Abstract
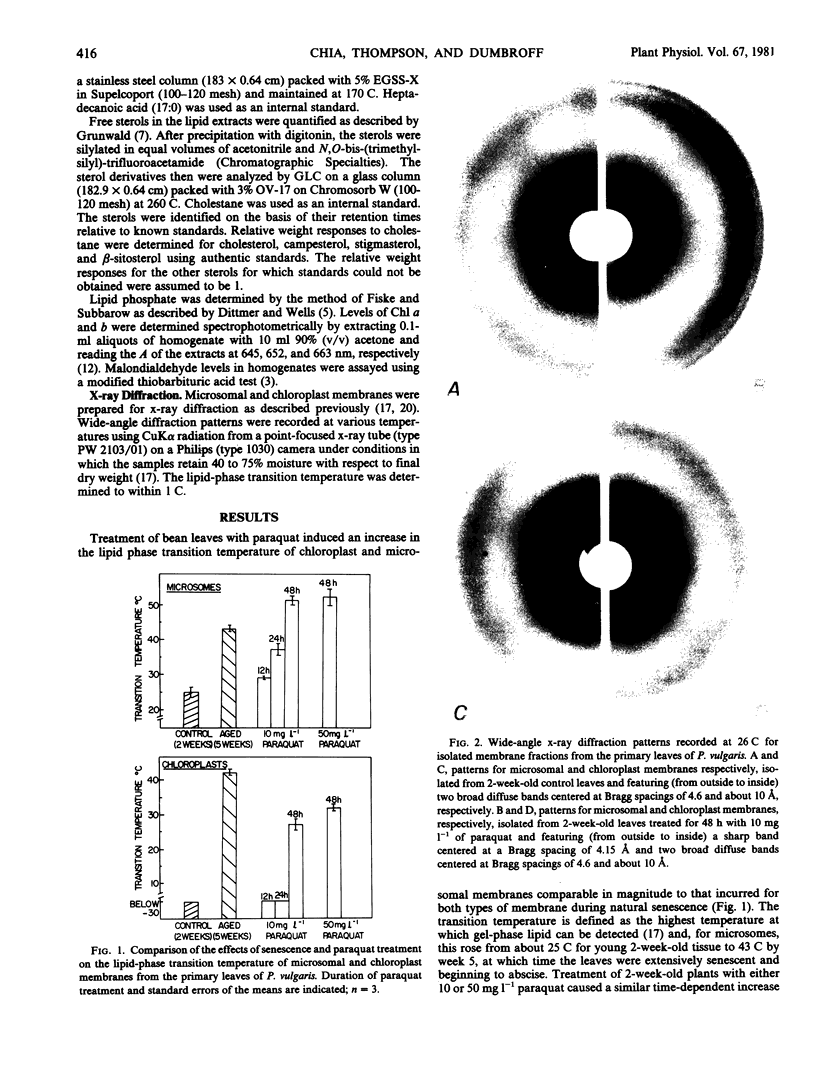
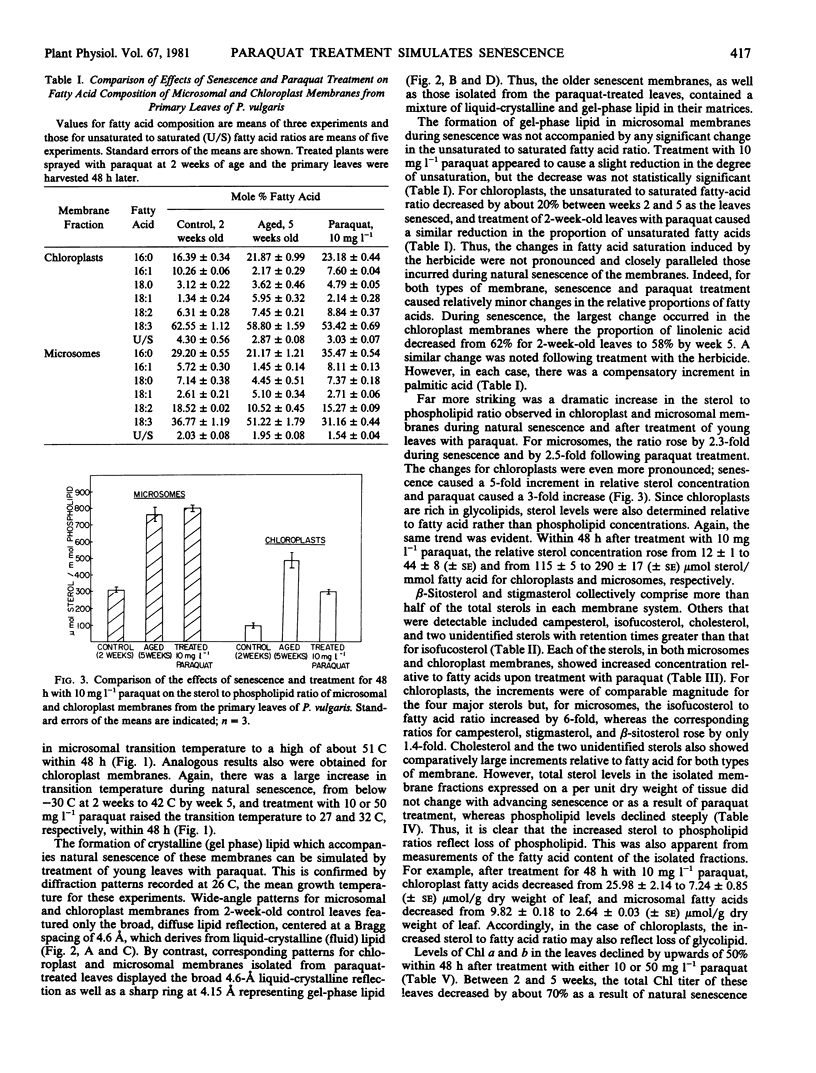
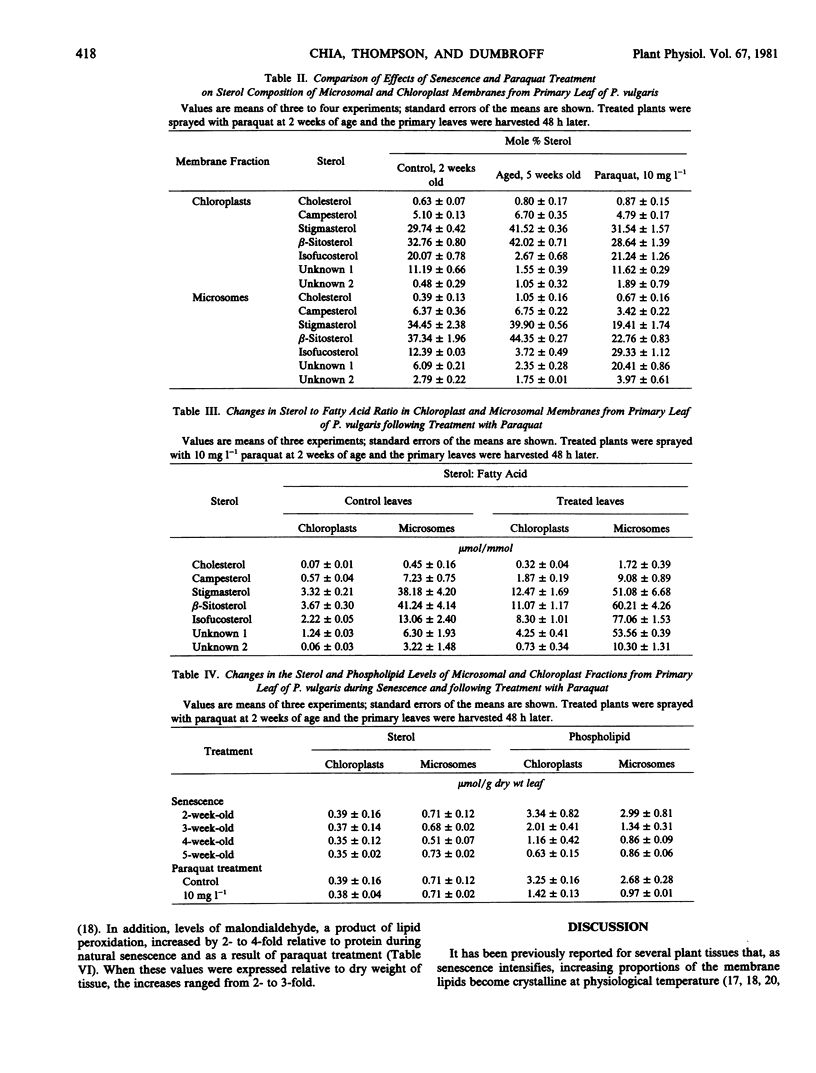
Chloroplast and microsomal membranes from the primary leaf of bean acquired increasing proportions of gel phase lipid as the tissue senesced. The lipid-phase transition temperature for microsomes rose from about 25 to 43 C and that for chloroplasts rose from below −30 C to about 52 C within 5 weeks of planting. This was accompanied by large increases (2- to 4-fold) in the sterol to phospholipid ratio of the membranes, which reflected breakdown of phospholipid. Changes in fatty acid saturation were of insufficient magnitude to account for the rise in transition temperature. All of these senescence-related changes in chloroplast and microsomal membranes were also induced by treating young, 2-week-old-plants with 10 milligrams per liter paraquat. Within 48 hours of treatment, the transition temperature rose from 25 to 57 C for microsomes and from below −30 to 24 C for chloroplasts. The membranes sustained only small changes in fatty acid saturation, comparable to those incurred during natural senescence, and there was a selective loss of phospholipid, resulting in augmented sterol to phospholipid ratios. Malondialdehyde, a product of lipid peroxidation, rose by 2- to 3-fold in both senescing and paraquat-treated leaves. Paraquat is known to form cation redicals that react with O2 to produce O2− and has been implicated as an agent of lipid peroxidation. Accordingly, these observations suggest that membrane deterioration during natural senescence may be due in part to free radical damage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. R., Bolton J. R. Superoxide formation in spinach chloroplasts: electron spin resonance detection by spin trapping. Biochem Biophys Res Commun. 1975 Jan 2;64(3):803–807. doi: 10.1016/0006-291x(75)90118-7. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marx J. L. Aging research (I): cellular theories of senescence. Science. 1974 Dec 20;186(4169):1105–1107. doi: 10.1126/science.186.4169.1105. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Lipid crystallization in senescent membranes from cotyledons. Plant Physiol. 1977 May;59(5):803–807. doi: 10.1104/pp.59.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase Behavior of Chloroplast and Microsomal Membranes during Leaf Senescence. Plant Physiol. 1978 Apr;61(4):639–643. doi: 10.1104/pp.61.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase properties of senescing plant membranes: role of the neutral lipids. Biochim Biophys Acta. 1979 Jan 5;550(1):48–58. doi: 10.1016/0005-2736(79)90114-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. In vitro simulation of senescence-related membrane damage by ozone-induced lipid peroxidation. Nature. 1980 Jan 31;283(5746):504–506. doi: 10.1038/283504a0. [DOI] [PubMed] [Google Scholar]
- Peiser G. D., Yang S. F. Ethylene and Ethane Production from Sulfur Dioxide-injured Plants. Plant Physiol. 1979 Jan;63(1):142–145. doi: 10.1104/pp.63.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijck P. W., Ververgaert P. H., Verkleij A. J., Van Deenen L. L., De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Nov 3;406(4):465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]



