Abstract
BACKGROUND
Umbilical-cord blood has been used as the source of hematopoietic stem cells in an estimated 30,000 transplants. The limited number of hematopoietic cells in a single cord-blood unit prevents its use in recipients with larger body mass and results in delayed hematopoietic recovery and higher mortality. Therefore, we hypothesized that the greater numbers of hematopoietic cells in two units of cord blood would be associated with improved outcomes after transplantation.
METHODS
Between December 1, 2006, and February 24, 2012, a total of 224 patients 1 to 21 years of age with hematologic cancer were randomly assigned to undergo double-unit (111 patients) or single-unit (113 patients) cord-blood transplantation after a uniform myeloablative conditioning regimen and immunoprophylaxis for graft-versus-host disease (GVHD). The primary end point was 1-year overall survival.
RESULTS
Treatment groups were matched for age, sex, self-reported race (white vs. nonwhite), performance status, degree of donor–recipient HLA matching, and disease type and status at transplantation. The 1-year overall survival rate was 65% (95% confidence interval [CI], 56 to 74) and 73% (95% CI, 63 to 80) among recipients of double and single cord-blood units, respectively (P = 0.17). Similar outcomes in the two groups were also observed with respect to the rates of disease-free survival, neutrophil recovery, transplantation-related death, relapse, infections, immunologic reconstitution, and grade II–IV acute GVHD. However, improved platelet recovery and lower incidences of grade III and IV acute and extensive chronic GVHD were observed among recipients of a single cord-blood unit.
CONCLUSIONS
We found that among children and adolescents with hematologic cancer, survival rates were similar after single-unit and double-unit cord-blood transplantation; however, a single-unit cord-blood transplant was associated with better platelet recovery and a lower risk of GVHD.
Since 1993, unrelated-donor umbilical-cord blood has been used as the source of hematopoietic stem cells for transplantation in an estimated 30,000 patients with malignant and nonmalignant diseases.1 As compared with stem-cell grafts from adult donors, cord blood has the advantages of more rapid availability, relative absence of donor attrition, and, after transplantation, a reduced risk of graft-versus-host disease (GVHD) despite donor–recipient HLA disparity.2,3 In addition, less restriction on HLA matching permits greater use of cord blood for members of racial minorities, who are less likely to have a suitably HLA-matched volunteer adult donor.4 However, the use of cord blood is limited by the finite number of hematopoietic progenitor cells that can be collected from a placenta, which restricts its application primarily to children and smaller adults. The doses of cryopreserved nucleated cells, colony-forming units, and CD34+ cells have been reported to be major determinants of neutrophil recovery and survival.5–9 For this reason, various strategies have been explored to increase the number of hematopoietic stem cells in a cord-blood graft, including the infusion of two cord-blood units from different partially HLA-matched donors.
On the basis of promising early studies involving single centers10,11 and registries,5,6 the National Heart, Lung, and Blood Institute proposed the Cord Blood Transplantation (COBLT) study, a phase 2 trial to determine whether cord blood from an unrelated donor could serve as an adequate source of hematopoietic stem cells. For children older than 2 years of age with hematologic cancer, the conditioning regimen consisted of 1350 cGy of total-body irradiation, 120 mg of cyclophosphamide per kilogram of body weight, and 90 mg of antithymocyte globulin (equine) per kilogram, along with cyclosporine and methylprednisolone for GVHD prophylaxis.12 The probability of disease-free survival in this group was 49.5% at 2 years, the incidence of neutrophil recovery at day 42 was 79.9%, the incidence of grade II–IV acute GVHD at day 100 was 19.5%, the incidence of chronic GVHD at 2 years was 20.8%, and the incidence of relapse at 2 years was 19.9%.12
Double-unit cord-blood transplantation, mainly for adults, was established during this same period at the University of Minnesota.5–7,13,14 The use of two partially HLA-matched cord-blood units was a straightforward strategy for achieving the desired cell dose of at least 2.5×107 nucleated cells per kilogram of body weight.15–17 Early results of studies involving patients with hematologic cancer who received a transplant of two cord-blood units after a modified conditioning regimen and GVHD prophylaxis (i.e., fludarabine rather than antithymocyte globulin and cyclosporine and mycophenolate mofetil rather than cyclosporine and methylprednisolone) suggested that engraftment and survival were better than those observed among children in the COBLT study.12,14
To determine whether two partially HLA-matched cord-blood units were better than one, a prospective clinical trial was developed by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0501) and conducted in collaboration with the Children’s Oncology Group.
METHODS
STUDY DESIGN
We conducted an open-label, phase 3, multicenter, randomized trial. Randomization was performed at the EMMES Corporation in a 1:1 ratio, with the use of random block sizes, and was stratified according to patient age and the expected volume of cord-blood transplantation procedures performed at each center. The primary end point was 1-year overall survival, as assessed by means of an intention-to-treat analysis. Prespecified secondary end points included incidences of neutrophil and platelet recovery, acute and chronic GVHD, transplantation-related mortality, disease-free survival, infection, and relapse. Other end points included an evaluation of immunologic reconstitution. Enrollment began on October 16, 2006, and ended after the accrual goal was met, on February 29, 2012; randomization began on December 1, 2006, and ended on February 24, 2012. The analysis includes data collected as of August 12, 2013. The median follow-up for surviving patients is 26.9 months (range, 0.3 to 48.6); the median follow-up for surviving patients after randomization into the double-unit group is 27.2 months, and that after randomization into the single-unit group is 26.7 months (P = 0.17).
PATIENTS
Eligible patients were 1 to 21 years of age (i.e., at least 1 and less than 22 years of age) and had high-risk acute leukemia, chronic myeloid leukemia, or myelodysplastic syndrome, a performance status of 70 or higher (on the Karnofsky scale for patients 16 to 21 years of age or the Lansky scale for patients younger than 16 years of age, with both scales ranging from 0 to 100 and lower scores indicating greater disability), adequate organ function, and availability of two cord-blood units that had adequate cell doses and were HLA-matched both to the patient and each other at at least four of six loci (i.e., a match score of 4/6, 5/6, or 6/6), considering only HLA-A and HLA-B at the antigen level and HLA-DRB1 at the allele level. At cryopreservation, the primary unit had to contain at least 2.5×107 nucleated cells per kilogram of the recipient’s actual body weight, with the second unit containing at least 1.5×107 nucleated cells per kilogram of body weight. Details of the inclusion and exclusion criteria are provided in the treatment protocol, available with the full text of this article at NEJM.org. Written informed consent was provided in all cases, either by the parents or guardians for children younger than 18 years of age or by the patients if they were 18 to 21 years of age. Assent was obtained from children who were 8 to 17 years of age. A total of 111 patients were randomly assigned to receive a double-unit transplant, with 108 undergoing transplantation (2 did not proceed to the transplantation stage, and 1 left the study); 113 patients were randomly assigned to receive a single-unit transplant, with 112 undergoing transplantation (1 did not proceed to the transplantation stage). One patient randomly assigned to receive a double-unit transplant and 2 patients randomly assigned to receive a single-unit transplant crossed over to the other treatment group.
TREATMENT
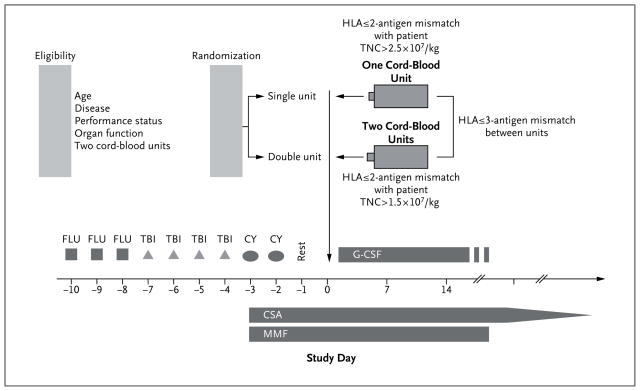
Patients were treated with 75 mg of fludarabine per square meter of body-surface area, 1320 cGy of total-body irradiation, and 120 mg of cyclophosphamide per kilogram, followed by the infusion of one or two cord-blood units (Fig. 1). Prophylaxis for GVHD consisted of cyclosporine administered through day 180, with subsequent tapering, and mycophenolate mofetil administered until day 45 (or longer if there was active GVHD).
Figure 1. Treatment Plan.
Eligible patients were at least 1 and less than 22 years of age and had a performance status of 70 or higher on the Lansky scale or the Karnofsky scale, adequate organ function, two HLA-matched cord-blood units of adequate cell dose available, and a diagnosis of high-risk acute leukemia, chronic myeloid leukemia, or myelodysplastic syndrome. CSA denotes cyclosporine, CY cyclophosphamide, FLU fludarabine, G-CSF granulocyte colony-stimulating factor, MMF mycophenolate mofetil, TBI total-body irradiation, and TNC total nucleated cells.
The protocol was designed and written by the authors, who vouch for the accuracy of the data and adherence to the protocol. No one who is not an author contributed to the writing of the manuscript. A protocol review committee appointed by the National Heart, Lung, and Blood Institute approved the research protocol, which was subsequently approved by the institutional review board at each participating center. An end-point review committee whose members were unaware of the treatment assignments adjudicated the primary causes of death, the presence of engraftment syndrome (defined on the basis of the development of fever and rash before day 28 without pathologic evidence of GVHD and effectively managed with topical or systemic glucocorticoids),18 and the maximum grade of acute and chronic GVHD.19,20 A data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute reviewed adverse events and outcome data at periodic intervals and the final analysis.
STATISTICAL ANALYSIS
The underlying hypothesis was that recipients of a double-unit cord-blood transplant would have a survival advantage over recipients of a single-unit transplant. The primary analysis was a comparison of overall survival at 1 year after randomization. The sample-size target of 110 patients in each treatment group was expected to be sufficient to maintain a type I error of 5% across all planned interim analyses, as well as to provide more than 86% statistical power for a two-sided test to detect an increase in the proportion of patients surviving at 1 year from 57% among recipients who received a single-unit graft to 77% among those who received a double-unit graft. Survival times were calculated from the day of randomization. All patients were followed for at least 1 year or until death. The only exception was a single patient who was randomly assigned to a treatment group but did not undergo transplantation (follow-up time, 0.3 months).
Categorical variables were compared with the use of the Pearson chi-square statistic, and comparisons with smaller numbers were analyzed with Fisher’s exact test. The Mann–Whitney test was used to analyze continuous variables in the two groups, and the Kaplan–Meier method was used to analyze the probability of overall and disease-free survival.21 The log-rank statistic was used for comparisons between the treatment groups. Overall and disease-free survival were evaluated in prespecified subgroup analyses involving Cox models.22 The subgroups tested were based on characteristics of recipients (age, sex, self-reported race, performance status, diagnosis, disease status, and results of serologic testing for cytomegalovirus) and the degree of donor–recipient HLA matching. The cumulative-incidence method was used to estimate the incidence of events in the presence of competing risks for neutrophil and platelet recovery, acute and chronic GVHD, treatment-related mortality, infection, and relapse.23 All P values are two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. Analyses were performed with the use of SAS software, version 9.2 (SAS Institute), with the exception of the cumulative-incidence analyses and Kaplan–Meier survival analyses, which were performed with the use of the cmprsk package of R software, version 2.13.1.
RESULTS
CHARACTERISTICS OF PATIENTS AND DONORS
The baseline characteristics of patients randomly assigned to receive a double-unit (111 patients) or single-unit (113 patients) transplant are shown in Table 1. The two treatment groups were balanced with respect to all baseline covariates, including sex, age, results of serologic testing for cytomegalovirus, disease type and status at transplantation, performance score, degree of donor–recipient HLA matching and ABO matching, and self-reported Hispanic or Latino background and race or ethnic group. The median age at transplantation was 9.9 years (range, 1.1 to 21.2) for double-unit recipients and 10.4 years (range, 1.4 to 21.4) for single-unit recipients, and the median body weight was 37.0 kg (range, 9.7 to 81.7) and 35.7 kg (range, 10.0 to 93.0), respectively. Each patient received a graft containing more than 2.5×107 nucleated cells per kilogram, as specified in the study protocol; the median number of infused total nucleated cells per kilogram was 7.2×107 for recipients of double-unit grafts and 3.9×107 for recipients of single-unit grafts, and the number of infused CD34+ cells per kilogram was 3.7×105 and 1.9×105, respectively (Table 2).
Table 1.
Characteristics of the Patients According to Randomly Assigned Treatment Group.*
| Characteristic | Double-Unit Treatment Group (N = 111) | Single-Unit Treatment Group (N = 113) |
|---|---|---|
| Acute myeloid leukemia diagnosis — no. (%)† | 38 (34) | 39 (35) |
| First complete remission | 14 (37) | 17 (44) |
| Second or later complete remission | 19 (50) | 16 (41) |
| First relapse | 3 (8) | 1 (3) |
| Morphologic complete remission before complete-blood-count recovery | 2 (5) | 2 (5) |
| Secondary or therapy-related | 0 | 3 (8) |
| Acute lymphocytic leukemia diagnosis — no. (%)† | 58 (52) | 61 (54) |
| First complete remission | 19 (33) | 19 (31) |
| Second complete remission | 28 (48) | 29 (48) |
| Subsequent complete remission | 10 (17) | 13 (21) |
| Morphologic complete remission before complete-blood-count recovery | 1 (2) | 0 |
| Acute biphenotypic leukemia diagnosis — no. (%)† | 2 (2) | 6 (5) |
| First complete remission | 1 (50) | 5 (83) |
| Second complete remission | 1 (50) | 1 (17) |
| Acute undifferentiated leukemia diagnosis — no. (%) | 0 | 1 (1) |
| Chronic myeloid leukemia diagnosis, second chronic phase — no. (%) | 0 | 1 (1) |
| Myelodysplastic syndrome diagnosis — no. (%)† | 13 (12) | 5 (4) |
| Refractory anemia | 0 | 2 (40) |
| Refractory cytopenia with multilineage dysplasia | 3 (23) | 2 (40) |
| Refractory anemia with excess blasts 1‡ | 3 (23) | 0 |
| Refractory anemia with excess blasts 2§ | 4 (31) | 0 |
| Myelodysplastic syndrome, unclassified | 3 (23) | 1 (20) |
| Cytomegalovirus serologic status of the recipient — no. (%) | ||
| Positive | 66 (59) | 58 (51) |
| Negative | 39 (35) | 51 (45) |
| Inconclusive | 1 (1) | 1 (1) |
| Unknown | 5 (5) | 3 (3) |
| Performance status — no. (%)¶ | ||
| 100 | 57 (51) | 58 (51) |
| 90 | 43 (39) | 38 (34) |
| 80 | 9 (8) | 13 (12) |
| 70 | 2 (2) | 4 (4) |
| Sex — no. (%) | ||
| Female | 41 (37) | 55 (49) |
| Male | 70 (63) | 58 (51) |
| Hispanic or Latino background — no. (%)|| | ||
| Hispanic or Latino | 20 (18) | 22 (19) |
| Not Hispanic or Latino | 88 (79) | 88 (78) |
| Unknown | 1 (1) | 1 (1) |
| Not reported | 2 (2) | 2 (2) |
| Race or ethnic group — no. (%)|| | ||
| American Indian or Alaskan Native | 0 | 1 (1) |
| Asian | 5 (5) | 4 (4) |
| Hawaiian or Pacific Islander | 0 | 0 |
| Black or African American | 11 (10) | 13 (12) |
| White | 85 (77) | 80 (71) |
| More than one race | 3 (3) | 3 (3) |
| Other | 3 (3) | 1 (1) |
| Unknown | 4 (4) | 7 (6) |
| Not reported | 0 | 4 (4) |
Percentages may not add to 100 because of rounding.
The statuses listed are the statuses at the time of transplantation.
This condition was defined by a blast percentage of 5 to 10%.
This condition was defined by a blast percentage of 11 to 20%.
Performance status was determined on the Lansky scale for children younger than 16 years of age and the Karnofsky scale for patients 16 to 21 years of age (on both scales, a score of 100 denotes no symptoms, and lower scores denote greater disability).
Hispanic or Latino background and race or ethnic group were self-reported.
Table 2.
Graft Characteristics According to Treatment Group among Patients Who Underwent Transplantation.*
| Characteristic | Double-Unit Treatment Group (N = 108) | Single-Unit Treatment Group (N = 112) | ||
|---|---|---|---|---|
| Unit A | Unit B | Combined | ||
|
| ||||
| HLA match score — no. of patients (%) | ||||
|
| ||||
| 3/6 | 2 (2) | 1 (1) | 2 (2)† | 1 (1) |
|
| ||||
| 4/6 | 33 (31) | 43 (40) | 48 (44)† | 44 (39) |
|
| ||||
| 5/6 | 59 (55) | 43 (40) | 46 (43)† | 51 (46) |
|
| ||||
| 6/6 | 14 (13) | 21 (19) | 12 (11)† | 16 (14) |
|
| ||||
| Cell dose — cells/kg | ||||
|
| ||||
| Cryopreserved total nucleated cells | 5.0×107±3.1×107 | 4.1×107±3.5×107 | 9.1×107 | 4.8×107±3.4×107 |
|
| ||||
| Infused total nucleated cells | 4.0×107±3.0×107 | 3.2×107±3.4×107 | 7.2×107 | 3.9×107±3.0×107 |
|
| ||||
| Cryopreserved CD34+ cells | 2.0×105±2.2×105 | 1.8×105±1.4×105 | 3.7×105 | 1.9×105±3.6×105 |
|
| ||||
| ABO match — no. of patients (%) | ||||
|
| ||||
| Major mismatch | 25 (23) | 31 (29) | — | 30 (27) |
|
| ||||
| Minor mismatch | 35 (32) | 29 (27) | — | 24 (21) |
|
| ||||
| Bidirectional mismatch | 8 (7) | 4 (4) | — | 10 (9) |
|
| ||||
| No mismatch | 36 (33) | 38 (35) | — | 44 (39) |
|
| ||||
| Unknown | 4 (4) | 6 (6) | — | 4 (4) |
Plus–minus values are means ± SD. Percentages may not add to 100 because of rounding.
The combined match score for patients in the double-unit treatment group is the minimum HLA match between the first unit and the recipient or the second unit and the recipient. For example, if Unit A was a 6/6 match with the recipient and Unit B was a 4/6 match, the match score assigned for that recipient was 4/6.
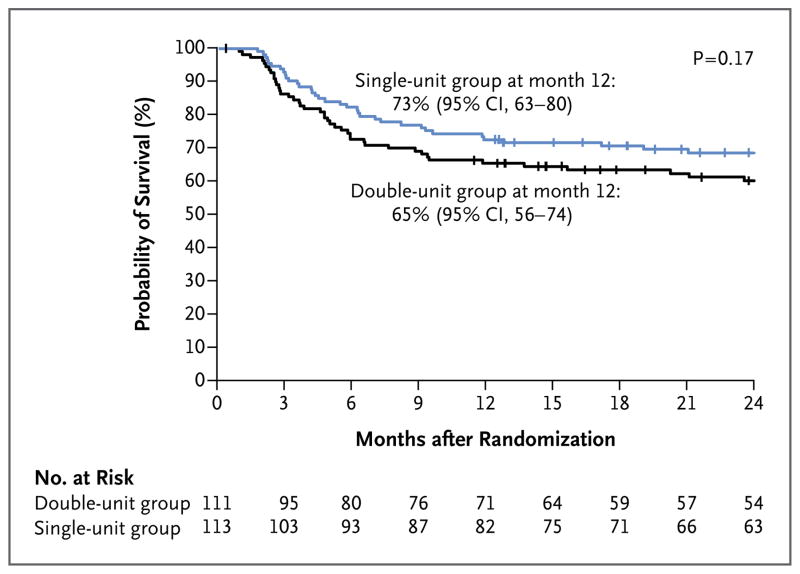
OVERALL SURVIVAL
The primary end point was 1-year overall survival after randomization. The overall survival rate was 65% (95% confidence interval [CI], 56 to 74) among double-unit recipients and 73% (95% CI, 63 to 80) among single-unit recipients (P = 0.17) (Fig. 2A). In a multivariate analysis, the risk of death did not differ significantly between recipients of double-unit transplants and recipients of single-unit transplants (hazard ratio, 1.34; 95% CI, 0.86 to 2.09; P = 0.20, with the single-unit group used as the referent), even after adjustment for disease type (acute myeloid leukemia vs. other), which was the only factor associated with an increased risk of death (Table S1 in the Supplementary Appendix, available at NEJM.org). In subgroup analyses, self-reported race (white vs. nonwhite), sex, age, and HLA matching had no discernible effect on survival (data not shown).
Figure 2. Survival after Randomization.
The probability of overall survival is shown for the two treatment groups in the intention-to-treat analysis.
DISEASE-FREE SURVIVAL
Among the patients who underwent transplantation, the 1-year disease-free survival rate was 64% (95% CI, 54 to 72) among double-unit recipients and 70% (95% CI, 60 to 77) among single-unit recipients (P = 0.11). In a multivariate analysis, the risk of relapse or death (i.e., the risk of treatment failure, the inverse of the disease-free survival rate) did not differ significantly between double-unit recipients and single-unit recipients (hazard ratio, 1.48; 95% CI, 0.95 to 2.29; P = 0.08, with the single-unit group used as the referent), even after adjustment for leukemia type, self-reported race (white vs. nonwhite), and HLA-match score, which were the factors associated with disease-free survival. As shown in Table S1 in the Supplementary Appendix, in subgroup analyses, disease type (acute myeloid leukemia vs. other), self-reported race (nonwhite vs. white), and HLA match (match score, 5/6 or 6/6 vs. 4/6) were associated with treatment failure.
HEMATOPOIETIC RECOVERY
The incidence of neutrophil recovery was 88% (95% CI, 82 to 94) among double-unit recipients and 89% (95% CI, 83 to 95) among single-unit recipients (P = 0.29), at a median of 23 days (range, 11 to 133) and 21 days (range, 11 to 62) after transplantation, respectively. No patients in either group had secondary graft failure. The incidence of platelet recovery, however, was significantly higher and recovery occurred more quickly among recipients of a single-unit transplant than among recipients of a double-unit transplant (incidence, 76% [95% CI, 68 to 85] vs. 65% [95% CI, 56 to 74]; P=0.04). The median time to platelet recovery was 58 days (range, 28 to 295) in the single-unit group and 84 days (range, 22 to 716) in the double-unit group.
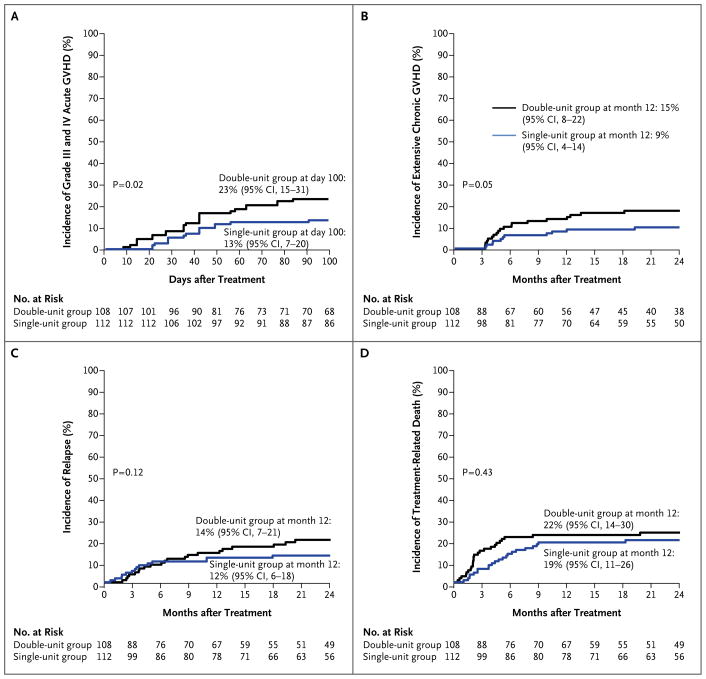
ACUTE AND CHRONIC GVHD
The incidence of grade II–IV acute GVHD was similar in the two treatment groups (P = 0.78); however, recipients of double-unit transplants had a higher incidence of grade III and IV acute GVHD (23% [95% CI, 15 to 31] vs. 13% [95% CI, 7 to 20], P = 0.02) (Fig. 3A). The incidence of any chronic GVHD at 1 year after transplantation was 32% (95% CI, 23 to 40) after double-unit transplantation and 30% (95% CI, 22 to 39) after single-unit transplantation (P = 0.51), with a higher incidence of extensive disease after double-unit transplantation (15% [95% CI, 8 to 22] vs. 9% [95% CI, 4 to 14], P = 0.05) (Fig. 3B).
Figure 3.
Incidence of Major Complications.
INFECTIONS, IMMUNOLOGIC RECOVERY, RELAPSE, AND TREATMENT-RELATED MORTALITY
A total of 201 of the 220 patients who underwent transplantation (91%) had at least one infectious episode. Among the 220 patients, 120 (55%) had a severe infection, and 32 (15%) had a life-threatening or fatal infection. No significant differences were observed between the treatment groups.
Absolute numbers of CD4+, CD8+, CD19+, and CD16/CD56+ cells were determined on days 100, 180, and 365 after cord-blood transplantation. The recovery rate for T-cell, B-cell, and natural killer–cell populations were similar in the two groups (Table S2 and Fig. S1 in the Supplementary Appendix). In addition, IgG, IgM, IgA, and IgE levels were similar in the two treatment groups at each time point.
The incidences of relapse and treatment-related death were also similar in the two treatment groups (Fig. 2C and 2D). The incidence of relapse at 1 year was low in both groups: 14% (95% CI, 7 to 21) in recipients of a double-unit transplant and 12% (95% CI, 6 to 18) in recipients of a single-unit transplant (P = 0.12). The incidence of treatment-related death at 1 year was 22% (95% CI, 14 to 30) among double-unit recipients and 19% (95% CI, 11 to 26) among single-unit recipients (P = 0.43). The primary causes of death were similar in recipients of double-unit and single-unit transplants and included GVHD (32% and 34%, respectively), infection (12% and 9%), and recurrent disease (37% in both groups).
DISCUSSION
In an attempt to broaden the application of cord-blood transplantation to adults and improve the outcomes of transplantation generally, a pilot study evaluating the safety and potential efficacy of double-unit cord-blood transplantation was performed, with very promising early results.16 Because patients enrolled in the pilot study also received a modified conditioning regimen and GVHD prophylaxis, it was possible that the apparent benefit of double-unit transplantation in that study may have been due to an improved treatment regimen, improved supportive care, enhanced quality of the cord-blood units, or other factors and not due to the use of the double-unit graft. Therefore, the aim of the current trial was to determine the effect of the graft composition (double-unit vs. single-unit) on 1-year survival among patients who received the same conditioning and GVHD prophylaxis regimen as that used in the pilot study.16 Since many adults would not have had an adequate single unit, conventionally defined as at least 2.5×107 nucleated cells per kilogram,24 the current trial involved children, and an adequate single unit and adequate double unit were available for all the patients.
There were several important findings in this randomized trial. First, recipients of double-unit transplants had no engraftment or survival benefit over recipients of an adequately dosed single-unit transplant. However, patients in both treatment groups in this study had better engraftment and survival outcomes than those previously reported in the COBLT trial, which suggests that perhaps some other factor, such as the use of fludarabine rather than antithymocyte globulin, the use of mycophenolate mofetil rather than methylprednisolone, or a combination of the two, was responsible for the promising findings in the pilot studies. Only a diagnosis of acute myeloid leukemia was associated with a lower rate of survival.
In addition, poorer platelet recovery and higher rates of grade III and IV acute and extensive chronic GVHD were observed after double-unit transplantation. These findings contrast with those reported in studies involving single centers and registries, in which platelet recovery was not affected or was marginally improved after double-unit transplantation, and rates of grade III and IV GVHD and extensive chronic GVHD were similar to those observed after single-unit transplantation.17,25–29 Perhaps the poorer platelet recovery after double-unit transplantation in this study was due to the higher rate of GVHD. Still, it must be recognized that most previous studies of double-unit transplantation involved adults, for whom the graft cell doses were lower. Although CD3+ cell dose has not been shown to affect the risk of GVHD, perhaps a threshold dose was achieved in these younger recipients, as compared with what can be achieved in larger adults, which resulted in higher rates of severe acute and chronic GVHD in these younger recipients. Further evaluation is not possible, since data on the infused graft CD3+ cell dose were not available. A higher incidence of grade II but not of grade III or IV acute GVHD after double-unit transplantation has been reported previously.29
In this analysis, the risk of relapse was similar in the two treatment groups. Despite a higher incidence of GVHD, an enhanced graft-versus-leukemia effect was not observed after double-unit transplantation in our study, a finding that is in contrast to that in some studies performed at single centers or described in registry reports.26–28,30,31 Whether the outcome in this study as compared with others reflects differences in disease or disease risk (e.g., greater proportions of adults with acute myeloid leukemia or increased testing for minimal residual disease to guide the timing of transplantation in more recent years), the younger patient age, or another factor or factors that might be present in non-randomized trials, remains unknown.
Other findings emerged in exploratory analyses. A diagnosis of acute myeloid leukemia, non-white race, and a better HLA-match score were associated with a lower rate of disease-free survival after cord-blood transplantation. The potential beneficial effect of greater HLA mismatch is provocative and merits further examination in a larger population. However, these results indicate that double-unit cord-blood transplantation does not confer a survival advantage in children and adolescents.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute and the National Cancer Institute; ClinicalTrials.gov number, NCT00412360.
Supported by a grant from the National Heart, Lung, and Blood Institute and National Cancer Institute (U10-HL069294, to the Blood and Marrow Transplant Clinical Trials Network) and by grants from the National Cancer Institute (P01CA065493-21, to Dr. Wagner, and U10CA098543, to the Children’s Oncology Group).
We thank the principal investigators and the skilled teams at each of the participating sites (for a complete list, see the Supplementary Appendix), as well as Jason Thompson and Adam Mendizabal from the EMMES Corporation for their oversight of this multi-institutional trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol. 2009;147:246–61. doi: 10.1111/j.1365-2141.2009.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheuk DKL. Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J Transplant. 2013;3:99–112. doi: 10.5500/wjt.v3.i4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–8. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 7.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 8.Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–22. [PubMed] [Google Scholar]
- 9.Page KM, Zhang L, Mendizabal A, et al. Total colony-forming units are a strong, independent predictor of neutrophil and platelet engraftment after unrelated umbilical cord blood transplantation: a single-center analysis of 435 cord blood transplants. Biol Blood Marrow Transplant. 2011;17:1362–74. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 12.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–27. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 14.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–60. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 16.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 17.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 20.Weisdorf DJ, Hurd D, Carter S, et al. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biol Blood Marrow Transplant. 2003;9:512–8. doi: 10.1016/s1083-8791(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 22.Cox DR. Regression model and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–200. [Google Scholar]
- 23.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Bart T, Boo M, Balabanova S, et al. Impact of selection of cord blood units from the United States and Swiss registries on the cost of banking operations. Transfus Med Hemother. 2013;40:14–20. doi: 10.1159/000345690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–8. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sideri A, Neokleous N, Brunet De La Grange P, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96:1213–20. doi: 10.3324/haematol.2010.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri A, Sanz G, Bittencourt H, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28:779–86. doi: 10.1038/leu.2013.259. [DOI] [PubMed] [Google Scholar]
- 28.Rocha V, Crotta A, Ruggeri A, et al. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23:223–9. doi: 10.1016/j.beha.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–5. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labopin M, Ruggeri A, Gorin NC, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99:535–40. doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.