Key Points
PI3K p110δ/γ inhibitor IPI-145 abrogates prosurvival signals and induces apoptosis in CLL cells.
IPI-145 overcomes BTK C481S mutation conferring ibrutinib resistance.
Abstract
Chronic lymphocytic leukemia (CLL) displays constitutive phosphatidylinositol 3-kinase (PI3K) activation resulting from aberrant regulation of B-cell receptor (BCR) signaling. Previous studies have shown that an oral PI3K p110δ inhibitor idelalisib exhibits promising activity in CLL. Here, we demonstrate that a dual PI3K p110δ and p110γ inhibitor, IPI-145, antagonizes BCR crosslinking activated prosurvival signals in primary CLL cells. IPI-145 causes direct killing in primary CLL cells in a dose- and time-dependent fashion, but does not generate direct cytotoxicity to normal B cells. However, IPI-145 does reduce the viability of normal T and natural killer cells and decrease activated T-cell production of various inflammatory and antiapoptotic cytokines. Furthermore, IPI-145 overcomes the ibrutinib resistance resulting from treatment-induced BTK C481S mutation. Collectively, these studies provide rationale for ongoing clinical evaluation of IPI-145 as a targeted therapy for CLL and related B-cell lymphoproliferative disorders.
Introduction
Chronic lymphocytic leukemia (CLL) cells display upregulated B-cell receptor (BCR) activation,1,2 which is integral for maintaining B-cell survival and proliferation through transmitting microenvironmental stimuli.1 Because of aberrant regulation of the BCR, CLL cells exhibit constitutively activated protein kinases, such as phosphoinositide-3 kinase (PI3K) and Bruton’s tyrosine kinase (BTK).3,4 Small molecules that target such kinases, such as idelalisib5 and ibrutinib,6 have shown promising activity in CLL patients. PI3Ks are enzymes that integrate extracellular stimuli from membrane receptors by generating phosphatidylinositol 3,4,5-trisphosphate which serves as a critical plasma membrane docking site for pleckstrin-homology domain containing proteins, including AKT and BTK. Class I PI3K has 4 catalytic isoforms: p110α, p110β, p110δ, and p110γ. Although p110α and p110β are ubiquitously expressed, p110δ and p110γ are enriched in the hematopoietic system. Previously, we demonstrated that selective inhibition of p110δ with idelalisib antagonizes prosurvival signals of CLL cells.3 IPI-145 is an oral PI3K p110δ and p110γ inhibitor whose structure and activity in inflammatory disease models have previously been described.7,8 It is well-tolerated and active in relapsed/refractory CLL patients and is currently being evaluated in the phase 3 setting as monotherapy for CLL (NCT02004522). Ibrutinib is an irreversible inhibitor of BTK that binds covalently to the cysteine residue (C481) in the kinase domain. It has been shown to be highly effective in CLL in various preclinical and clinical studies.4,6 However, a small proportion of patients develop resistance.6 Our group has recently reported that resistant patients harbor a C481S mutation on the BTK gene that allows ibrutinib to reversibly bind to BTK.9 It is of interest to explore whether IPI-145 can inhibit prosurvival signaling through AKT in the setting of this C481S mutation. To better understand the mechanism of IPI-145 and whether it can overcome C481S ibrutinib resistance, we evaluated the activity of IPI-145 in various preclinical and ibrutinib-resistance models.
Methods
Cell culture and treatment reagents
Blood was obtained from patients with CLL under the approval of the Institutional Review Board at The Ohio State University3 with informed consent in accordance with the Declaration of Helsinki. B, T, or natural killer (NK) were selected and maintained as previously described.3 The XLA cell line was obtained from the Coriell Institute. IPI-145 was supplied by Infinity Pharmaceuticals.
Immunoblot analysis
Immunoblots were performed as previously described.3 Antibodies included: anti-AKT, anti-phospho-AKT (ser473), anti-phospho-AKT (thr308), anti-ERK1/2, anti-phospho-ERK1/2 (thr202/tyr204), and anti-BTK from Cell Signaling Technologies; anti-phospho-BTK (tyr223) from Abcam; and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Millipore.
Flow cytometry
Cell viability was also measured using annexin-V/propidium iodide flow cytometry (Beckman-Coulter) as previously described.3
Cytokine analysis
Th1/Th2/Th17 cytokines were measured using BD Cytometric Bead Array (BD Biosciences) according to the manufacturer’s published protocol.
Retroviral vectors and generations of BTK cell lines
The inducible Tet-on transactivator (Clontech) was stably introduced into the XLA cell line as previously described.10 The retroviral construct pRetroX-Tight-Puro was used to stably transfect XLA cells with BTK. A mutation was made using QuikChange site directed mutagenesis (Stratagene) in the kinase domain at cysteine 481 to serine (see the primer sequence in supplemental Methods on the Blood Web site). Confirmation of the DNA sequence, production of the viral particles, and infection of the XLA cell line were performed as previously described.11 Cells were selected with puromycin and G418. BTK expression was induced with doxycycline for 48 hours.
Results and discussion
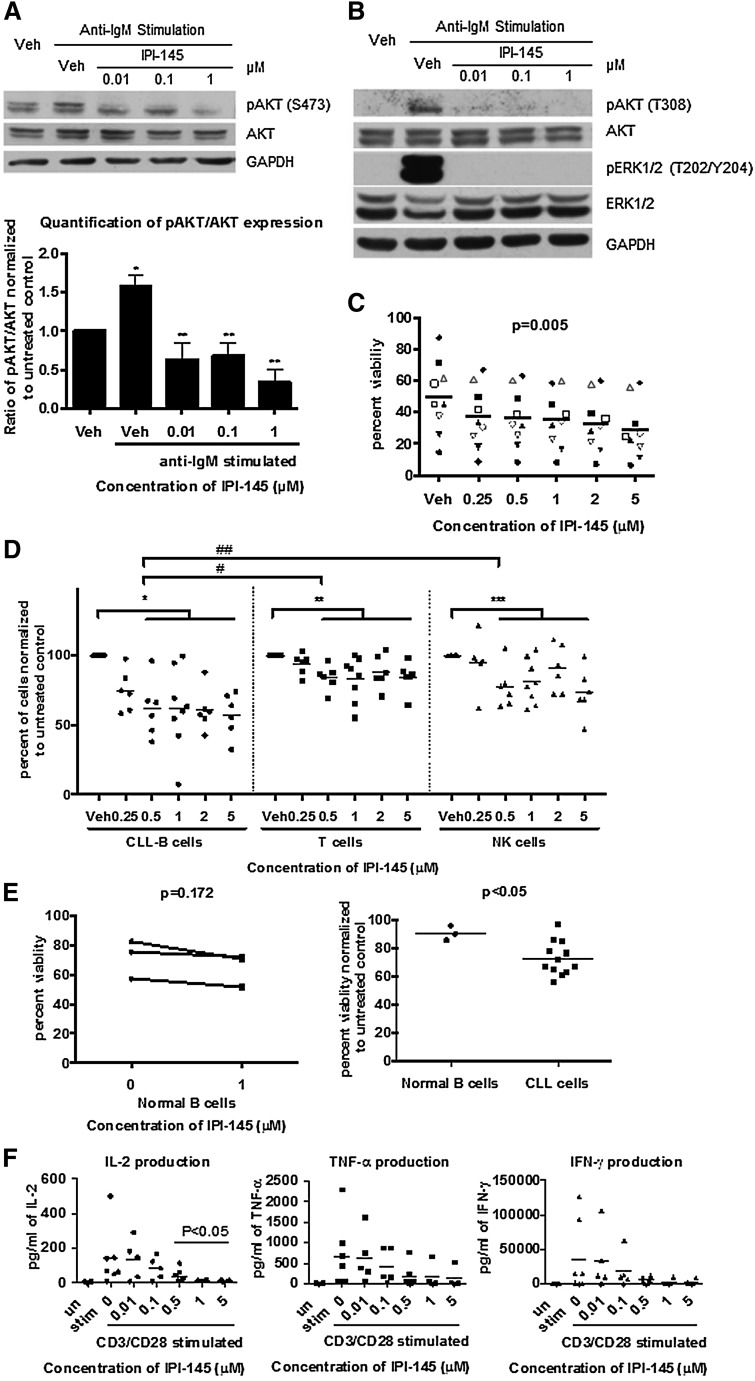
To determine whether IPI-145 antagonizes BCR-crosslinking stimulation, we activated primary CLL cells using plate-bound anti-immunoglobulin M (IgM) and treated with IPI-145. Anti-IgM crosslinking resulted in the activation of pAKTS473 in 4 of 6 patients samples tested. pAKTS473 was significantly inhibited by IPI-145 at a concentration as low as 0.01 μM in 4 of 4 patients (Figure 1A). Moreover, IPI-145 completely abrogated the anti-IgM activated pAKTT308 and downstream pERKT202/Y204 signaling (Figure 1B). These data suggest that IPI-145 is effective at blocking PI3K activity and overcoming BCR-dependent microenvironmental protection.
Figure 1.
IPI-145 inhibits constitutive PI3K signaling, induces selective cytotoxicity in CLL cells, and does not cause direct cytotoxicity toward other normal immune cells but alters cytokine production. (A) B cells were enriched from peripheral blood of patients with CLL and stimulated with plate-bound anti-IgM (n = 4 of 6) and incubated with or without IPI-145 (0.01 to 1 μM) for 1 hour. Cell lysates were immunoblotted for pAKTS473, total AKT, and GAPDH. (Upper) Representative blots from 4 independent experiments. (Lower) Quantification results. All data were normalized to unstimulated control. Stimulated samples from 4 patients were included for statistical analysis. CLL cells were significantly stimulated compared with untreated control *P < .005. After anti-IgM stimulation, pAKTS473/AKT were significantly inhibited by IPI-145 compared with vehicle (veh)-treated control **P < .05. Paired t tests were used. (B) B cells from patients with CLL (n = 4-6) were stimulated with plate-bound anti-IgM and incubated with or without IPI-145 (0.01 to 1 μM) for 1 hour. Cell lysates were immunoblotted for pAKTT308, total AKT, pERK1/2T202/Y204, total ERK1/2, and GAPDH. The representative blots are from 4 (pAKTT308, total AKT) and 6 (pERK1/2T202/Y204, total ERK1/2) independent experiments. (C) B cells from patients with CLL (n = 12) were incubated with or without IPI-145 (0.25 to 5 μM) for 24 to 72 hours. Viability was determined by annexin/PI flow cytometry. A dose-dependent graph from the 48-hour time point is shown on the left (P = .005 for dose-dependent). Each icon represents individual patients. Horizontal bars represent averages. IPI-145 causes significant linear and quadratic cytotoxicity over both time and dose. P < .01 for statistical analysis for linear trend for time. (D) Whole blood from CLL patients (n = 7) was incubated with IPI-145 (0.25 to 5 μM) for 48 hours. Absolute count of live CD19+ B cells, CD3+ T cells, and CD56+ NK cells were measured by flow cytometry. Different icons represent different cell types. For all cell types, there was no statistically significant killing at 0.25 μM. Average viability of CD19+ B cells, CD3+ T cells, and CD56+ NK cells with 0.5 to 5 μM IPI-145 is significant lower than untreated. *, **, ***P < .001. CD19+ cytotoxicity to CD19+ B cells is significantly more than CD3+ T cells (#P < .001) or CD56+ NK cells (##P = .001) at 0.5 to 5 μM. (E) CD19+ B cells (n = 3) from healthy volunteers were incubated with or without IPI-145 (1 μM) for 48 hours. Viability was determined by annexin/PI flow cytometry and was normalized to time-matched untreated controls. Horizontal bars represent averages. IPI-145 caused significantly more cytotoxicity to CLL cells vs normal B cells *P < .05. (F) CD3+ T cells (n = 4-7) from healthy volunteers were stimulated with plate-bound anti-CD3 and soluble anti-CD28 and incubated with or without IPI-145 (0.01 to 5 μM). Cytokine levels were measured by BD Cytometric Bead Array. 0.5 to 5 μM IPI-145 significantly inhibited IL-2 and a trend for inhibition for both IFN-γ and TNF-α. *P < .05.
The functional activity of IPI-145 was confirmed by viability analysis. Cell viability was measured every 24 hours (up to 72 hours); 0.25 to 5 μM IPI-145 exhibited concentration- (Figure 1C) and time-dependent (supplemental Figure 1) induction of cytotoxicity in CLL. This concentration range covers the plasma steady-state concentration (0.9 μM) and plasma peak concentration (Cmax) (2.4 μM) for the patients who receive doses at 25 mg twice daily.12 The cytotoxic effect observed is independent of patients’ Ig heavy chain variable mutational status (supplemental Figure 2).
To examine the overall cytotoxicity of IPI-145 to immune cells, we incubated whole blood from CLL patients with 0.25 to 5 μM IPI-145 for 48 hours and analyzed it by flow cytometry for absolute count of live T, NK, and B cells. T and NK cells were sensitive to IPI-145; however the B-cell population was more sensitive (Figure 1D). To specifically examine normal B cells, we isolated B cells from healthy volunteer blood and incubated them with 1 μM IPI-145 for 48 hours and observed no cytotoxicity (Figure 1E), despite observing a significant decrease in CLL cells viability under the same conditions.
Previous studies have shown that PI3K p110δ, and p110γ are essential to cytokine production by immune effector cells. Although IPI-145 did not cause cytotoxicity to T cells (supplemental Figure 3), we sought to determine whether it affects the function of T cells. Upon CD3/CD28 stimulation, T cells showed significant increases in interleukin-1 (IL-2), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) production. IL-2 was inhibited by IPI-145 starting at 0.5 μM, and a trend in inhibition of TNF-α and IFN-γ was also observed (Figure 1F). Because these inflammatory cytokines are prosurvival to CLL cells, this inhibitory effect of IPI-145 could further enhance CLL cytotoxicity in vivo. However, these cytokines are also important for the normal inflammatory response, and the inhibitory effect could associate with opportunistic infection.
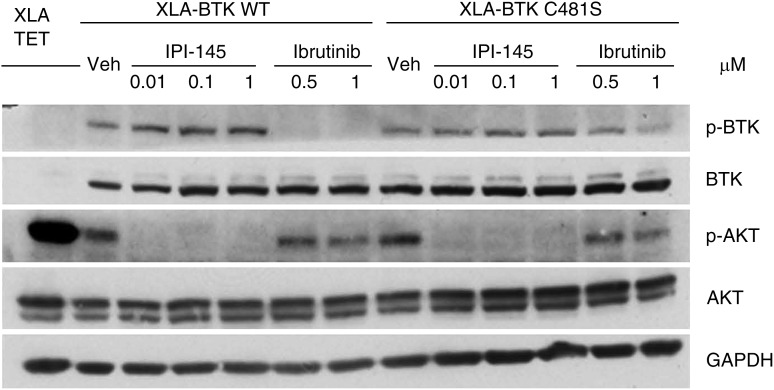
Finally, to understand whether IPI-145 can potentially overcome ibrutinib resistance, we established an in vitro ibrutinib resistant cell line model. BTK-null XLA cells were transfected with wild-type or C481S BTK.11 Phosphorylation of wild-type BTK was inhibited by short-term ibrutinib treatment; however, phosphorylation of C481S mutant BTK could not be inhibited to the same extent. AKT phosphorylation in wild-type cells but not C481S BTK cells can be inhibited by ibrutinib. In contrast, IPI-145 completely abrogated AKT phosphorylation irrespective of BTK mutation status (Figure 2). We observed similar signaling blockade in accordance with cytotoxicity in primary CLL cells that were isolated from BTK C481S mutated ibrutinib resistant patients (supplemental Figure 4).
Figure 2.
IPI-145 abrogates the prosurvival AKT signal in XLA-BTK C481S cells. The inducible XLA-BTK wild-type (WT) and XLA-BTK C481S cell lines were induced with 1 μg/mL of doxycycline for 48 hours. Both cells were left untreated or treated with IPI-145 at various concentrations (0.01 to 1 μM) for 1 hour or with ibrutinib (0.5 μM and 1 μM) for 30 minutes followed by washout and incubated in media for another 30 minutes. Cell lysates were immunoblotted for pBTKY223, total BTK, pATKS473, total AKT, and GAPDH. The blots are representative of 3 independent experiments.
Here we provide data characterizing IPI-145 in CLL for the first time and as the first report describing the activity of IPI-145 in an ibrutinib resistance model. We demonstrated that IPI-145, an inhibitor of PI3K p110δ and p110γ, antagonized prosurvival signaling in BCR cross-linked CLL cells and promoted apoptosis in a dose- and time-dependent fashion, whereas not generating direct cytotoxicity to normal B cells. These apoptosis results are similar to those previously reported by us with idelalisib.3 IPI-145 also reduces the viability of normal T and NK cells and decreases activated T-cell production of various inflammatory and antiapoptotic cytokines. Moreover, we proved that IPI-145 is effective at reducing downstream PI3K signaling as evidenced by diminished AKT phosphorylation even in the setting of BTK C481S mutation. This study provides solid rationale for ongoing clinical development of IPI-145 in CLL patients. Additionally, this study provides justification for clinical use of IPI-145 in the subset of patients that harbor C481S BTK mutation, most likely in combination, given the potential of this subset of CLL patients to develop resistance to monotherapy kinase inhibitors.
Acknowledgments
The authors thank the families who provided samples for this work.
This work was supported by the Specialized Center of Research from the Leukemia and Lymphoma Society, the National Institutes of Health National Cancer Institute (K12 CA133250, P50-CA140158, P01 CA95426, P01 CA81534, and P01 CA101956), and the D. Warren Brown Foundation, the Four Winds Foundation, the Sullivan Chronic Lymphocytic Leukemia Research Fund, Mr and Mrs Al Lipkin, Mr and Mrs Michael Thomas, and The Harry T. Mangurian Foundation.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.D., A.J.J., and J.C.B. designed the experiments, analyzed the data, wrote the article, and reviewed and approved the final version; D.G. established, characterized, and provided the ibrutinib resistance cell line model, reviewed and modified versions of the article, and approved the final version; and J.A.D., Y.Z., A.L., J.K., and J.A.W. planned and contributed to components of the experimental work presented, reviewed and modified versions of the article, and approved the final version.
Conflict-of-interest disclosure: J.K. is an employee of Infinity Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Amy J. Johnson, Ohio State University Comprehensive Cancer Center Bldg. Room 455C, 410 West 12th Avenue, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.
References
- 1.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler DG, Faia KL, DiNitto JP, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Boyle DL, Kim HR, Topolewski K, Bartok B, Firestein GS. Novel phosphoinositide 3-kinase δ,γ inhibitor: potent anti-inflammatory effects and joint protection in models of rheumatoid arthritis. J Pharmacol Exp Ther. 2014;348(2):271–280. doi: 10.1124/jpet.113.205955. [DOI] [PubMed] [Google Scholar]
- 9.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertlein E, Beckwith KA, Lozanski G, et al. Characterization of a new chronic lymphocytic leukemia cell line for mechanistic in vitro and in vivo studies relevant to disease. PLoS ONE. 2013;8(10):e76607. doi: 10.1371/journal.pone.0076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapalombella R, Yeh YY, Wang L, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21(5):694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel M, Kahl B, Horwitz S, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ in patients with relapsed/refractory CLL [abstract]. American Society of Clinical Oncology Annual Meeting. Chicago, IL; 2013. Abstract 7070. [Google Scholar]