Abstract
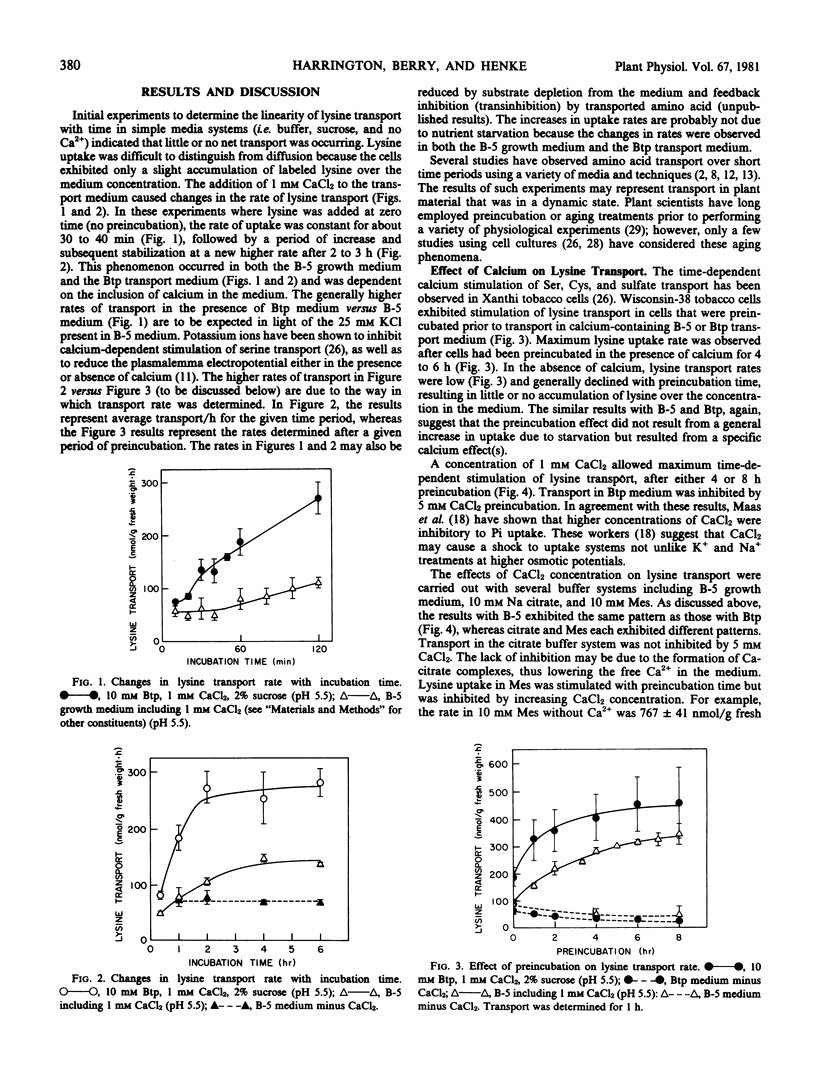
The effects of calcium ions on lysine transport into cultured Wisconsin-38 tobacco cells were examined. In the presence of calcium, lysine was transported at a relatively low rate for 30 to 40 minutes followed by a period of increasing rates and subsequent stabilization at a higher rate after 2 to 3 hours. In the absence of calcium, transport was uniformly low.
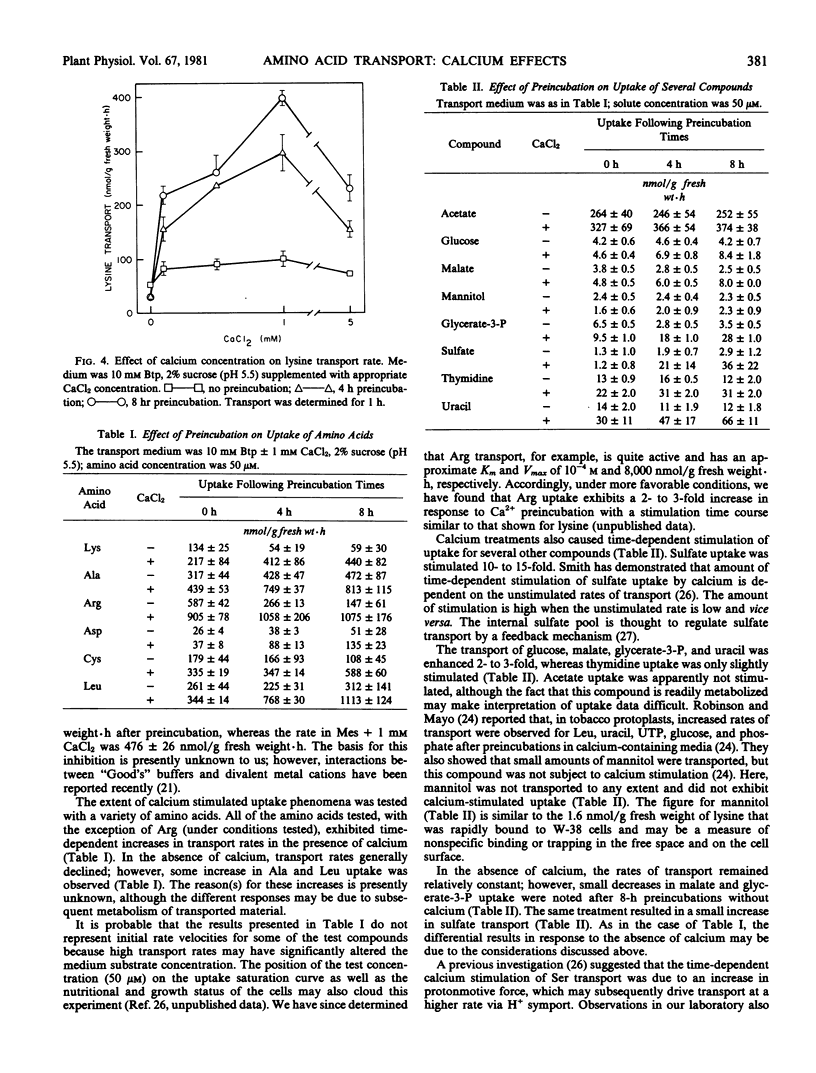
Time-dependent stimulation of lysine transport rate was observed after the cells had been preincubated in calcium-containing media. Similar treatments also resulted in the stimulated uptake of a variety of other amino acids, organic compounds, and sulfate. The stimulation of lysine uptake was apparently not due to nutrient starvation.
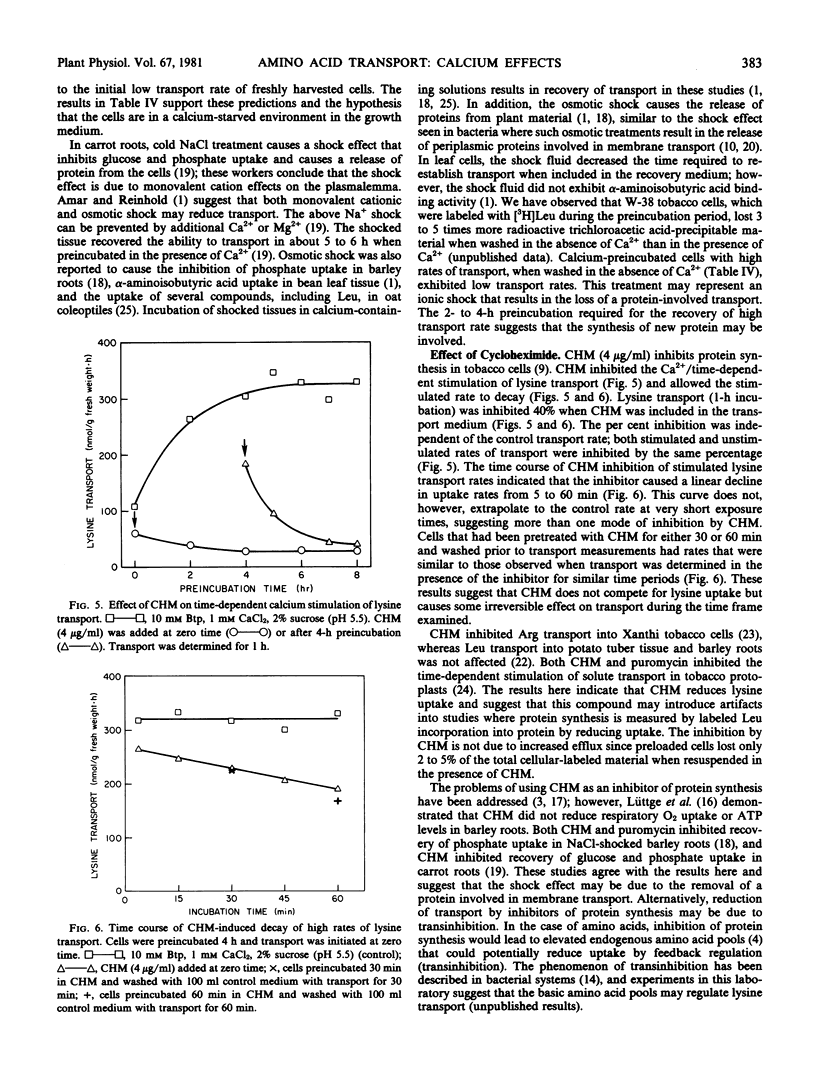
Lysine transport was not stimulated in a time-dependent fashion by K+, La3+, Mg2+, or Mn2+. Cells with stimulated transport rates continued to exhibit high rates when washed with calcium-containing media followed by transport in calcium-containing media. All other cation wash treatments were inhibitory, although magnesium treatments resulted in partial preservation of stimulated transport rates. Cycloheximide inhibited the calcium/time-dependent stimulation of lysine transport and caused the stimulated rate to decay.
The initial experimental treatments or the culture conditions may represent some form of shock that alters the membrane transport mechanism, thus reducing transport. The observed calcium/time-dependent stimulation may require protein synthesis and represents damage repair.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. S., Beevers H. Influence of cycloheximide on the synthesis and utilization of amino acids in suspension cultures. Plant Physiol. 1971 Sep;48(3):261–264. doi: 10.1104/pp.48.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Henke R. R. Amino Acid Transport into Cultured Tobacco Cells: I. LYSINE TRANSPORT. Plant Physiol. 1981 Feb;67(2):373–378. doi: 10.1104/pp.67.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine transport into cultured tobacco cells. Plant Physiol. 1977 Dec;60(6):807–811. doi: 10.1104/pp.60.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich W., Ring K. Regulation of amino acid transport in growing cells of Streptomyces hydrogenans. I. Modulation of transport capacity and amino acid pool composition during the growth cycle. Arch Microbiol. 1976 Sep 1;109(3):227–235. doi: 10.1007/BF00446633. [DOI] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. HCO(3) Influx Across the Plasmalemma of Chara corallina: Divalent Cation Requirement. Plant Physiol. 1977 Dec;60(6):862–867. doi: 10.1104/pp.60.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Läuchli A., Ball E., Pitman M. G. Cycloheximide: a specific inhibitor of protein synthesis and intercellular ion transport in plant roots. Experientia. 1974 May 15;30(5):470–471. doi: 10.1007/BF01926298. [DOI] [PubMed] [Google Scholar]
- Maas E. V., Ogata G., Finkel M. H. Salt-induced Inhibition of Phosphate Transport and Release of Membrane Proteins from Barley Roots. Plant Physiol. 1979 Jul;64(1):139–143. doi: 10.1104/pp.64.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman R. H., Willis C. Correlation between the Suppression of Glucose and Phosphate Uptake and the Release of Protein from Viable Carrot Root Cells Treated with Monovalent Cations. Plant Physiol. 1971 Sep;48(3):287–293. doi: 10.1104/pp.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Pope J. M., Stevens P. R., Angotti M. T., Nakon R. Free metal ion depletion by "Good's" buffers. II. N-(2-acetamido)-2-aminoethanesulfonic acid (ACESH): complexes with calcium(II), magnesium(II), manganese(II), cobalt(II), zinc(II), nickel(II), and copper(II). Anal Biochem. 1980 Apr;103(2):214–221. doi: 10.1016/0003-2697(80)90258-4. [DOI] [PubMed] [Google Scholar]
- Renosto F., Ferrari G. Mechanism of sulfate transport inhibition by cycloheximide in plant tissues. Plant Physiol. 1975 Oct;56(4):478–480. doi: 10.1104/pp.56.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977 Mar 25;252(6):1858–1864. [PubMed] [Google Scholar]
- Rubinstein B., Mahar P. Effects of Osmotic Shock on Some Membrane-regulated Events of Oat Coleoptile Cells. Plant Physiol. 1977 Mar;59(3):365–368. doi: 10.1104/pp.59.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Role of calcium in serine transport into tobacco cells. Plant Physiol. 1978 Dec;62(6):941–948. doi: 10.1104/pp.62.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Sulfate transport in cultured tobacco cells. Plant Physiol. 1975 Feb;55(2):303–307. doi: 10.1104/pp.55.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


