Abstract
Environmental enrichment attenuates the response to psychostimulants and has been shown to reduce both anxiety and stress-related behaviors. Since stress is a major vulnerability factor for addiction, we investigated whether enrichment could reverse stress profiles in high anxious rats as well as reduce their amphetamine sensitivity. Using selectively-bred high and low anxiety males (filial 3) from enriched, social or isolated environments, we tested elevated plus maze exploration, novelty place preference and amphetamine (AMPH; 0.5 mg/kg, IP)-induced hyperactivity. We measured plasma corticosterone (CORT) response after forced novel object exposure, phosphorylation of the tropomyosin-related kinase B receptor (pTrkB) in the hippocampus and striatum, and dopamine (D2) receptor mRNA levels in the striatum and nucleus accumbens. Results indicate that high anxiety animals reared in social or enriched environments spent more time on open arms of the EPM while low anxiety animals raised in enriched environments spent more time on open arms when compared to either isolated or social groups. There were no group differences or interactions found for novelty place preference. Enriched environments decreased the response to AMPH and stress-induced CORT regardless of trait but selectively decreased pTrkB and increased D2 mRNA levels in high anxiety animals. The results suggest that selectively-bred trait anxiety rats show state anxiety that is influenced by rearing environments, and D2 protein levels and BDNF/TrkB signaling may differentially contribute to integrating these effects.
Keywords: ANXIETY, BDNF, ELEVATED PLUS MAZE, ADDICTION, DOPAMINE RECEPTOR
1. Introduction
Since Hall [1] first demonstrated that defecation scores in an open-field apparatus could distinguish anxiety levels in rat lines, investigations of anxiety-like behavior in rodent models have revealed significant neural, endocrine, and behavioral responses associated with an animal’s innate level of anxiety [2]. Currently, studies of anxiety-like behavior in rodents use a number of tasks including the elevated plus maze [3, 4], forced swim test [5], social interaction test [6] and novel environment exposure test [7, 8]. In addition, a number of studies have described a selectively-bred line of rats that show low (LAB) and high anxiety-like behavior (HAB) on the elevated plus maze [9]. HAB animals exhibit increased CRF mRNA expression in hypothalamic nuclei following prenatal stress [10] and increased stress susceptibility [11] as well as hyperactivity along the hypothalamic pituitary adrenal (HPA) axis following stress [12]. Moreover, HAB rats also show elevated Fos immunoreactivity in the hypothalamus following anxiety provoking stimuli [13] and increased expression of the neuropeptide cocaine and amphetamine regulated transcript (CART) in the hypothalamus [14] which may contribute to increased sensitivity to amphetamine-induced locomotion [15]. These findings suggest that individual differences in anxiety-like behavior involve activation along the HPA axis that may contribute to psychostimulant drug vulnerability.
It remains to be seen whether early environments can modify individual differences in both anxiety and drug vulnerability. Postnatal environments that are enriched (e.g., toys, wheels) can influence an animal’s coping skills [16] as well as its vulnerability to drugs of abuse such as cocaine [17], morphine [18] and amphetamine [19, 20]. Conflicting findings exist on enriched environment’s (EE) impact on stress with some reporting no difference in corticosterone levels at baseline or after stressor [21] and others indicating a reduction in Sprague-Dawley rats [22] or even elevated levels in enriched Wistar rats [23]. Konkle et al. [24] indicated that a minimum of 40 days in EE, particular strain and type of test impacted HPA and behavioral responses to stress but not reward, with EE rats showing greater flexibility in the face of stress. EE rats that show diminished response to psychostimulants display alterations in dopamine synthesis and turnover, and dopamine transporter (DAT) [20, 25, 26]. Differences in D2 are also observed in anxiety and social dominance models [27, 28]. Further, a role for neurotrophins in the reversal of amphetamine sensitivity in EE rats has been reported [25] as well as increases in dendritic branching and hippocampal neurogenesis [29]. However, there are few studies examining whether enriched environment can modulate stress as a major vulnerability factor for amphetamine sensitivity.
In order to determine whether hippocampal neurogenesis or neuroplasticity in the dorsal striatum contributes to the effects of EE on anxiety-like behavior and amphetamine susceptibility in high anxiety rats, we measured an indirect marker of BDNF activity, the high-affinity neurotrophin intra-cellular signaling by phosphorylation of the tropomyosin-related kinase B receptor (TrkB). cAMP-dependent TrkB phosphorylation has been demonstrated to mediate the structural and functional changes in synaptic development and plasticity associated with BDNF [30]. Further, since numerous studies on anxiety [28] and amphetamine sensitization [31] implicate D2 receptor as a mechanism, we also explored whether D2 protein levels may contribute to environmental enrichment’s modulation of the stress response and amphetamine-induced locomotion. We used the percent open arm time on the EPM as a behavioral screen to phenotype low and high anxiety Long Evans rats. We selectively bred non-sibling pairs of low (above median split/upper quartile, greater %OA time) and high (below median split/lower quartile) then housed same-anxiety groups of unrelated male offspring in enriched (EE), social (SE) or impoverished environments (IE). We subsequently tested the ability of EE to correct anxiety-like behavior on the elevated plus maze, novelty place preference, amphetamine sensitivity and the stress-induced adrenocortical response following a novel environment stressor.
2. Methods and Materials
2.1 Subjects
A total of 165 Long-Evans rats were used in the current study. The stock parental lines (N=85) were purchased from Charles River Breeding Labs (Wilmington, MA), consisting of adult males (n=40) and adult females (n=45) (250-275 g). The subsequent outbred generational lines were bred at the University of Massachusetts Boston where they received standard handling, care and husbandry. Animals were housed in pairs, unless pregnant, and given free access to food and water, while maintained in a temperature- and humidity-controlled environment on a 12 hr light/dark cycle (lights on at 0700 h). All protocols were approved by the University of Massachusetts IACUC and were in adherence to the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals”.
2.2 Husbandry
After a minimum of 21 d in the UMass Boston vivarium, the parental lines were subjected to the elevated plus maze (Med Associates, St. Albans, VT) to establish emotionality or extreme traits of high anxiety (HAn) and low anxiety (LAn) phenotypes using a median split. Subsequently, HAn males were mated with HAn females, LAn males were mated with LAn females. A total of ten males and ten females were mated per anxiety line, each female received a vaginal lavage to determine sexual receptivity and was mated with only one male. After verifying pregnancy with the appearance of a copulation plug, designated Day 0, female rats were monitored for appropriate weight gain and then allowed to deliver and nurture pups through the lactation period. There were no differences in litter size or sex distribution between litters. At weaning, pups were tested on the elevated plus maze which allowed us to confirm HAn and LAn designation and to choose animals that were in the lower quartile (those animals showing the least %OA time) and upper quartile (those animals showing the most %OA time) of the median split for further mating with unrelated same phenotyped animals. All data for the current study include findings from the 3rd generational line of animals.
2.3 Postnatal Housing Environments
Two to four days after weaning (PND 23-25), F2 pups (N=60; a maximum of 2 males per litter) were used to establish Trait × Postnatal Housing Environments (impoverished environment (IE, n=10), social environment (SE, n=10) and enriched environment (EE, n=10). A total of thirty LAn males were placed in IE, SE and EE; similar housing groups were established for the HAn phenotype (n=30; 10 per group). Animals remained in the housing environment for a minimum of three weeks before the onset of behavioral testing and remained there for a total of 18 weeks.
For the IE, single animals were housed in a standard Plexiglas cage (48 × 25 × 22 cm) where each rat had visual and olfactory cues from neighboring animals, but no physical contact.
On average, 10 animals per cage were housed in the SE, which consisted of a large Plexiglas cage (52 × 47 × 38 cm). While initially a total of ten rats were housed per cage, this cage-size only remained constant until with weight gain the rats exceeded the state and federal space recommendations; at that time, the cage size was changed to a minimum of 5 rats per cage (average PND 70); this change occurred after all anxiety tests were completed and only impacted LMA testing.
In the EE, 10 animals were group-housed in each of two large cages, 122 × 71 × 46 cm. The cages were constructed of wire, metal and mesh, allowing for visualization of animals by facility staff and easy attachment of a hanging water bottle and nozzle to remain in place. Constitution of this environment was adapted from Bowling and Bardo [32] in brief, laboratory chow was continuously added to a variety of food containers inside the cage along with ten objects made of metal, Nalgene or hard plastic (e.g., rattles, buckets, Lego blocks, wheels, ladders) and acrobatic objects such as hanging chains, ropes, and interlocking chambers. These objects were rearranged weekly, with new objects introduced and old/worn objects removed.
2.4 Generational Anxiety Screening
We measured the anxiety level of the original, parental line of male and female rats and three other generations around PND 60 or younger. Four days after weaning (PND 25), F2 animals were placed into the select housing environment. Behavioral testing occurred during the light cycle between 0900 and 1400 h. The timeline for in-house breeding through study termination, blood and brain tissue harvesting is depicted in Figure 2. After weaning, male animals were placed in select housing (between PND 23 - 25), 21 days later animals received an EPM screen. One week later rats were tested in the novel place paradigm (NPP). On PND 100, all rats were habituated to the locomotor activity apparatus and tested for amphetamine-induced hyperactivity. Finally, animals were sacrificed and tissue and blood were harvested on PND 126.
Fig. 2.
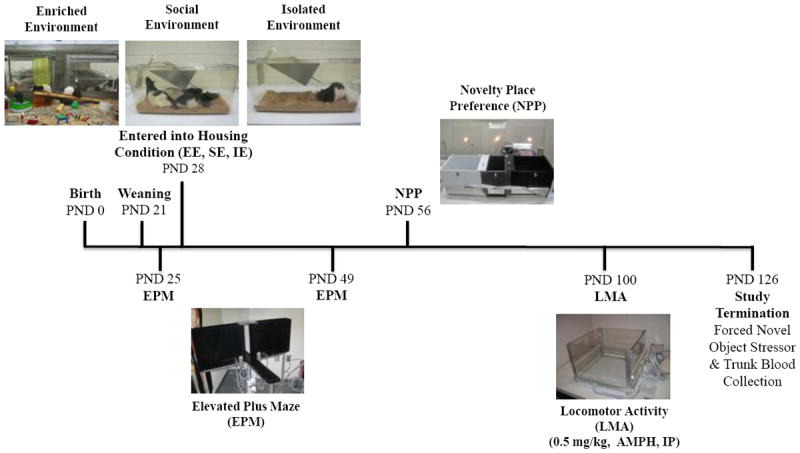
Timeline showing study procedures and postnatal days for behavioral testing and biological assays. Abbreviations: PND=postnatal day, EE=enriched environment, SE=social environment, IE=isolated environment, EPM=elevated plus maze, NPP=novelty place preference, LMA=locomotor activity. At study termination, 8-10 animals per group were sacrificed for brain and terminal blood harvesting.
2.5 Elevated Plus Maze
The elevated plus maze (EPM) apparatus was purchased from Med Associates (St. Alban, VT) and consisted of a central platform surrounded by two open arms and two high, enclosed arms. The apparatus was elevated 70 cm above the floor in a large room with normal light intensity as testing occurred during the animals’ light phase. Rats were kept in their home cage and habituated to the experimental room for 15 min before the onset of testing. Prior to the start of each session, the maze was cleaned with a mixture of mild detergent and water, and dried. A rat was then placed on the central platform facing an open or closed arm, alternating the direction for subsequent rats. The following parameters were collected from MED-SYST-8 interface and software: (1) the time spent on open and closed arms (calculated as percent time on OA), and (2) the number of entries in open and closed arms (calculated as total entries and %OA entries).
2.6 Novelty Place Preference
The NPP apparatus (Med Associates adapted Condition Place Preference box) consisted of two rectangular compartments, equally sized, and separated by a smaller compartment with guillotine doors. Each compartment was equipped with distinguishable visual and tactile cues. One white compartment contained a grid floor, while the other black compartment had a mesh floor. The open guillotine doors allowed the rat free access to the other compartment during testing but were closed during training sessions. Each rat was habituated to one of the two compartments in the NPP apparatus, for two consecutive days (Day 1-2). The initial compartment was counter-balanced for each anxiety/housing group of rats and each rat was allowed to explore its compartment for 30 min on each day. During the test session (Day 3), the rat was placed in the gray center chamber and allowed to move freely in the NPP box for 15 min with the guillotine doors recessed. The data for time spent in each of the three compartments was gathered. However, only data on percent time spent in the novel chamber and total entries were used for statistical analyses.
2.7 Locomotor Activity
Rats were run in a commercial locomotor activity (LMA) system (Med Associates) with activity measured by the number of photobeam breaks over a 90 min period on the test day. Initially, animals were habituated to the LMA apparatus for 30 min and then removed for injection with a low dose of amphetamine (0.5 mg/kg, IP) and returned to the LMA chamber for a 60-min post-injection period. Data are reported as group means ±SEM for distance traveled and rearing bouts.
2.8 Determination of Plasma Corticosterone
Following 30 minutes of forced exposure to a novel object (a colored polystyrene cup) in an open field, animals were rapidly decapitated and trunk blood was collected into heparinized tubes. Plasma was prepared and stored at -80°C until assay. Plasma corticosterone was measured using the Coat-A-Count(r) Rat Corticosterone RIA Kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA) according to package insert instructions. Assay detection limit was 5.7 ng/ml, and average intra-assay variability (between duplicates) was 2.25%.
2.9 Western Blot Analysis
Following trunk blood collection, brains were rapidly dissected and frozen in dry ice and stored at −80°C. Based on the rat brain atlas [33] the brain areas containing the nucleus accumbens (NAc), dorsal lateral caudate putamen (CPu) and hippocampus were collected from each animal after thawing the brains.
For protein extraction, brain tissues were thawed and homogenized in cold TPER (Fisher, catalog 78510) with a protease inhibitor (Sigma-Aldrich). Tissue was homogenized, centrifuged and total protein concentrations were determined by BCA (bicinchoninic acid) Protein Assays (Pierce Chemical Co., Rockford, IL). Samples from the dorsal lateral region of the CPu (15 μg protein), NAc (15 μg protein), and hippocampus (25 μg protein) were subjected to electrophoresis on 10% polyacrylamide-SDS gels and transferred to PVDF membranes. Membranes were blocked for 1h then rinsed with 5% milk and incubated with dopamine D2 receptor antibody (1:5000; Millipore, Billerica, MA), TrkB (1:1000; Cell Signaling, catalog 80E3), or phosphor-TrkA (Tyr 674/675)/TrkB (Tyr 706/707); catalog 4621) overnight at 4°C. Additional samples from the CPu (15 μg protein) were subjected to electrophoresis on 7.5% polyacrylamide-SDS gels and transferred to nitrocellulose membranes. These membranes were blocked for 1h then rinsed and incubated with TrkB and phospho-TrkA (Tyr 674/675)/TrkB (Tyr 706/707) overnight at 4°C. phospho-TrkA/TrkB signaling was measured as a control since activation at this receptor site has not been linked to structural and biological functioning of BDNF acting at TrkB [34]. After rinsing, all blots were incubated for 1h in a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:10,000; Jackson), followed by detection with SuperSignal® West Pico Chemiluminescent Substrate (Pierce Chemical Co.). All blots were reprobed with antibody against β-actin (1:100,000; Sigma-Aldrich Corp.), and after rinsing, the blots were incubated for 1h in an HRP-conjugated goat anti-mouse IgG secondary antibody (1:10,000; Jackson) followed by chemiluminescent detection. The intensities of each of the candidate proteins and β-actin were visualized and quantified using the Bio-Rad Chemi Doc XRS+ Imager and Image Lab software. Levels of each of the proteins were normalized to those of β-actin in each sample and a standard that was run on every gel. The standard used to normalize between blots was the protein concentration of the CPu of a non-experimental animal.
2.10 Data Analysis
Statistical analyses were carried out with GraphPad Prism (v. 12.0, San Diego, CA). Separate two-way ANOVA with Housing and Trait factors were performed for each of the behavioral and biological assays. For the generational screening data, a two-way ANOVA was run on the percent time spent on the open arms of the EPM across multiple generations (Trait × Generation). Behavioral measures included percent time on open arm, percent open arm entries and total entries in the EPM; percent novel chamber entries for NPP; and average distance traveled (habituation and post-AMPH) as well as average rearing bouts in the locomotor activity monitors. Post-hoc comparisons were performed using Bonferroni multiple comparisons (with alpha adjustments) where appropriate. We also performed bivariate correlations to determine any associations among behavioral assays (all animals, N=40) and neural markers using Pearson R correlations. This correlation analysis was performed to determine the relationship between variables and the direction of this relationship. A significance level of p < 0.05 was used throughout the study. Data are presented as group means (n=10, unless otherwise noted) ± SEM.
3. Results
3.1 Generational Anxiety Screening
A reduction in anxiety-like behavior (i.e. increased percent time on open arms) was observed in the LAn phenotype across multiple generations, while increased anxiety-like behavior (i.e. decreased percent time on open arms) was observed in the HAn phenotype across multiple generations. Two-way ANOVA indicated a significant main effect of Trait [F(1,69)=101.5, p<0.0001] as well as a Trait × Generation interaction effect [F(3,69)=8.776, p<0.0001], with F3 and F5 generations of LAn and F5 generation of HAn animals displaying significant differences when compared to parental lines (p<0.05 for LAn, HAn Parental relative to F3 and HAn Parental compared to F5; (p<0.01) LAn Parental relative to F5 (Fig. 1).
Fig. 1.
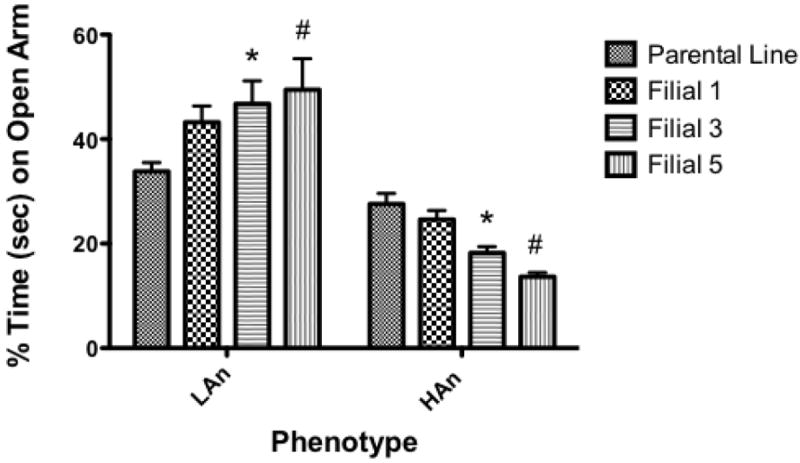
Selectively outbred high and low anxiety rats show exaggerated responses on the elevated plus maze across generations. Bar graph comparing males’ (n=10 per group) percent time on open arm of EPM for Parental line and filial 1 (F1) at PND 35, F3 and F5 at PND 36 selectively bred HAn and LAn lines. *p<0.05 (same phenotype) Parental compared to F3 #p<0.01 (same phenotype) Parental versus F5.
3.2 Elevated Plus Maze
To assess the effects of trait anxiety and environmental housing environment on anxiety-like behavior, percent time on open arms was analyzed using a one-way ANOVA. A main effect of Housing was obtained for percent time on open arms [F(2,54)=33.84, p<0.0001]; a trend towards significance for Trait was found [F(1,54)=3.404, p=0.0705] as well as a significant interaction [F(2,54)=6.812, p<0.01] (Figure 3a). Post-hoc multiple comparisons (Bonferroni) revealed HAn SE rats spent more time on the open arms than IE rats (p<0.001); both HAn and LAn rats reared in enriched environments differed from those from IE, with increased percent time on the open arms (p<0.0001); HAn rats reared in EE were not different from those reared in SE, however, LAn rats reared in EE spent more time on the open arms than those reared in SE (p<0.01). We also measured open arm entries and found a main effect of Housing [F(2,54)=17.10, p<0.0001]. Post-hoc analyses revealed HAn SE and EE had more %OA entries than the HAn IE group (p<0.01, p<0.001, respectively); LAn EE animals also had greater %OA entries relative to LAn IE animals (p<0.001) (Fig. 3b).
Fig. 3.
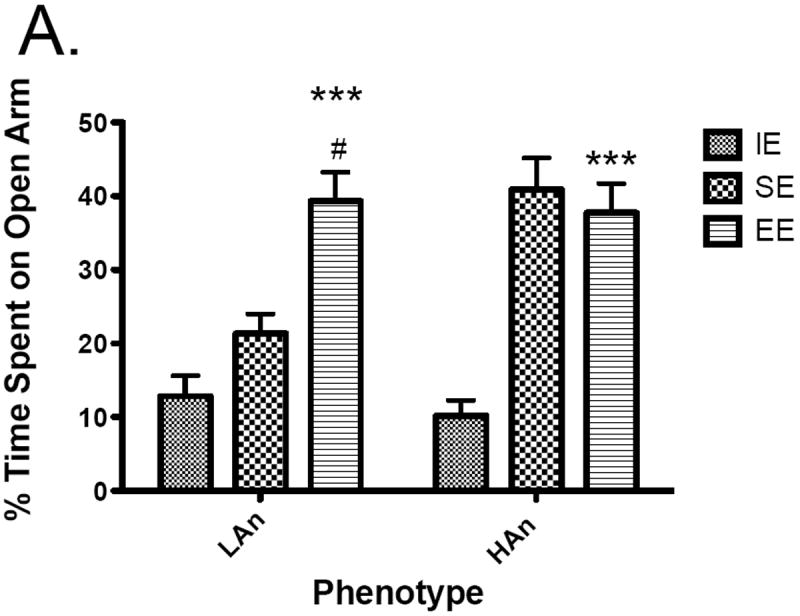
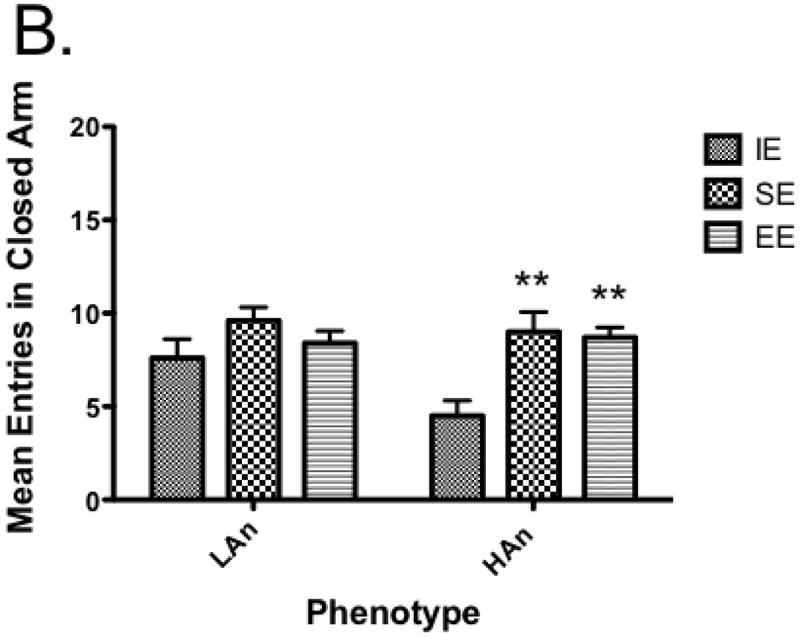
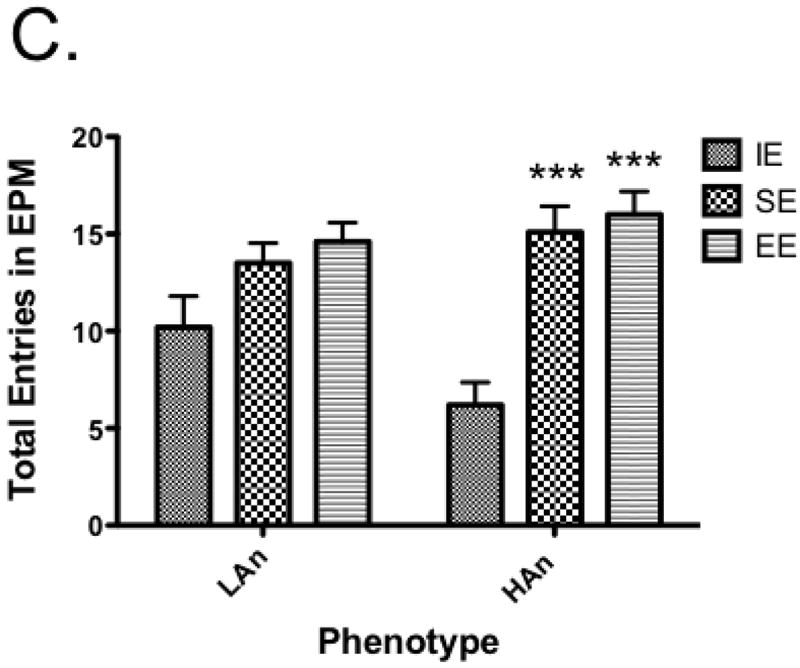
a-c Anxiety-like responses and general activity on the EPM of HAn/LAn animals following select housing conditions. (a) HAn animals reared in SE and EE spent more time on the open arms compared to IE; LAn animals from EE also had more percent time on open arm, (b) HAn EE and SE reared rats entered the open arms more often than HAn animals reared in isolated environments, (c) HAn animals reared in SE and EE had an increase in total arm entries over HAn animals in isolation ***p<0.001 (same phenotype, compared to IE).
For activity on EPM, we analyzed total entries separately using a two-way ANOVA. Results show Housing [F(2,54)=19.52, p<0.0001] and Housing × Trait interaction effects [F(2,54)=3.336, p<0.05), with post-hoc tests indicating HAn animals reared in SE and EE had more total entries than HAn IE reared animals, p<0.001 (Figure 3c).
3.3 Novel Place Preference
To determine if any differences existed between trait anxiety and housing environment on free-choice novel place preference, we analyzed percent time and total entries in novel chamber separately using two-way ANOVAs. No significant main or interaction effects were found in either test (data not shown).
3.4 Locomotor Activity
We analyzed the distance traveled data using a three-way ANOVA for Trait, Housing and Time (averaging the habituation and post-amphetamine blocks). We observed significant effects of Housing [F(2,108)=13.826, p<0.0001] and Time [F(1,108)=27.030, p<0.0001] as well as trend towards a significant Trait × Housing effect [F(2,108)=2.469, p<0.089] and Housing × Time [F(2,108,)=4.141, p<0.05] effect. Post hoc tests revealed significant differences between the IE and EE groups (p<0.0001) and the SE and EE groups (p<0.001). No significant differences were observed for rearing bouts (data not shown).
3.5 Determination of Plasma Corticosterone
Plasma corticosterone responses were measured thirty minutes following a forced novel object exposure. Two-way ANOVAs run on group CORT responses showed a significant Housing effect [F(2,54),=11. 87, p<0.001] (Figure 5). Planned post-hoc comparisons indicated that HAn animals reared in the EE had significantly lower CORT levels than HAn rats from IE (p<0.001) and SE (p<0.05). In a similar fashion, LAn animals from EE had significantly lower CORT than those from SE (p<0.5) and marginally lower than those from IE (p=0.064).
Fig. 5.
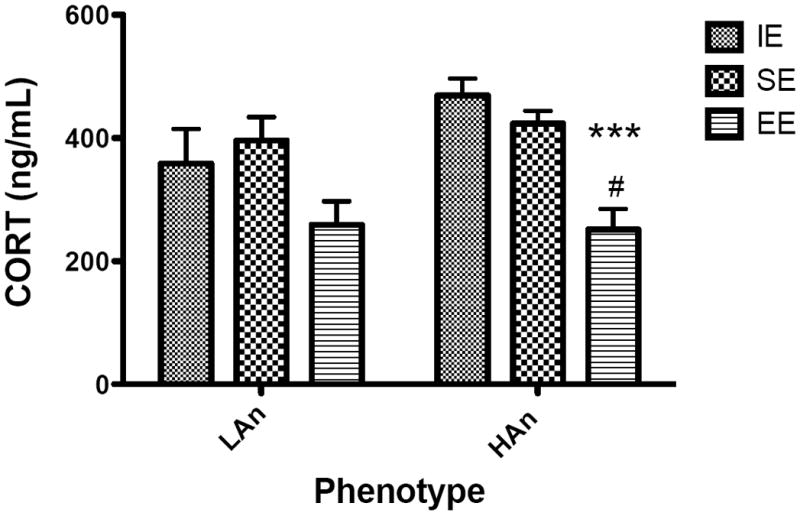
Plasma corticosterone levels (ng/ml) in response to a 30 min forced novel object exposure varied across high anxiety selective-housed groups (mean ± SEM). Assay detection limit was 5.7 ng/ml, and average intra-assay variability (between duplicates) was 2.25%. Data shown are mean response ng/ml ± SEM. After stress, animals of the EE group (both HAn and LAn) showed the smallest stress-induced corticosterone secretion, whereas this secretion was elevated in all other groups. HAn from EE relative to HAn animals from isolated environments (***p<0.001) and compared to HAn SE (#p<0.05).
3.6 Dopamine Receptor (D2) Levels in the Caudate Putamen (CPu) and Nucleus Accumbens (NAc)
A two-way ANOVA of the Western blots of the dopamine receptor (D2) from CPu tissue revealed that there were main effects of Trait [F(1,23)=8.360, p<0.01] and Housing [F(2,23)=6.445, p<0.01]. Post hoc analyses showed that D2 levels were relatively higher in HAn animals from IE and EE than those from SE (p<0.05; Fig. 6a). Analysis of NAc D2 protein levels uncovered main effects for Housing [F(2,25)=2.037, p<0.01] and Trait [F(1,25)=6.516, p<0.05], with HAn rats from EE displaying higher D2 levels relative to those from IE (Bonferroni, p<0.01, Fig. 6b).
Fig. 6.
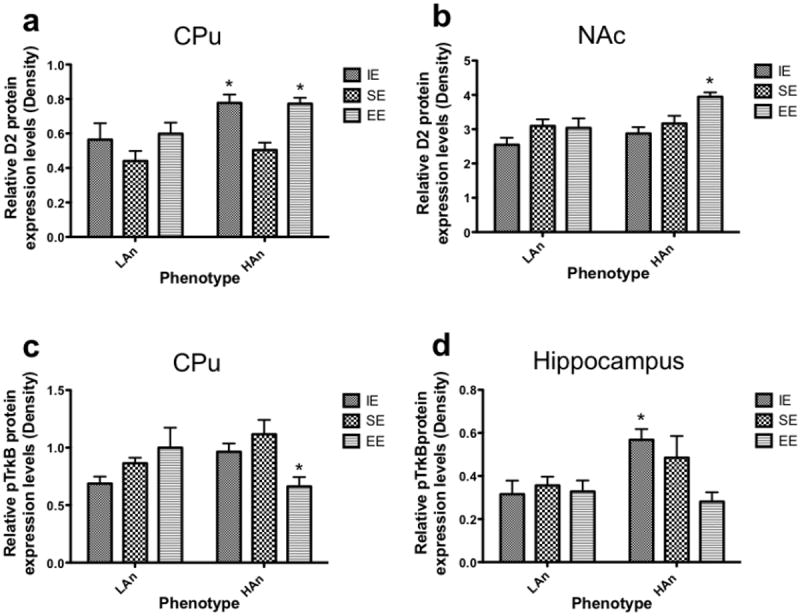
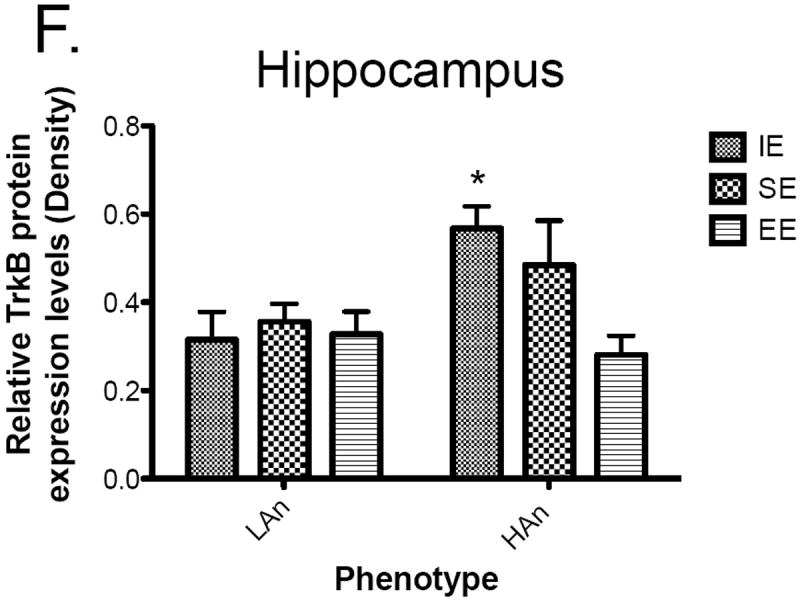
a-d Housing and Trait differentially affected protein levels (Western blots) (n=4-6 brains per group). (a) Dopamine D2 receptor expression in the CPu was significantly higher in HAn animals reared in isolation and in enrichment relative to HAn rats from isolated rearing environments (p<0.05). (b) Dopamine D2 receptor expression (±SEM) in the NAc of was significantly higher in HAn animals from enriched rearing compared to HAn animals reared as isolates (p<0.05). (c) Phosphorylation of TrkB (pTrkB) in the CPu was significantly higher in the HAn animals from social environments relative to the HAn enriched animals, (*p<0.05. relative to HAn EE). (d) HAn animals from isolation rearing had higher levels of pTrkB in the hippocampus than the HAn EE group (*p<0.05). All data are expressed in means ± SEM, and levels of each of the proteins were normalized to those of β-actin in each sample. There were no significant main effects or interaction for total TrkB receptor protein levels in the CPu or hippocampus; data not shown.
3.7 TrkB and Phosphorylation of TrkB Levels in the CPu and Hippocampus
No significant main effects or interaction for total TrkB receptor protein levels in the CPu were found (data not shown). By contrast, pTrkB protein levels in the CPu were increased in HAn animals from social environments relative to those receiving enrichment (Figure 6c); an interaction effect was obtained [F(2,23)=5.661, p<0.01], (post-hoc tests, HAn SE to HAn EE, p<0.05). Analysis of TrkB protein levels in the hippocampus did not reveal any significant main effects or an interaction; data not shown. On the other hand, a significant effect for Trait was found for pTrkB receptor protein levels in the hippocampus, [F(1,28)=5.214, p<0.05], a marginally significant main effect of Housing [F(2,28)=3.045, p = 0.0636] as well as a marginally significant interaction effect [F(2,28)=3.214, p=0.0554]. Post hoc analyses indicated that HAn animals from isolated environments had markedly greater pTrkB protein levels than those from enriched environments (Fig. 6D), p<0.05. Given that there were no main or interaction effects for TrkB levels in either the CPu or the hippocampus it is likely that the differences in pTrkB were not due to potential differences in BDNF receptor levels.
3.8 Pearson r Correlations
Correlation analysis indicated a negative correlation between corticosterone levels after a mild stressor and percent time spent on the open arms of the elevated plus-maze (Pearson’s r = −0.370; p < 0.01); open arm time was also negatively correlated with distance traveled after amphetamine injection (Pearson’s r = -0.38; p < 0.01; two-tailed) and with pTrkB protein levels in the caudate putamen (Pearson’s r = -0.49; p < 0.01; two-tailed) (Table 1).
Table 1. Correlations among the eight variables assayed for animals from selectively-bred high and low anxiety lines.
Correlation matrix showing relatedness of variables used in behavioral assays and detection of hormone and protein levels for animals selectively bred for high and low anxiety using the elevated plus maze.
| Mean | SEM | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) % Time on OA | 22.62 | 1.96 | --- | |||||||
| (2) LMA | 9143 | 739 | -.438** | --- | ||||||
| (3) CORT (ng/ml) | 359.70 | 17.99 | -.370** | .238 | --- | |||||
| (4) % Time NPP | 36.64 | 9.56 | .142 | .117 | .109 | --- | ||||
| (5) CPu pTrkB mRNA | 0.904 | 0.05 | -.404* | .290 | .301 | .198 | --- | |||
| (6) Hip pTrkB mRNA | 0.921 | 0.05 | -.102 | .275 | .052 | -.056 | .135 | --- | ||
| (7) CPu D2 mRNA | 0.6 | 0.04 | .180 | -.101 | -.094 | -.413+ | -.010 | -.197 | --- | |
| (8) NAc D2 | 2.995 | 0.102 | .214 | -.123 | -.044 | -.165 | -.173 | -.122 | .133 | --- |
Means (±SEM) represent all animals’ responses (N=40) for each assay, allowing for the assumption of normal distribution of variables to be met. Abbreviations: OA=open arms of the elevated plus maze, CORT=corticosterone, CPu=caudate putamen, pTrkB= phosphorylation of the tropomyosin-related kinase B receptor, D2=dopamine 2 receptor, Hip=hippocampus, NAc=nucleus accumbens,
p < 0.05,
p < 0.01,
p=0.053.
4. Discussion
Our findings demonstrate that Long Evans male rats selectively bred (unrelated pairs) for extreme anxiety on the elevated plus maze show increased divergence across generations and that the enriched environment (EE) can interact with trait anxiety to modify behavioral as well as physiological and neural responses. In the current study, animals were placed in select environments shortly after weaning, a critical period of neural development. Developmental timing of and duration of exposure to EE are factors that have been shown to markedly impact neural and behavioral outcomes [34, 35]. Indeed, developing neural systems are highly plastic and particularly sensitive to both insults [37] and enrichment [38].
4.1 Environment Enrichment Effects on Novelty Preference and Elevated Plus Maze Performance
In the current experiment, rearing environment, did not impact novel environment preference. Earlier work by Hall et al. [39] found isolation rearing increased novel environment preference, especially in environments that were favorable and less aversive. Similar effects were not observed in the current study. It is possible that additional conditioning sessions or the use of more distinct novel environment would have elicited effects. Others have reported no effect of EE on sucrose preference, which may suggest that rearing environment does not alter responding to natural rewards, such as food or novelty preference. Overall, these data suggest that neither trait anxiety nor post-weaning rearing environment significantly modifies this form of place preference [39].
EE impacted anxiety-like behavior on the EPM by increasing percent open arm exploration, most notably among HAn animals. In our initial parental animals, we did a median split separating HAn (lower quartile, low %OA time) and LAn (upper quartile, high %OA time) lines displaying the anxiety-like behavior and less anxiety-like profiles on the EPM, respectively. In the subsequent offspring from unrelated breeding lines, HAn animals show significantly less activity on the open arms of the EPM, averaging about 25% for F3 (Fig. 1). When tested on the maze following 3 weeks in distinct housing environments, however, high anxiety animals reared in SE and EE had significantly greater time on the open arms compared to IE reared rats, and the averages for these HAn animals (>40%; Fig. 3a) was higher than that observed in similar HAn animals maintained in standard double housing conditions and tested as part of the initial inter-generational study (see Fig. 1). These findings extend previous work demonstrating that stressed Long Evans rats reared for a minimum of 40 days in EE exhibit a reduced behavioral stress response [24]. Prior exposure to SE in a large cage with social interaction and EE in which a large cage with social peers is equipped with toys that are manipulated (e.g., added or deleted), present these animals with ample opportunities for social interaction, exercise, manipulation of and interaction with objects, all of which have been shown to improve resilience to stress [41]. This exposure may better equip highly anxious animals in particular [41] to navigate potentially stressful areas such as the open arms on the EPM, and while other labs have reported EE animals show less overall exploration in novel environments, EE appears to interact with trait anxiety in the current study, increasing exploration and reducing anxiety-like behavior suggesting the anxiety may be mediated by changes in exploration. It is interesting that in the LAn animals reared in SE, no differences in anxiety-like behavior were observed.
4.2 Environmental Enrichment Influences Anxiety-Induced Amphetamine Sensitivity
Several lines of research highlight the putative role of the stress domain as well as early life experience (e.g., EE) on vulnerability to drugs of abuse [10, 17, 11]. To test whether extreme trait anxiety and postnatal housing might interact to influence drug sensitivity, we recorded the locomotor-activating effects to a low dose of amphetamine in our extreme trait anxiety animals after 10 weeks of select housing. Initially, there were significant housing differences during the 30-min habituation to the locomotor cages with higher activity in the HAn- SE relative to HAn-EE rats. These results are not in agreement with that of others, demonstrating no differences in activity levels between animals showing high (HAB) and low anxiety-like behavior (LAB) [19]. The findings are consistent with the reported differences in activity in the EPM for the HAn animals in the current study. Following amphetamine injection, activity levels were greatest in the HAn-SE animals, a profile similar to that observed in the LAn IE rats in that they, too, differed in AMPH-induced activity from LAn SE and EE groups. Bardo and colleagues reported that EE reduced amphetamine self-administration in rats [19]. Drug-induced locomotion was lowest in the HAn and LAn EE rats, implying that rats bred along an anxiety domain show recovery from drug sensitivity if exposed to significantly enriched rearing environments. Indeed, using a validated model of ADHD, the inbred spontaneously hyperactive rats (SHR), de Carvalho et al. [40] found that SHR rats living in EE showed less novelty-seeking and failed to (1) develop conditioned place preference to ethanol, (2) show sucrose preference and (3) self-administer ethanol. We also found that the low anxiety-like behavior on the EPM was negatively correlated with AMPH-induced locomotion, and that was observed regardless of trait anxiety (Table 1).
4.3 Enriched Environments Alter Stress-Induced Neuroendocrine Response
In the current work, after forced exposure to a novel object, the adrenocortical response was markedly elevated in high anxiety groups reared in impoverished and social environments compared to HAn EE. These findings suggest that EE’s restorative effects on emotional responding in the EPM likely involve diminished neuroendocrine levels in these animals. Other labs have reported higher basal levels of CORT in Long Evans rats housed in EE but a more rapid return to baseline following an acute stressor, successive forced swim tests and in prenatally-stressed rats reared in EE, suggestive of greater adaptability in these animals [23, 45]. Currently, EPM open arm responses were inversely related to CORT levels (Table 1), implying in the HAn and LAn lines that increased activity on the open arms may represent low anxiety-like behavior.
4.4 Environmental Enrichment Effects on BDNF/pTrKB and D2 Protein Levels
Recent findings by Schloesser et al. [43] indicate the adaptive adrenocortical response in EE mice is influenced by neurogenesis in the dentate gyrus. Additional evidence includes recent work by Jeanneteau et al. [46] that shows glucocorticoids in Trk neurotrophin activation and neuroprotection. Currently, we used the indirect measure of phosphorylation of the BDNF high affinity tropomyosin-receptor-kinase B (TrkB) [47] and found elevated protein levels of pTrkB in the caudate putamen only, and just in animals from the high anxiety phenotype housed with same-sex social peers, not the EE group. Perhaps an alternate, non-BDNF mechanism mediates the increased neurogenesis reported by Schloesser and colleagues [43] or that differing variables influence the disparate findings in that they used mice, and distinct behavioral assays (e.g., social defeat paradigm, a model of depression) than those used presently. It may be the case that looking at whole hippocampal tissue affected our findings as well because while researchers have reported similar decreases in BDNF mRNA levels in CA3 and dentate gyrus of stressed rats, they have not seen any changes in CA1 [48]. Further, perhaps the analysis of amygdala may have provided evidence for a relationship between anxiety and neurogenesis by targeting a brain region long implicated in anxiety.
Consistent with our findings, using transgenic mice Govindarajan and colleagues [49] reported that BDNF over-expression was linked to increased anxiety-like responses in the open field test. As well, Branchi et al. [38] found that in mice reared in a communal nest, increased BDNF levels and protracted survival of BrdU-positive cells accompanied elevated stress responses in the open field and elevated plus maze. Finally, Meshi and colleagues [50] found EE improvements in anxiety were neurogenesis-independent. Therefore, the current findings are in agreement with a body of evidence from research models that focus on anxiety as indexed by open field and elevated plus maze tests.
We also observed increases in protein levels for D2 in the nucleus accumbens and CPu, with higher levels for the HAn EE group in both regions, and additionally, for the HAn animals reared in the isolated environment for the CPu region (Fig 6a). Other work supports this inverse relationship, indicating decreased D2 protein levels in anxious and social dominance animal models [26, 27], with no changes in EE reared animals [24], the same profile we observed with the HAn animals reared in SE showing decreased D2 protein levels. Although HAn SE animals did not present as anxious in the EPM, the LAn SE did trend towards greater anxiety-like behavior and decreased D2 levels. Although not tested presently, it is probable that social hierarchy contributed to these findings and the EE ameliorated these effects. These results also extend that of others showing EE decreased DAT levels and functionality along the mesocorticolimbic pathway [24, 25], while not affecting striatal DA transmission [51] following an environmental enrichment condition postnatally. Moreover, literature on stress implicates decreases in striatal D2 sensitivity and D2 mRNA levels in isolation-reared animals [52] and in anxiety and social dominance models [26, 27].
4.5 Pearson r Correlations
The correlation analysis of the behavioral, hormonal and protein data showed that the time spent on the open arms of the elevated plus-maze was negatively correlated with the corticosterone secretion after stress and total distance traveled following amphetamine injection. In a similar fashion, others found EE-reared rodents display blunted adrenocortical responses to early life or adult stress [23, 45]. Further, percent time on open arms negatively correlated with pTrkB protein levels in the caudate putamen and showed a near-significant negative correlation with D2 protein levels in that region as well. These data implicate less anxiety-like behavior mapping on to decreased BDNF / TrkB signaling and more anxiety-like behavior associated with decreased D2 protein levels as implicated by others [52].
5. Conclusion
Taken together, we report that phenotyping outbred animals based on response on the EPM and mating selectively thereafter resulted in an increase in extreme anxiety responses on the EPM across generations (i.e., for F3 and F5; see Fig. 1). This, however, did not extend to the 3rd generation animals reared in select housing presented in the current work. We did not find trait differences in anxiety-like behavior or exploration along the elevated plus maze, novelty place preference or locomotor response to amphetamine. It is probable that the multiple-handling and repeated testing paradigms may have contributed to this discrepancy. Perhaps these multiple exposures reduced the trait-specific effects in this generational line. This is in contrast to the extensive work done on the inbred Wister line of high (HAB) and low anxiety-like behavior (LAB) rats where animals show an anxious profile in multiple tests [12]. It is also likely that the discrepancy is due to the genetic diversity of the HAn/LAn lines as well as the use of only the 3rd generational line. Of interest, we show that decreased anxiety-like responding on the EPM (i.e. increased percent open arm time) is negatively correlated with corticosterone responses and AMPH-induced locomotor responding. Our findings provide evidence for a putative role of rearing environmental manipulations in ameliorating the vulnerability for drug addiction associated with high anxiety. High anxiety animals reared in EE or SE were found to have a behavioral profile more closely resembling that of LAn animals in EE (increased percent open arm time, decreased AMPH locomotor response and a diminished CORT response) while low anxiety animals reared in IE behaviorally resemble their HAn IE counterparts. Taken together, this strongly suggests that post-weaning environmental manipulation can augment the behavioral profile of adult trait anxiety animals, causing the extreme behavioral profiles of HAn and LAn animals to become moderate over time. EE has been successfully shown to alter psychostimulant drug sensitivity elsewhere [24] but had not yet been shown to do so in animals with particular drug vulnerability. At least changes in protein levels for D2 that are consistent with decreased CORT changes may underlie EE’s effects on AMPH resilience and also contribute to stress adaptability. Additional work is necessary to determine whether immediate changes in neurotrophin activity / TrkB phosphorylation in other brain areas is another critical mechanism for EE’s beneficial effects for the current model.
Fig. 4.
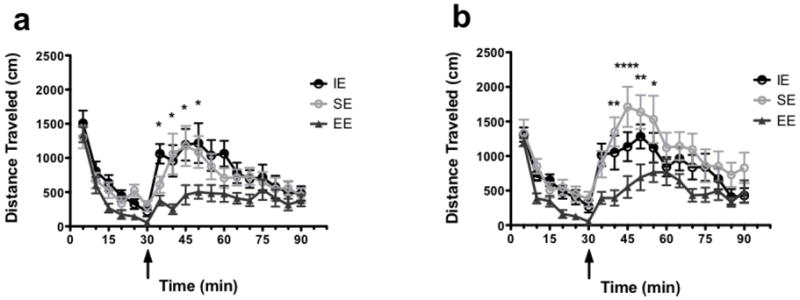
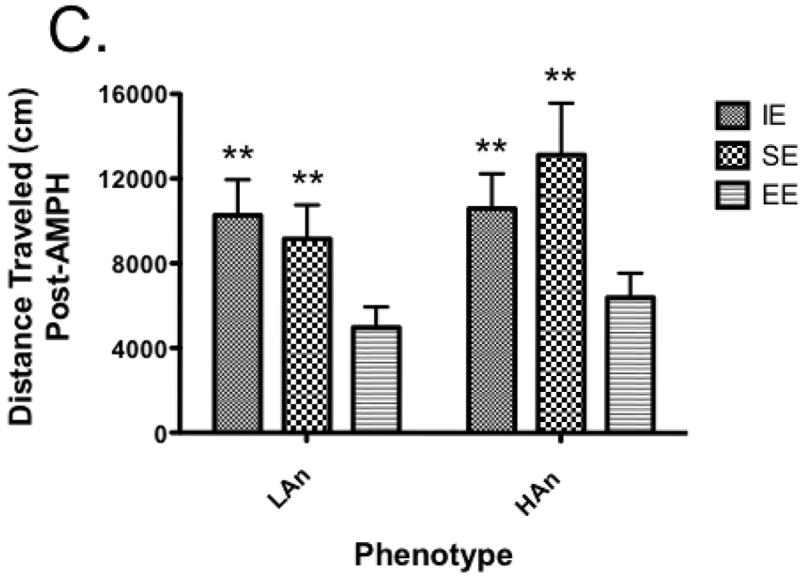
a-b Amphetamine-induced locomotion is elevated in animals from isolated (solid, black lines) and social (dashed, grey lines) environments. (a) Time-course of habituation (0-30 min) followed by amphetamine (0.5 mg/kg, IP; injection at 30 min (arrow))-induced motor activity in LAn and HAn animals from select housing conditions. (b) Average total activity during habituation (0-30 min) (c) and post-AMPH injection. Animals from IE and SE differed from those reared in EE (**p<0.001).
Highlights.
Selective outbred high anxiety (HAn) rats show greater anxiety across generations
Enriched housing lowers anxiety and corticosterone levels in HAn animals
Isolate and social housing increase amphetamine-induced activity in HAn rats
Striatal BDNF pTrkB receptor levels were elevated in HAn rats reared socially
Striatal D2 levels were markedly higher in social and enriched HAn-reared rats
Acknowledgments
The authors gratefully acknowledge Elizabeth Boates, Mitzi Sweeney and Dr. Nichole Neugebauer for assistance with animal maintenance and husbandry, technical help and comments on the manuscript. STD was supported by Award Number P20MD002290 from the National Institute on Minority Health and Health Disparities (Celia Moore, Ph.D., P.I.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health. JHP was supported by NIH grant 5R00HD056041-05. JJB and EMB were supported by NIH grant R01DA25674.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- 2.Broadhurst PL. Determinants of emotionality in the rat: II. Strain differences. J Comp Physiol Psychol. 1958;51:55–59. doi: 10.1037/h0047266. [DOI] [PubMed] [Google Scholar]
- 3.Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 4.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–28. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M. Is immobility of rats in the forced swim test “behavioral despair?”. Physiol Behav. 1988;42:93–95. doi: 10.1016/0031-9384(88)90266-1. [DOI] [PubMed] [Google Scholar]
- 6.File SE, Hyde JRG. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–13. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 8.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 9.Landgraf R, Wigger A. High vs low anxiety-related behavior in rats: an animal model of extremes in rat anxiety. Behav Genet. 2002;32:301–14. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- 10.Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 11.Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- 12.Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- 13.Salchner P, Sartori SB, Sinner C, Wigger A, Frank E, Landgraf R, Singewald N. Airjet and FG-7142-induced Fos expression differs in rats selectively bred for high and low anxiety-related behavior. Neuropharmacology. 2006;50:1048–1058. doi: 10.1016/j.neuropharm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Wiehager S, Beiderbeck DI, Gruber SHM, El-Khoury A, Wamsteeker J, Neumann ID, Petersén Å, Mathé AA. Increased levels of cocaine and amphetamine related transcript in two animal models of depression and anxiety. Neurobiol Disease. 2009;34:375–380. doi: 10.1016/j.nbd.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Yen Y, Anderzhanova E, Kleinknecht K, Bunck M, Micale V, Landgraf R, Wotjak CT. Inbred low anxiety-related behavior (LAB) mice – a mouse model of attention-deficit hyperactivity (ADHD)? Pharmacopsychiatry. :21–A124. [Google Scholar]
- 16.Diamond MC. Response of the brain to enrichment. An Acad Bras Ciênc. 2001;73:211–20. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- 17.Magalhaes A, Summavielle T, Tavares MA, de Sousa L. Effects of postnatal cocaine exposure and environmental enrichment on rat behavior in a forced swim test. Ann NY Acad Sci. 2004;1025:619–29. doi: 10.1196/annals.1316.077. [DOI] [PubMed] [Google Scholar]
- 18.Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–79. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- 19.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self- administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 20.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 21.Pham TM, Ickes B, Albeck D, Söderström S, Granholm AC, Mohammed A. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–86. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- 22.Belz EE, Kennell JS, Czambel K, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–86. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–31. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 24.Konkle AT, Kentner AC, Baker SL, Stewart A, Bielajew C. Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J Am Assoc Lab Anim Sci. 2010;49:427–36. [PMC free article] [PubMed] [Google Scholar]
- 25.Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–1007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–23. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–72. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 28.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self- administration. Nature Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 29.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nature Neurosci. 2005;8:164–72. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: Effects on D2 autoreceptors, transporters and synaptic vesicles. J Neurosci. 2001;21:5916–24. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social and isolated rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego CA: Academic Press; 2004. [Google Scholar]
- 34.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 35.Branchi I, D’Andrea I, Fiore M, Di Fausto V, Aloe L, Enrico A. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60:690–96. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Pereira LO, Strapasson ACP, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but no in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–66. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology. 2002;23:375–84. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 38.Branchi I, Karpova NN, D’Andrea I, Castrén E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–72. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on preference by rats for a novel environment. Physiol Behav. 1997;62:299–303. doi: 10.1016/s0031-9384(97)00117-0. [DOI] [PubMed] [Google Scholar]
- 40.de Carvalho CR, Pandolfo P, Pamplona FA, Takahashi RM. Environmental enrichment reduces the impact of novelty and motivational properties of ethanol in spontaneously hypertensive rats. Behav Brain Res. 2010;208:231–36. doi: 10.1016/j.bbr.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Green TA, Cain ME, Gehrke BJ, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 43.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–63. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–32. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behavior and HPA axis activity in rats. Eur J Neurosci. 2003;18:3367–74. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- 46.Jeanneteau F, Garabedian MJ, Chao MS. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. PNAS. 2008;105:4862–67. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poo MM. Neurotrophins as synaptic modulators. Nature Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 48.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. PNAS. 2006;35:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 51.Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–11. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- 52.Bjørnebekk A, Mathé AA, Brené S. Isolated Flinders Sensitive Line rats have decreased dopamine D2 receptor mRNA. NeuroReport. 2007;18:1039–43. doi: 10.1097/WNR.0b013e3281668bf7. [DOI] [PubMed] [Google Scholar]


