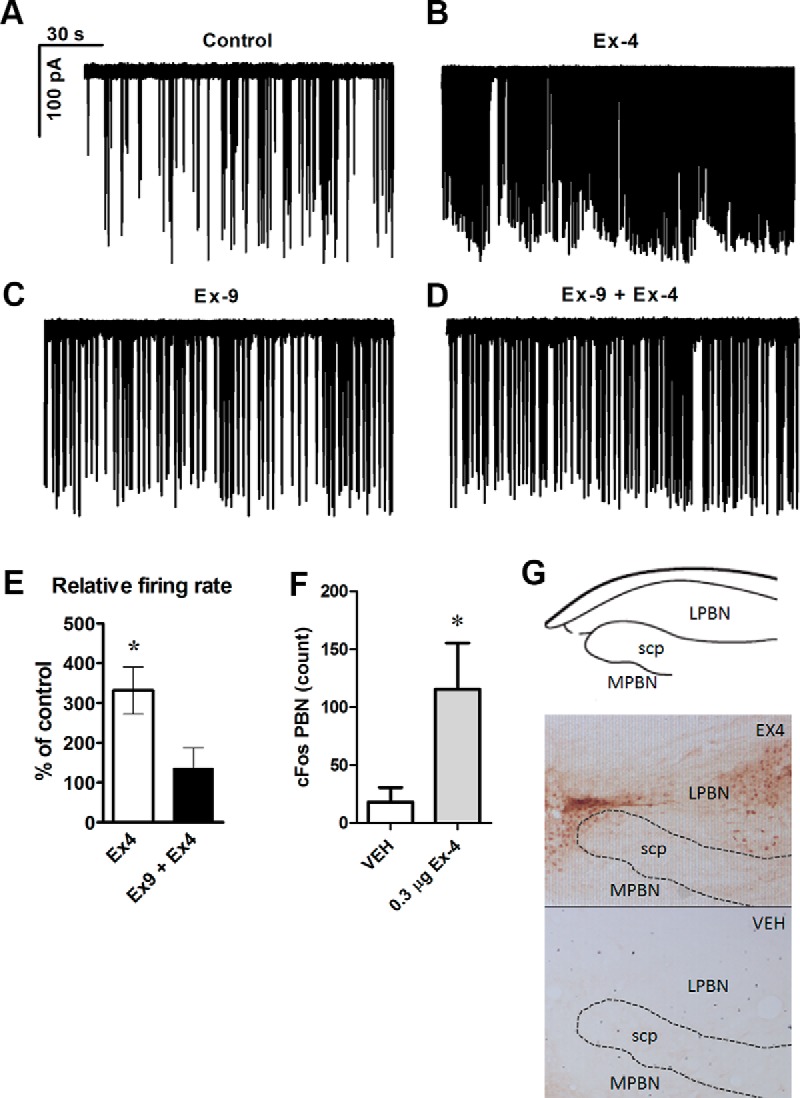
Loose-patch clamp recordings of action currents in the neurons of the external lPBN. Application of GLP-1R agonist Ex-4 (1μM) in the extracellular solution increased the firing rate (A and B). Extracellular administration of the GLP-1R antagonist Exendin-9(9-39) (Ex-9) (1μM), blocked this effect of Ex-4 (C and D). Histogram shows the relative percentages of firing rate after application of Ex-4 with and without Ex-9 (E). Central GLP-1R stimulation by lateral ventricle injection of Ex-4 increased c-Fos activation in the PBN. Quantified immunoreactivity of Fos-positive neurons in the PBN after Ex-4 treatment in ad libitum-fed rats (F) and representative images of the c-Fos study (G). Data are expressed as mean ± SEM. c-Fos data were expressed as average of total c-Fos count of all rats; total number per rat was calculated by adding total number of c-Fos-positive cells from the left and right lPBN. Each electrophysiological experimental group contained 10 recorded cells from 6–7 animals. *, P < .05. scp, superior cerebellar peduncles; VEH, vehicle.
c-Fos protein expression
To confirm the electrophysiology results from rat PBN slices in vivo, we determined whether a central injection of Ex-4 can activate PBN neurons. Central GLP-1R stimulation via lateral ventricle injection of Ex-4 increased the number of detected c-Fos-positive cells in the lPBN (Figure 4, F and G).
Gene expression
Central activation of GLP-1Rs resulted in a 62% increase in expression of mRNA encoding CGRP (Calca) (Figure 5A), an anorexic peptide that is expressed in the intra PBN- and amygdala-projecting PBN neurons, in ad libitum-fed rats. The expression of the gene encoding melanocortin receptor 4, the stimulation of which in lPBN was previously shown to result in anorexia, was not altered. Similarly, the expression of genes encoding receptors for N-methyl D-aspartate receptor subtype 2B (Grin2b) and GABA-A receptor δ (Gabrd), as well as the gene encoding glutamate decarboxylase (Gad1), remained unchanged after Ex-4 treatment in this experimental paradigm. We next determined whether Ex-4 treatment increased the expression of IL1β and IL6, 2 molecules that mediate a part of the anorexic effects of Ex-4 in the hypothalamus and the hindbrain (28), and showed that Ex-4 increased IL6, but not IL1β, gene expression in the PBN in both ad libitum-fed and food-deprived rats (Figure 5, C and D). The next average ΔCt values (±SEM) relative to β-actin were detected for ad libitum-fed rats: Calca (3.0 ± 0.3, 2.3 ± 0.1, P < .01), Gabrd (5.6 ± 0.2, 5.6 ± 0.2), Gad1 (5.2 ± 0.2, 5.3 ± 0.1), Grin2b (6.6 ± 0.2, 6.4 ± 0.1), Mc4r (9.0 ± 0.3, 8.7 ± 0.2), IL1 β (9.2 ± 0.2, 8.9 ± 0.7), and IL6 (11.9 ± 0.2, 9.4 ± 0.7) (P < .005); values are given for vehicle and Ex-4, respectively. The next average ΔCt values relative to β-actin were detected for overnight food-restricted rats: Calca (2.6 ± 0.2, 2.7 ± 0.1), Gabrd (5.9 ± 0.3, 5.2 ± 0.3), Gad1 (5.6 ± 0.3, 5.3 ± 0.2), Grin2b (6.3 ± 0.2, 6.4 ± 0.1), Mc4r (8.9 ± 0.2, 8.9 ± 0.1), IL1β (8.5 ± 0.1, 8.1 ± 0.4), and IL6 (11.3 ± 0.2, 9.1 ± 0.5) (P < .005); values are given for vehicle and Ex-4, respectively.
Figure 5.
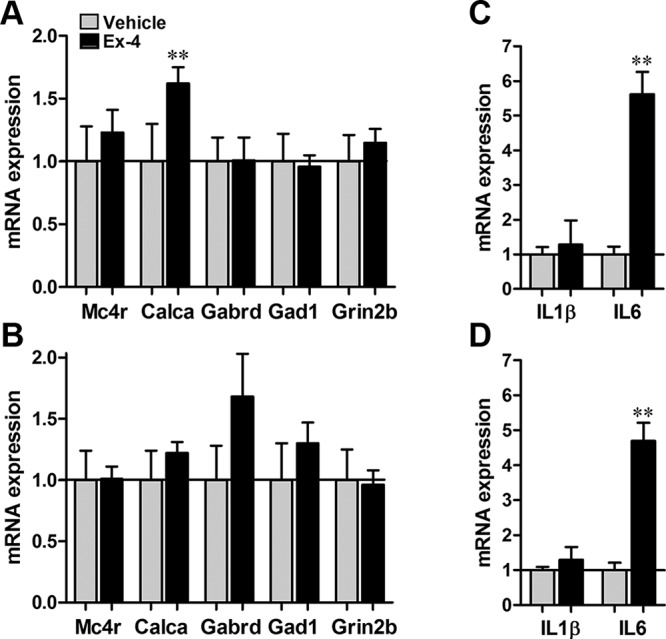
Gene expression after central GLP-1R stimulation. In ad libitum-fed rats, GLP-1R activation by Ex-4 increased the mRNA expression of the gene that encodes CGRP (Calca), without significantly changing the mRNA expression of other genes previously shown to be associated with changes in food intake in the PBN (A). In overnight-fasted rats, Ex-4 did not significantly change the mRNA expression of any of the genes measured (B). Ex-4 increased the expression of IL6 (but not IL1β), central mediators of GLP-1R-induced anorexia in both ad libitum-fed (C) and fasted (D) rats. Data are expressed as mean ± SEM. **, P < .01.
Innervation of lPBN CGRP-positive cells and effect of intra-lPBN CGRP injections on food intake and body weight
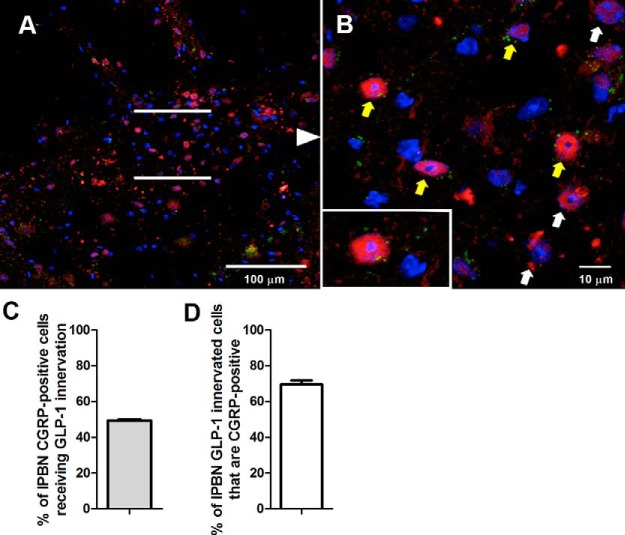
Guided by results indicating an elevation in CGRP in the PBN, we set out to determine whether the GLP-1 fibers provide direct innervation to the lPBN CGRP neurons. Our results indicate that most lPBN-projecting GLP-1 fibers innervate CGRP-positive cells, and nearly half of the CGRP-expressing cells in the lPBN receive GLP-1 innervation (Figure 6, A–D). Furthermore, we determined whether elevated CGRP is sufficient to alter food intake when applied directly and selectively to the lPBN. Intra-lPBN CGRP injections resulted in a significant short-term (1–2 h) food intake reduction (Figure 7A). Food intake and body weight at 16 hours after CGRP injections were not altered (Figure 7, B and C).
Figure 6.
GLP-1 innervation of CGRP neurons in the lPBN. Many YFP-immunoreactive axons (green) closely apposed the CGRP neurons (red) of the lPBN. Yellow arrows indicate CGRP-labeled lPBN cell bodies closely apposed by the GLP-1 fibers, whereas white arrows indicate CGRP-labeled cell bodies in this region that were not apposed by the GLP-1 fibers. B, Higher magnification of the lPBN region presented in A. Inset in B shows the interaction at a single cell level. Blue color represents DAPI the nuclear stain. Nearly half of the CGRP-positive cells in the lPBN receive GLP-1 innervation (C), and most cells in the lPBN that were innervated by GLP-1 fibers were CGRP-positive (D).
Figure 7.
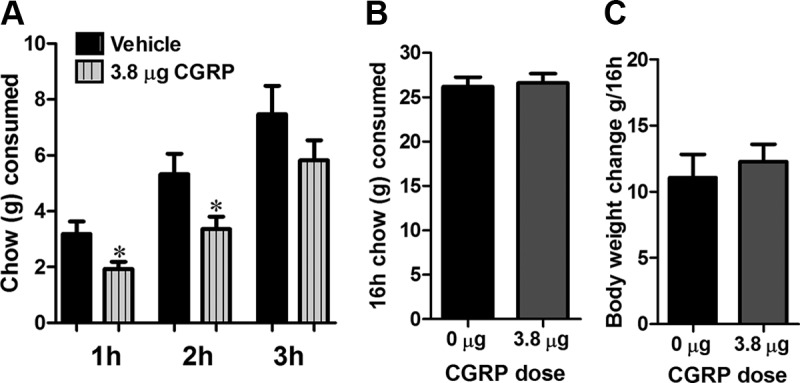
CGRP reduces food intake via a direct action in lPBN. Intra-lPBN delivery of CGRP reduced chow consumption up to 2 hours after injections (A). This effect was short lasting, because chow intake (B) and body weight (C) measured 16 hours after injections were not altered. Data are expressed as mean ± SEM. *, P < .05.
Discussion
Viscerosensory stimuli result in neuronal activation of the PBN, but the origin and neurochemical identity of the excitatory neuronal input to the PBN remain largely unexplored. Here, we provide data implicating NTS-originating GLP-1-producing neurons as one source of excitatory projections to the PBN. Moreover, we demonstrate a functional role for parabrachial GLP-1R activation in food intake control. Several lines of evidence support this conclusion. Direct activation of lPBN GLP-1R inhibited food intake and body weight gain, and conversely lPBN GLP-1R blockade increased food intake and body weight gain in rats. These data indicate that lPBN GLP-1R are necessary and sufficient for food intake control. Stimulation of PBN GLP-1R potently activated PBN neurons, the activation of which has previously been linked to anorexia. The activation of lPBN neurons via GLP-1R is underscored both by an activational effect shown via electrophysiology in rat brain slices, and as increased activity of PBN neurons, reflected by a significant increase in the number of c-Fos-positive cells in the lPBN after central Ex-4 injection. Moreover, our neuroanatomical data implicate solitary tract GLP-1 neurons as the source of endogenous agonist for the GLP-1R in the lPBN. Using a unique and well-validated Venus PPG reporter mouse (3, 20, 29, 30), we show GLP-1-containing fibers in the lPBN that are very likely to originate from the NTS, the only major source of GLP-1-producing neurons in the brain. We also identify the downstream neurochemical mechanism of the anorexic effect of GLP-1 in the lPBN. We show that in lPBN, GLP-1R activation increased the expression of CGRP, and most NTS-originating GLP-1 fibers innervated lPBN CGRP-producing neurons. Moreover, increased CGRP signaling in the lPBN induced anorexia and body weight reduction.
Stimulation of GLP-1R in the PBN increased the activity of neurons in this area, as implied by our electrophysiology and c-Fos data. These data fit remarkably well with previous studies showing that activation of neurons in the PBN, by removal of the hypothalamic inhibitory GABAergic projections to the PBN or by optogenetic activation of lPBN CGRP-expressing neurons, resulted in hypophagia in mice (17, 18). Here, we propose that activation of lPBN neurons by GLP-1 may be one of the excitatory mechanisms that are uncovered when the hypothalamic brake on the PBN neurons is removed. It is likely, however, that GLP-1 transmission is not the only excitatory input to the PBN. Glutamatergic input may also be involved, because knockdown of N-methyl-D-aspartate glutamate receptors in the PBN can prevent the hypophagia resulting from GABAergic signal removal (16). Moreover, GLP-1-producing neurons may also produce a fast neurotransmitter, which could be glutamate. Even though Ex-4, a potent and selective agonist for GLP-1R, was used in the current study our results are likely relevant for the GLP-1 peptide. In fact, one previous report already indicated that central injections of the native peptide, GLP-1, at the start of the dark cycle in rats also induces c-Fos in the PBN (31).
Until recently, detection of the distribution of axonal fibers of the GLP-1 neurons was hindered by the need to use antibodies with neuronal tracers. This process was facilitated by the creation of a transgenic mouse that expresses a fluorescent signal, YFP, in PPG-expressing cells; this mouse model has been used to identify GLP-1-producing neurons in the brain (3, 20, 29, 30). The transgenic YFP-PPG mice offer an advantage over previous methods in the form of the strong YFP expression that allows for clear visualization of the GLP-1-producing neuron axon fibers and terminals. We detected dense YFP-positive axons at several levels of the PBN. GLP-1 innervation was detected throughout the rostro-caudal extent of the PBN, with denser innervation detected in the rostral region. YFP-positive fibers were also found in the dorso-lateral PBN, the external-lateral PBN, and the mPBN. Thus, we show neuroanatomical grounds for NTS GLP-1 neuron communication to all levels of the PBN. This potentially allows GLP-1 to influence a wide range of physiological responses controlled by different nuclei of the PBN. One potential downside of using the YFP mice in the current study is that there may be a species difference in the projection targets of the GLP-1-producing neurons. For example, leptin control of GLP-1 neurons has been shown to differ between mice and rats (32). In mice, leptin receptors are located directly on the GLP-1-producing neurons in the NTS, whereas in rats, leptin may only be influencing GLP-1 neuron activity indirectly (32). Nonetheless, some literature already exists to support a direct, monosynaptic, connection between the caudal NTS and the PBN in a rat. Indeed, it seems that PBN, especially the lateral subdivisions, receives very dense innervation from the NTS also in the rat (2). Innervation from the NTS was shown to overlap with GLP-1-positive terminals, and a retrograde tracer injected into the lPBN was indicated to colocalize with NTS GLP-1-producing neurons in a rat (2, 33). Combined with other data showing expression of GLP-1R in the rat PBN (10) and the strong behavioral effect of GLP-1R activation in this species, direct projection to the PBN from the NTS seems rather likely in the rat.
GLP-1 activation in the lPBN suppressed chow intake, intake of palatable chocolate pellets, and also intake of noncaloric saccharine solution. This indicates that GLP-1R signaling in the lPBN can reduce food intake across the palatability and caloric density spectrum. Importantly PBN Ex-4 injections did not reduce water intake. Collectively, these data indicate that PBN GLP-1 signaling may interact with the caloric density, taste, and hedonic properties of food. The PBN is a crucial relay for the hedonic value of food; lesion of the PBN blunts nucleus accumbens dopamine elevation in response to palatable food (34). Moreover, one recent study indicates that lPBN-directed Ex-4 injections reduce high-fat food intake and suppress the motivation to work for a high-fat reward in rats (33). Both taste and caloric value, processed in the PBN, may contribute to the PBN-relayed dopamine response. Activation of PBN neurons, akin to that observed here with Ex-4, can reduce the hedonic properties of food and inhibition of PBN neurons by microinjections of GABA-A receptor agonists into the PBN increases hedonic responses to oral sucrose (35).
The PBN is a heterogeneous nucleus with at least 12 distinct subnuclei and subdivisions of the PBN can be clearly differentiated based on their neuronal inputs and outputs (36). The gustatory afferents are represented in the medial subdivision, and the viscerosensory, the cardiovascular, and the respiratory functions in the lateral subdivision. In the current study, the decision to target the lPBN was based on the idea that caudally located GLP-1 neurons are likely to project to the viscerosensory lPBN rather than the mPBN, because inputs from the caudal viscerosensory NTS are segregated from the gustatory inputs from the rostral NTS to the mPBN. However, the segregation of inputs does not prevent some cross-communication, because even the gustatory PBN displays sensitivity to the metabolic status, and the lPBN, especially the dorsal part, is activated by noncaloric gustatory stimuli like saccharine (37). This may be the reason why both caloric food (chocolate and chow) and noncaloric saccharine consumption were reduced in the current study. Our study shows that activation of the GLP-1R can suppress intake of a sweet noncaloric solution.
The PBN plays a critical role in relaying visceral signals to the forebrain (38). The parabrachial-associated effect of a gut/brain peptide to reduce food intake, demonstrated here, fits well with previous studies showing that lPBN lesion impairs cholecystikinin (CCK)- and amylin-induced food intake suppression and attenuates the c-Fos activation normally expected from CCK and amylin action in the central nucleus of the amygdala (39, 40). Here, we show a direct effect of GLP-1R in the PBN. This is different from the previous studies, performed with peripheral injections of CCK and amylin, which could not determine whether these peptides exert direct or indirect feeding effects at the lPBN.
Signaling at the central GLP-1R is necessary for hypophagia induced by satiety and metabolic signals, like CCK and leptin, that are recruited in health, but it is also a key mediator of hypophagia induced by aversive stimuli, like lithium chloride and lipopolysaccharide (41–43). Interestingly, the same sickness-associated hypophagic stimuli can activate PBN neurons, and this activation may be a necessary component of the feeding suppression they cause (44–46). These 2 components are tied together by data indicating that hindbrain selective blockade of GLP-1R prevents lipopolysaccharide-induced hypophagia (47). Thus, it is possible that the GLP-1 neuron projections to the PBN and the hypophagia resulting from lPBN GLP-1R activation are relevant relays for sickness-induced hypophagia and not only for homeostatic appetite during health, as discussed above.
PBN neurons project to the hypothalamus, limbic system, and other forebrain regions. In order to begin to understand the potential downstream circuitry activated by GLP-1R stimulation in the PBN, we determined gene expression levels for candidate genes recently shown to be key for appetite suppression in the PBN. We found that Calca, the gene that encodes CGRP, an anorexic peptide, was elevated by Ex-4 treatment. CGRP neurons, found exclusively in the lPBN, play a key role in appetite suppression (18, 48, 49). Optogenetic stimulation of these neurons suppresses food intake in fed and food-deprived rats. c-Fos studies indicate that these neurons are activated by satiety signals, like amylin and CCK, and also by illness inducing signals like lithium chloride and lipopolysaccharide. Here, we show that GLP-1R activation can also stimulate CGRP gene expression. Our neuroanatomical and immunohistochemical data suggest that this effect could be exerted by direct inputs from GLP-1 releasing fibers onto the CGRP-producing cells, because we found that most NTS-originating GLP-1-producing fibers innervated CGRP neurons in the lPBN. The elevation of CGRP levels could contribute to the anorexic and weight-suppressing effect of GLP-1, because direct intra-lPBN injections of CGRP reduced both food intake and body weight gain. It is noteworthy that GLP-1R stimulation increased CGRP only in ad libitum-fed rats, a more physiological situation for endogenous GLP-1 release. The lack of effect of GLP-1R activation on CGRP in fasted rats may indicate that orexigenic signals abundant during fasting may inhibit GLP-1's ability to induce CGRP, thereby reducing its ability to suppress food intake. This result is in line with a previous report showing a nearly complete suppression of GLP-1 neuron activation by food deprivation (50). Taken together, the present and previous studies suggest that fasting can suppress both production/release of GLP-1 and GLP-1-stimulated downstream anorexic pathways.
IL-1 and IL-6 are key regulators of the inflammatory response (51, 52), but they may also play an important role in healthy animals to regulate metabolic function. Mice lacking IL-1R or IL-6 develop late-onset obesity as well as disturbed glucose metabolism (53–56). Recently, we identified IL-1β and IL-6 as key mediators of the appetite-suppressive effects of GLP-1 (28). The results of the current study complement the previous data by showing that GLP-1R activation can increase IL6 gene expression not only in the hypothalamus and caudal brainstem but also in the PBN. The elevation of IL-6 in the PBN was detected irrespective of the feeding state of the animal, indicating that the relationship between Ex-4 and IL-6 is robust. Previous data link CGRP and IL-6 in the pituitary (57). Although our data do not directly examine this connection, CGRP may not be necessary for the Ex-4 induction of IL-6, because IL-6 levels were elevated in fasted rats, whereas CGRP remained unchanged. IL-1β mRNA was not altered in the PBN, in line with previous data showing that GLP-1 can induce IL-1β in the hypothalamus but not the caudal brainstem (28).
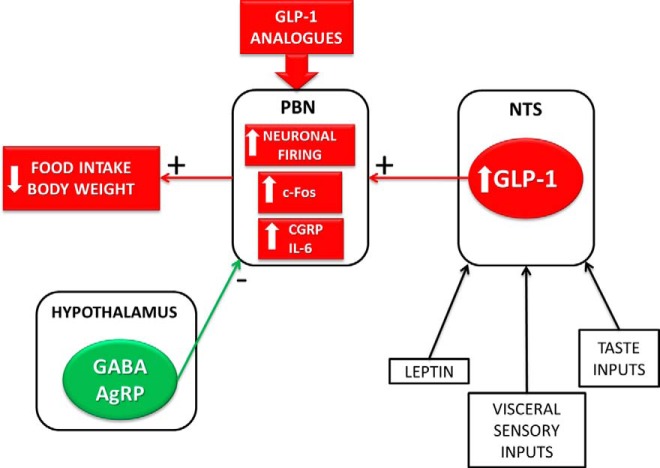
Collectively, this study reveals lPBN as a neural substrate for the feeding suppression effect of GLP-1 and identifies the mechanisms involved (Figure 8). This mechanism of action may be relevant to patients receiving Ex-4, or other GLP-1 analogues, given that these pharmaceuticals can cross the blood-brain barrier after peripheral application, and c-Fos results indicate that peripheral Ex-4 injections in rodents can activate the PBN (58).
Figure 8.
Graphical summary of results. Collectively, our data reveal the lPBN as a neural substrate for the feeding and body weight suppression effect of GLP-1 and identify the mechanisms involved. Elements of this novel energy balance relevant circuit identified in the current study are indicated in red. In contrast to the excitatory GLP-1 projections to the PBN, the projections from the hypothalamus (green) to the PBN provide inhibitory inputs, and their activation results in an orexigenic response.
Acknowledgments
We thank the Centre for Cellular Imaging at the University of Gothenburg for the use of imaging equipment as well as the technical support received from Julia Fernandez-Rodriguez, Maria Smedh, Carolina Tängemo, and Marjorie Nicholson.
This work was supported by a Novo Nordisk Foundation Excellence project grant (K.P.S.); Swedish Research Council Grants 2011-3054 (K.P.S.), K2007/54X/09894/16/3 (J.-O.J.), and 2012-1758 (to S.L.D.); Läkarutbildningsavtalet Göteborg grant at Sahlgrenska Hospital SU7601 (to J.-O.J.) and ALFGBG-138741 (to S.L.D.); European Union Seventh Framework Programme FP7-KBBE-2010-4-266408 (to J.-O.J., S.L.D., and Z.L.) and FP7-KBBE-2013-607310 (to S.L.D.) under Grant Agreement 266408; Fru Mary von Sydow's Foundation; and Harald Jeanssons Stiftelse with Harald and Greta Jeanssons Stftelse (K.P.S.). This work was also supported by grants from the Hungarian Scientific Research Fund (OTKA K100722), the National Development Agency of Hungary (NFUBONUS-HU08/2-2011-0006), and the European Community's Seventh Framework Programme (FP7/2007-2013, number 245009). F.M.G. and F.R. were funded by the Wellcome Trust (WT088357/Z/09/Z and WT084210/Z/07/Z).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- CCK
- cholecystikinin
- CGRP
- calcitonin gene-related peptide
- DAPI
- diamidino-2-phenylindole
- GABA
- γ-aminobutyric acid
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- GLP-1 receptor
- lPBN
- lateral PBN
- mPBN
- medial PBN
- naCSF
- normal aCSF
- NTS
- nucleus of the solitary tract
- PB
- phosphate buffer
- PBN
- parabrachial nucleus
- PPG
- preproglucagon
- TNT-buffer
- TRIS NaCl Tween 20-buffer
- YFP
- yellow fluorescent protein.
References
- 1. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. [DOI] [PubMed] [Google Scholar]
- 2. Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turton MD, O'Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. [DOI] [PubMed] [Google Scholar]
- 5. Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1427–R1435. [DOI] [PubMed] [Google Scholar]
- 6. Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. [DOI] [PubMed] [Google Scholar]
- 11. Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150(12):5351–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28(39):9702–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1055–R1065. [DOI] [PubMed] [Google Scholar]
- 14. Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190(2):259–282. [DOI] [PubMed] [Google Scholar]
- 15. Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA. 2013;110(36):14765–14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173(4002):1136–1139. [DOI] [PubMed] [Google Scholar]
- 20. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell metabolism. 2008;8(6):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skibicka KP, Alhadeff AL, Leichner TM, Grill HJ. Neural controls of prostaglandin 2 pyrogenic, tachycardic, and anorexic actions are anatomically distributed. Endocrinology. 2011;152(6):2400–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farkas I, Vastagh C, Sarvari M, Liposits Z. Ghrelin decreases firing activity of gonadotropin-releasing hormone (GnRH) neurons in an estrous cycle and endocannabinoid signaling dependent manner. PLoS One. 2013;8(10):e78178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farkas I, Kalló I, Deli L, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(12):5818–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbert H, Bellintani-Guardia B. Morphology and dendritic domains of neurons in the lateral parabrachial nucleus of the rat. J Comp Neurol. 1995;354(3):377–394. [DOI] [PubMed] [Google Scholar]
- 25. Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24(37):8141–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tung YC, Hewson AK, Carter RN, Dickson SL. Central responsiveness to a ghrelin mimetic (GHRP-6) is rapidly altered by acute changes in nutritional status in rats. J Neuroendocrinol. 2005;17(6):387–393. [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 28. Shirazi R, Palsdottir V, Collander J, et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci USA. 2013;110(40):16199–16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes. 2010;59(8):1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2013;229:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Dijk G, Thiele TE, Seeley RJ, Woods SC, Bernstein IL. Glucagon-like peptide-1 and satiety. Nature. 1997;385(6613):214. [DOI] [PubMed] [Google Scholar]
- 32. Huo L, Gamber KM, Grill HJ, Bjørbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology. 2008;149(2):492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39(9):2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84(3):363–369. [DOI] [PubMed] [Google Scholar]
- 35. Söderpalm AH, Berridge KC. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000;877(2):288–297. [DOI] [PubMed] [Google Scholar]
- 36. Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197(2):291–317. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto T, Sawa K. Comparison of c-fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res. 2000;866(1–2):144–151. [DOI] [PubMed] [Google Scholar]
- 38. Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46(1):17–23. [PubMed] [Google Scholar]
- 39. Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res. 2007;1162:76–84. [DOI] [PubMed] [Google Scholar]
- 40. Trifunovic R, Reilly S. Medial versus lateral parabrachial nucleus lesions in the rat: effects on cholecystokinin- and D-fenfluramine-induced anorexia. Brain Res. 2001;894(2):288–296. [DOI] [PubMed] [Google Scholar]
- 41. Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277(2 pt 2):R582–R590. [DOI] [PubMed] [Google Scholar]
- 42. Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999;277(5 pt 2):R1537–R1540. [DOI] [PubMed] [Google Scholar]
- 43. Seeley RJ, Blake K, Rushing PA, et al. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20(4):1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sclafani A, Azzara AV, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci. 2001;115(4):920–933. [DOI] [PubMed] [Google Scholar]
- 45. Engblom D, Ek M, Ericsson-Dahlstrand A, Blomqvist A. Activation of prostanoid EP(3) and EP(4) receptor mRNA-expressing neurons in the rat parabrachial nucleus by intravenous injection of bacterial wall lipopolysaccharide. J Comp Neurol. 2001;440(4):378–386. [DOI] [PubMed] [Google Scholar]
- 46. Sagar SM, Price KJ, Kasting NW, Sharp FR. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res Bull. 1995;36(4):381–392. [DOI] [PubMed] [Google Scholar]
- 47. Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1190–R1193. [DOI] [PubMed] [Google Scholar]
- 48. Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Evidence for a physiological role of central calcitonin gene-related peptide (CGRP) receptors in the control of food intake in rats. Neurosci Lett. 1997;230(3):159–162. [DOI] [PubMed] [Google Scholar]
- 49. Paues J, Engblom D, Mackerlova L, Ericsson-Dahlstrand A, Blomqvist A. Feeding-related immune responsive brain stem neurons: association with CGRP. Neuroreport. 2001;12(11):2399–2403. [DOI] [PubMed] [Google Scholar]
- 50. Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013;121:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. [DOI] [PubMed] [Google Scholar]
- 52. Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. [DOI] [PubMed] [Google Scholar]
- 53. Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75–79. [DOI] [PubMed] [Google Scholar]
- 54. McGillicuddy FC, Harford KA, Reynolds CM, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60(6):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. García MC, Wernstedt I, Berndtsson A, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55(5):1205–1213. [DOI] [PubMed] [Google Scholar]
- 57. Tatsuno I, Somogyvari-Vigh A, Mizuno K, Gottschall PE, Hidaka H, Arimura A. Neuropeptide regulation of interleukin-6 production from the pituitary: stimulation by pituitary adenylate cyclase activating polypeptide and calcitonin gene-related peptide. Endocrinology. 1991;129(4):1797–1804. [DOI] [PubMed] [Google Scholar]
- 58. Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-López G. Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin-4. J Neuroendocrinol. 2012;24(12):1505–1516. [DOI] [PubMed] [Google Scholar]