Abstract
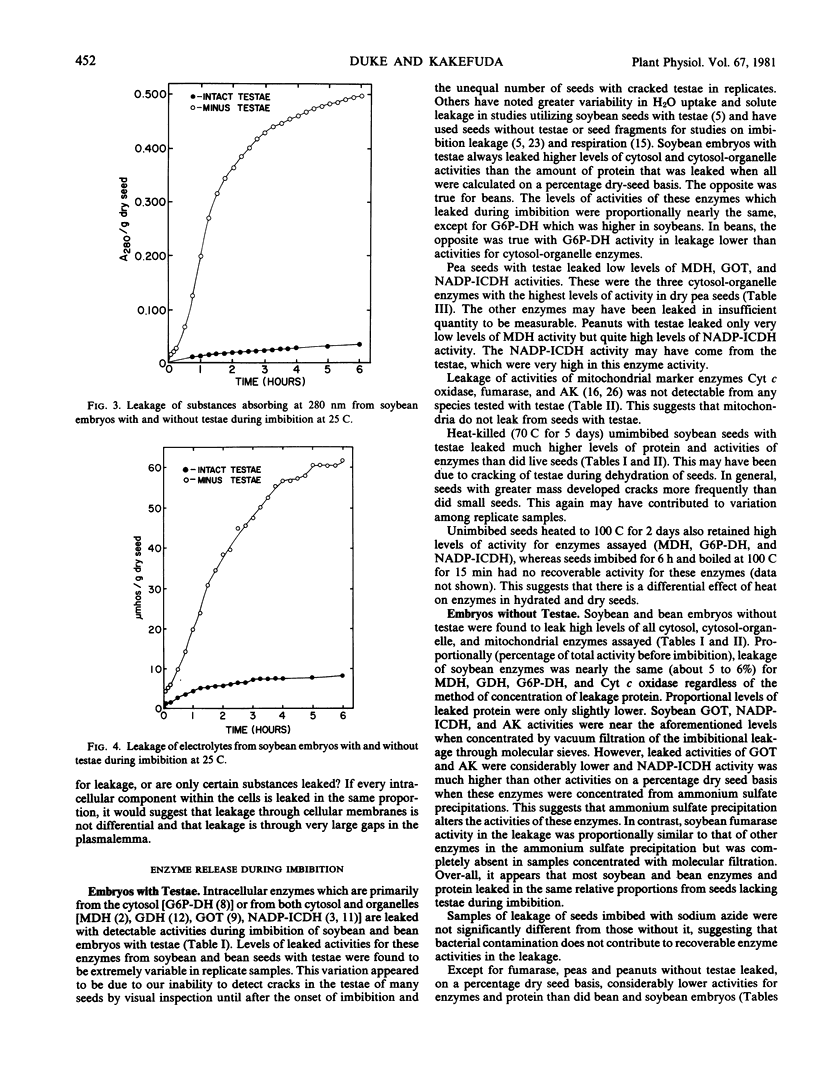
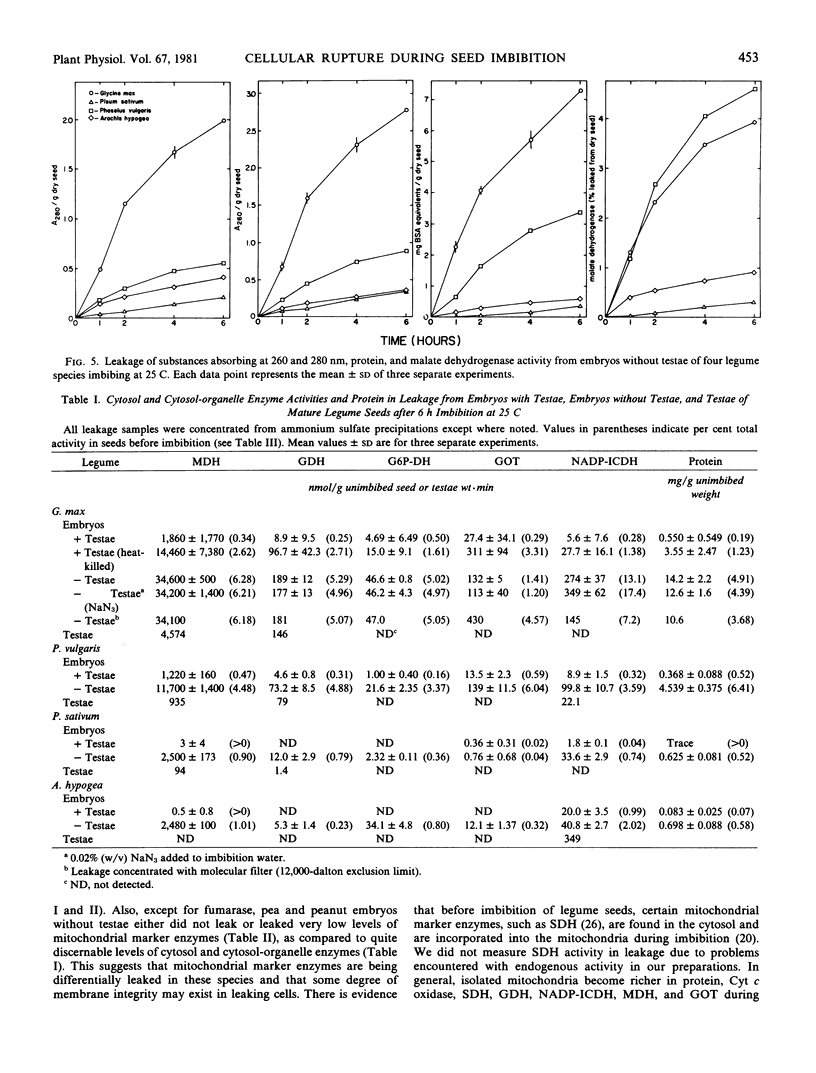
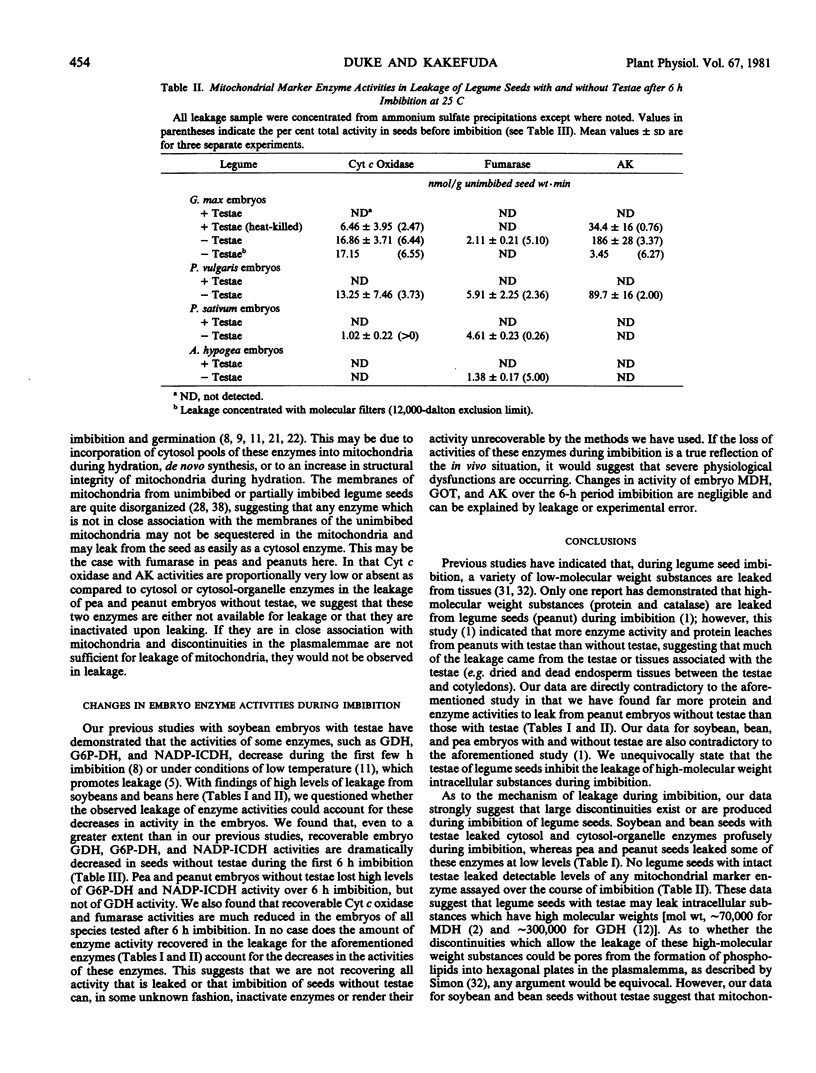
Studies with the seeds of soybean, navy bean, pea, and peanut were made to determine the extent of leakage of intracellular enzymes during imbition. Embryos with intact testae from all four species were found to leak detectable activities of either intracellular enzymes of the cytosol (glucose-6-phosphate dehydrogenase) or enzymes found in both the cytosol and organelles (malate dehydrogenase, glutamate dehydrogenase, glutamate oxaloacetate transaminase, and NADP-isocitrate dehydrogenase) after 6 hours imbition at 25 C. Pea and peanut embryos with testae leaked considerably lower levels of activity for these enzymes than did those of soybean and bean. Leakage of mitochondrial marker enzymes (fumarase, cytochrome c oxidase, and adenylate kinase) was not detected from embryos with testae, suggesting that a differential diffusion of intracellular components out of cells occurred. Soybean and bean embryos without testae leaked high, and proportionally (per cent dry seed basis) similar, levels of all cytosol, cytosol-organelle, and mitochondrial marker enzymes and protein during imbibition, indicating that cell membranes were not differential to leakage and that they had ruptured. Pea and peanut embryos without testae leaked detectable activities of all cytosol and cytosol-organelle enzymes, although fumarase was the only detectable mitochondrial marker enzyme leaked, suggesting that some degree of differential leakage may have occurred in these species. The outermost layers of embryo cells of seeds without testae of all four species absorbed and sequestered the nonpermeating pigment Evan's blue after 5 to 15 minutes imbibition, indicating that membranes had ruptured. This occurred to a much lesser extent in seeds with intact testae. Both soybean and bean embryos without testae were observed to disintegrate during imbibition, whereas those of pea and peanut did not. These data indicate that seeds of certain legumes are susceptible to cellular rupture during imbibition when seed coats are damaged or missing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman E. J., Ikuma H., Stein H. J. Citric Acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976 Sep;58(3):426–432. doi: 10.1104/pp.58.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bramlage W. J., Leopold A. C., Parrish D. J. Chilling Stress to Soybeans during Imhibition. Plant Physiol. 1978 Apr;61(4):525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Free Amino Acid Pools during Germination. Plant Physiol. 1978 Oct;62(4):642–647. doi: 10.1104/pp.62.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Mitochondrial Respiration and Several Dehydrogenases during Imbibition and Germination. Plant Physiol. 1977 Nov;60(5):716–722. doi: 10.1104/pp.60.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L. A. The effect soaking pea seeds with or without seedcoats has on seedling growth. Plant Physiol. 1968 Feb;43(2):255–259. doi: 10.1104/pp.43.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Musgrave M. E. Respiratory changes with chilling injury of soybeans. Plant Physiol. 1979 Nov;64(5):702–705. doi: 10.1104/pp.64.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. R. Nutritive Role of the Seedcoats during Embryo Development in Pisum sativum L. Plant Physiol. 1979 Nov;64(5):763–769. doi: 10.1104/pp.64.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N., Sugimoto I., Asahi T. Presence in Dry Pea Cotyledons of Soluble Succinate Dehydrogenase That Is Assembled into the Mitochondrial Inner Membrane during Seed Imbibition. Plant Physiol. 1980 Feb;65(2):229–233. doi: 10.1104/pp.65.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Transient changes during soybean imbibition. Plant Physiol. 1977 Jun;59(6):1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Asahi T. Biochemical Properties of Mitochondrial Membrane from Dry Pea Seeds and Changes in the Properties during Imbibition. Plant Physiol. 1975 Dec;56(6):816–820. doi: 10.1104/pp.56.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Rapid isolation of mesophyll cells from leaves of soybean for photosynthetic studies. Plant Physiol. 1977 Apr;59(4):587–590. doi: 10.1104/pp.59.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek L. Distribution and Properties of a Potassium-dependent Asparaginase Isolated from Developing Seeds of Pisum sativum and Other Plants. Plant Physiol. 1980 Jan;65(1):22–26. doi: 10.1104/pp.65.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner P. E., Parlange J. Y. Water uptake and water diffusivity of seeds. Plant Physiol. 1976 Feb;57(2):153–156. doi: 10.1104/pp.57.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]