Abstract
A nonaqueous procedure using glycerol and 3-chloro-1,2-propanediol was developed for the isolation from maize of starch granules with associated metabolites. In this procedure, immature endosperm tissue was quickly frozen at −156 C, freeze-dried, homogenized in cold glycerol, filtered through Miracloth, and centrifuged through a higher density medium of 3-chloro-1,2-propanediol. The procedure was used to isolate starch granules from the endosperm of normal and the mutant amylose-extender dull waxy. Starch and water-soluble polysaccharide recovery was high with low cytoplasmic (RNA) and nuclear (DNA) contamination.
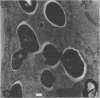
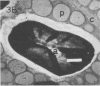
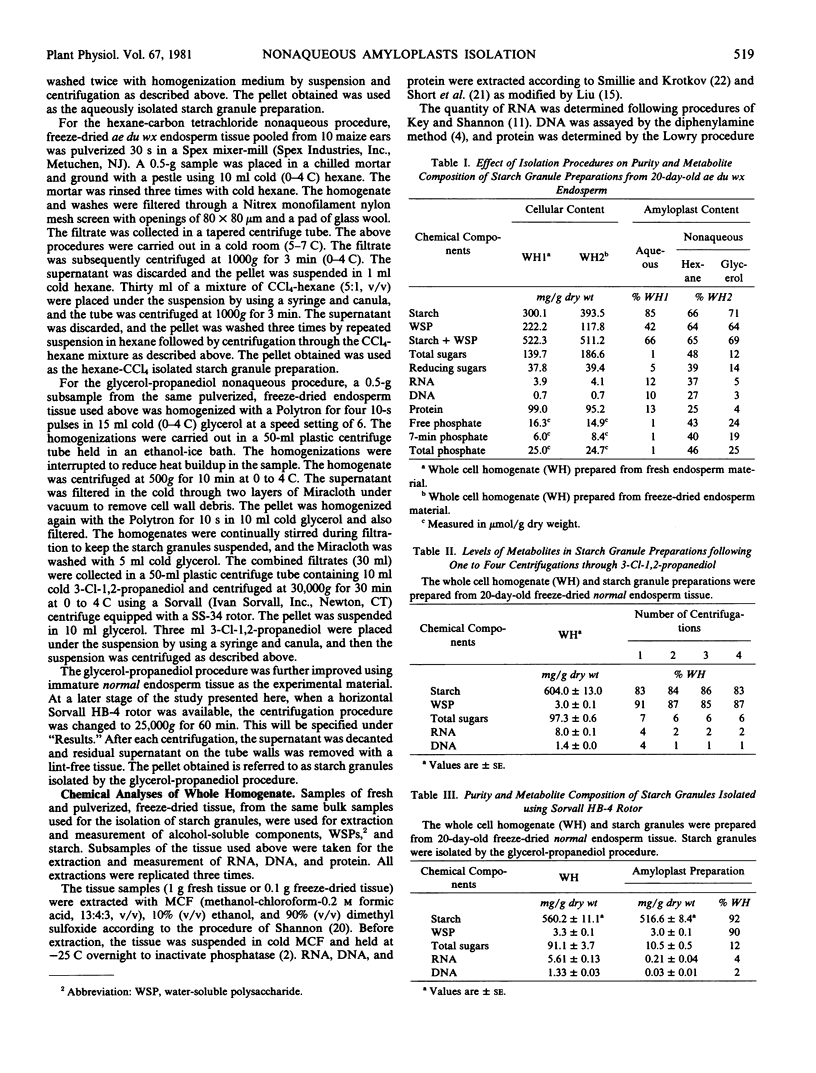
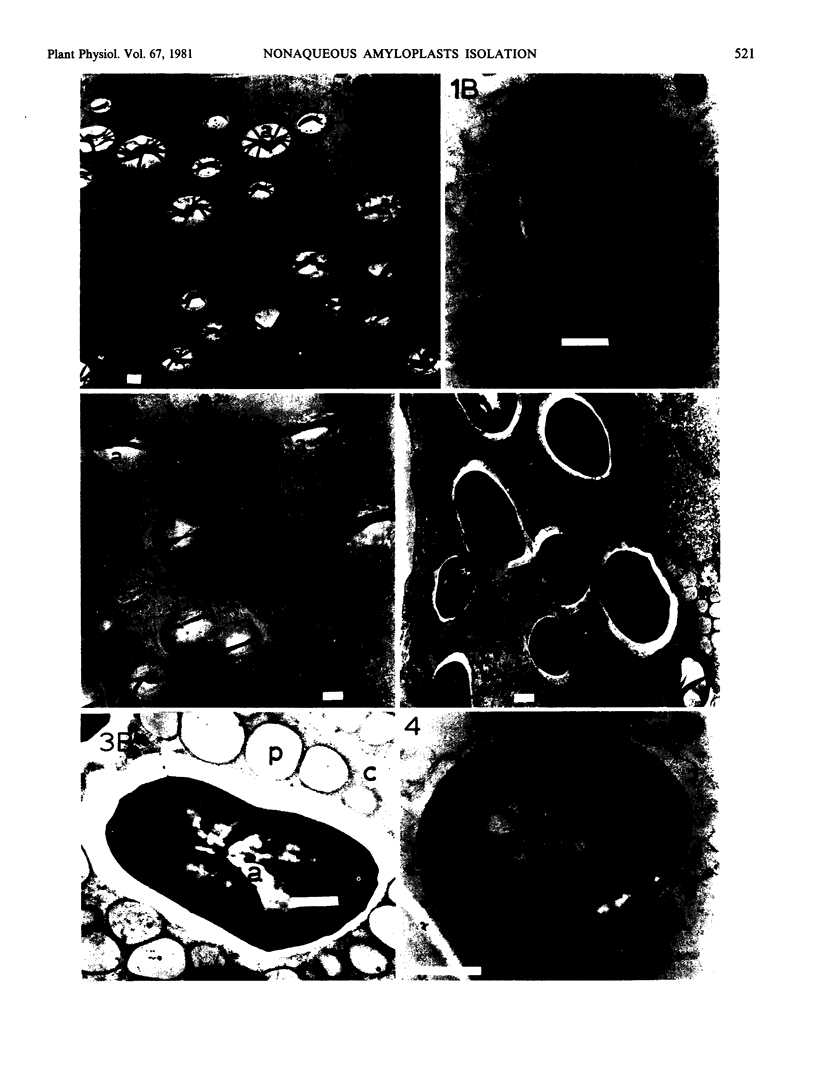
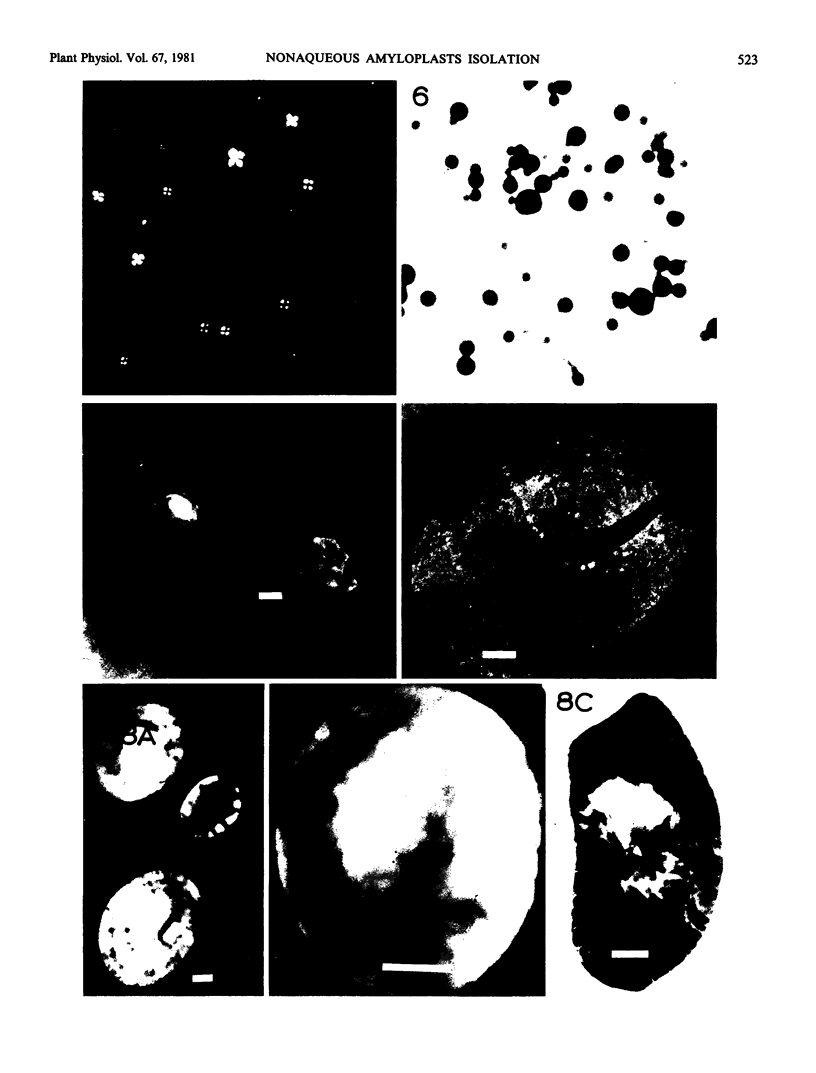
Electron microscopic examination of the isolated starch granules failed to demonstrate the presence of the amyloplast's membrane. However, based on an examination of fresh, freeze-dried, and rehydrated freeze-dried normal endosperm, it is suggested that the amyloplast membrane and enclosed stroma metabolites were dried onto the surface of the starch granules during the freeze-drying procedure. Chemical analysis of the glycerol-propanediol isolated granules showed the presence of alcohol-soluble sugars, inorganic phosphate, and phosphate-containing compounds. These soluble metabolites may represent amyloplast stroma metabolites which became bound to the starch granules during freeze-drying. Thus, this isolation procedure should be useful when metabolites closely associated with starch granules in situ are to be evaluated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIELESKI R. L. THE PROBLEM OF HALTING ENZYME ACTION WHEN EXTRACTING PLANT TISSUES. Anal Biochem. 1964 Dec;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- BUTTROSE M. S. Submicroscopic development and structure of starch granules in cereal endosperms. J Ultrastruct Res. 1960 Dec;4:231–257. doi: 10.1016/s0022-5320(60)80021-4. [DOI] [PubMed] [Google Scholar]
- Duffus C. M., Rosie R. Purification and fractionation of potato amyloplasts. Anal Biochem. 1975 May 12;65(1-2):11–18. doi: 10.1016/0003-2697(75)90484-4. [DOI] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch W. M., Leitner J. W., Gainey M., Schulz D., Lasher R., Nakane P. Bulk isolation in nonaqueous media of nuclei from lyophilized cells. Science. 1970 Jun 26;168(3939):1592–1595. doi: 10.1126/science.168.3939.1592. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. T., Shannon J. C. Measurement of Metabolites Associated with Nonaqueously Isolated Starch Granules from Immature Zea mays L. Endosperm. Plant Physiol. 1981 Mar;67(3):525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Carbon-14 Distribution in Carbohydrates of Immature Zea mays. Kernels Following CO(2) Treatment of Intact Plants. Plant Physiol. 1968 Aug;43(8):1215–1220. doi: 10.1104/pp.43.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Duffus C. M. Separation and Some Properties of Large and Small Amyloplasts throughout Development in Barley Endosperm. Plant Physiol. 1977 Feb;59(2):189–192. doi: 10.1104/pp.59.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]