Abstract
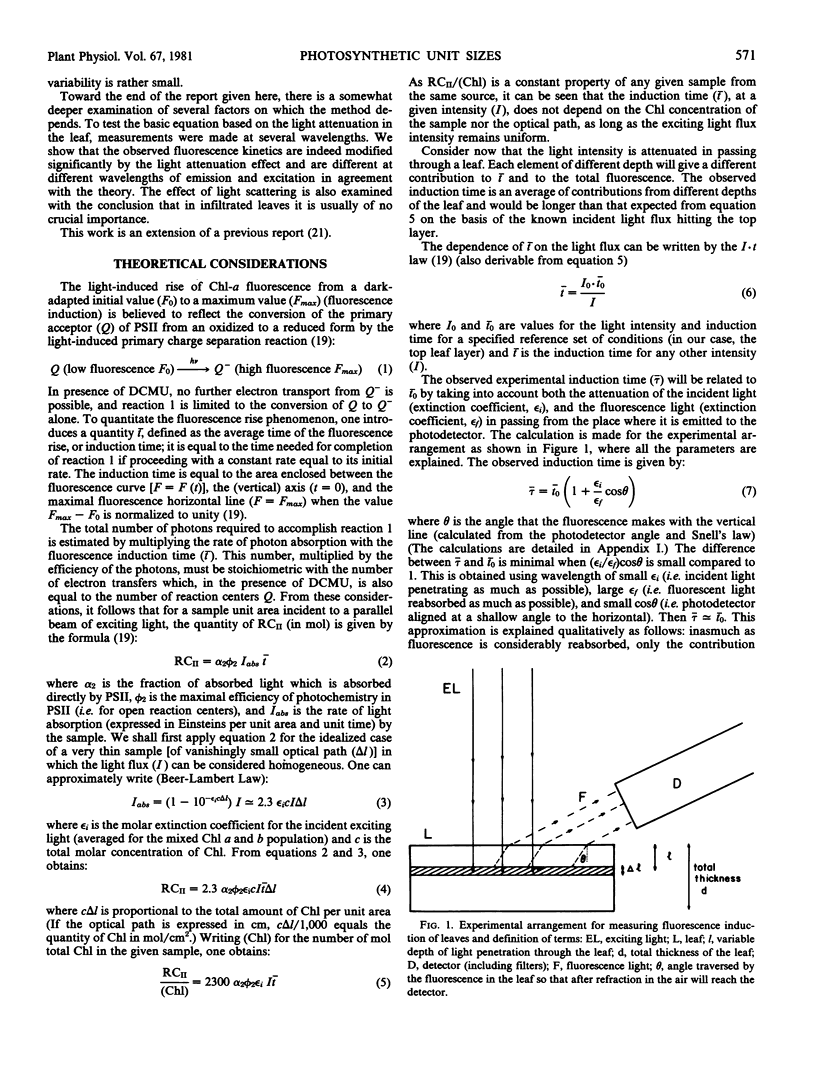
The use of fluorescence induction measurements in leaves infiltrated with 3-(3,4-dichlorophenyl)-1,1-dimethylurea has been evaluated as a routine method for estimation of the concentration of the reaction centers of photosystem II relative to total chlorophyll in a wide variety of plant species. The procedure is based on a simple theory that takes into account the attenuation of light in passing through the leaf and the linear dependence of the fluorescence induction time from different parts of the leaf on the inverse of the local light intensity. A formula to calculate the reaction center concentration of photosystem II was obtained. The effect of the light attenuation is accounted for by a correction factor which could become practically insignificant by an optimal choice of the excitation and emission wavelengths and the geometry of the photodetector with respect to the sample. Estimation of quantum yields for primary photochemistry and influence of light scattering were considered. The results demonstrate the effect of the above factors under various circumstances and are in agreement, to a first approximation, with the theory.
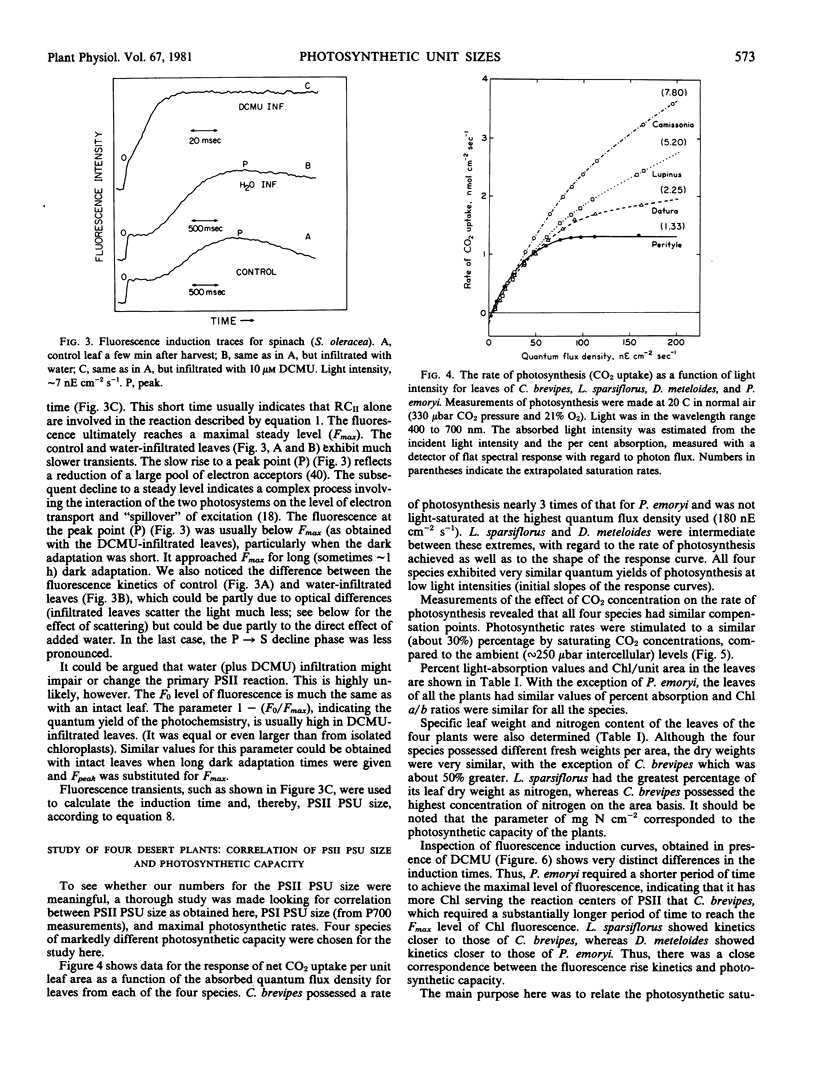
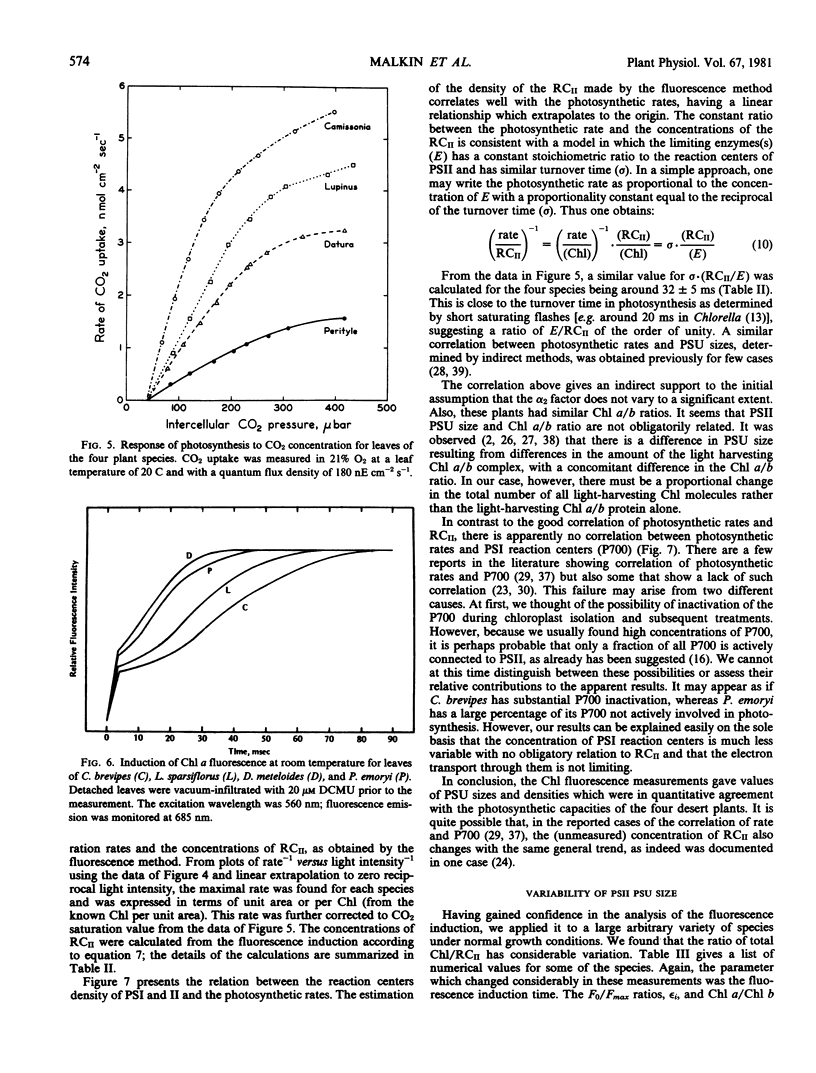
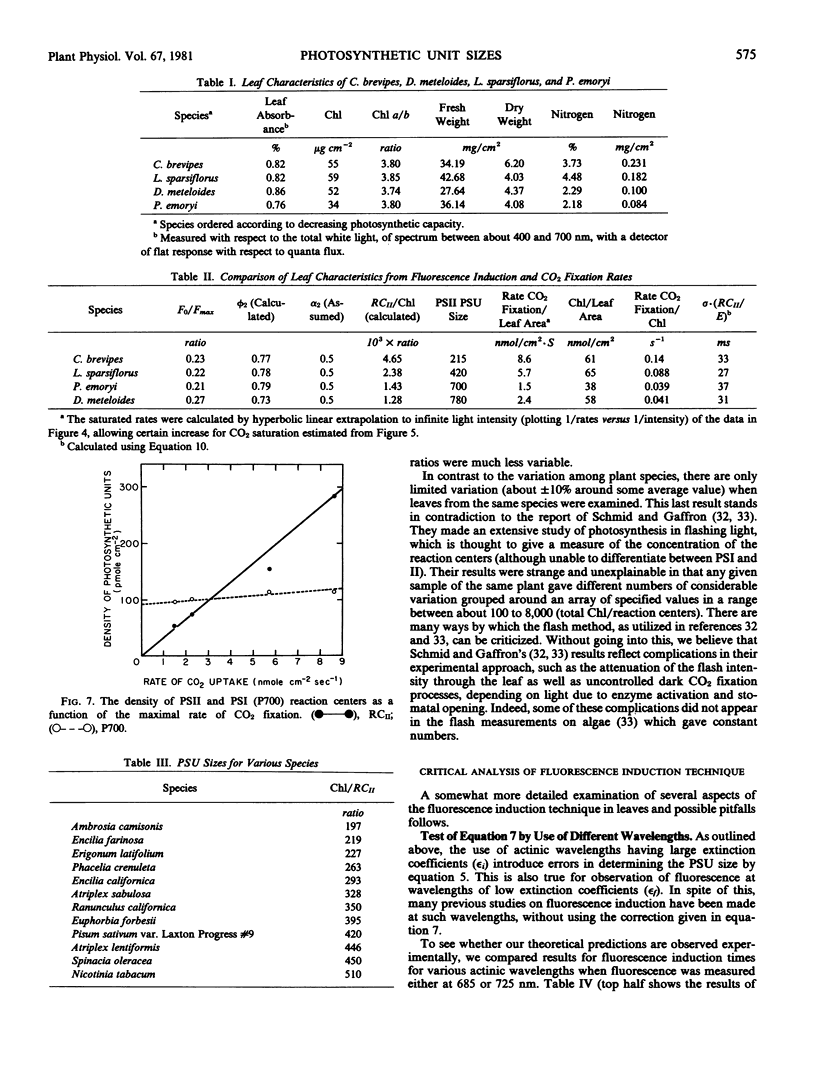
The utility of the method is demonstrated by a detailed study of four desert plant species: estimation of reaction center concentrations of both photosystem I (by estimation of P700) and photosystem II (by the fluorescence induction method) were made and were compared to the rates of CO2 fixation. There was a good quantitative correlation between the photosynthetic rates and the concentration of photosystem II reaction centers (expressed as per chlorophyll or per unit area of the leaf), but no such correlation was found with photosystem I reaction centers.
The ratio of total chlorophyll per reaction centers II varied in the range of about 200 to 800 in different species, but there was no variation of this parameter in any single species.
Full text
PDF

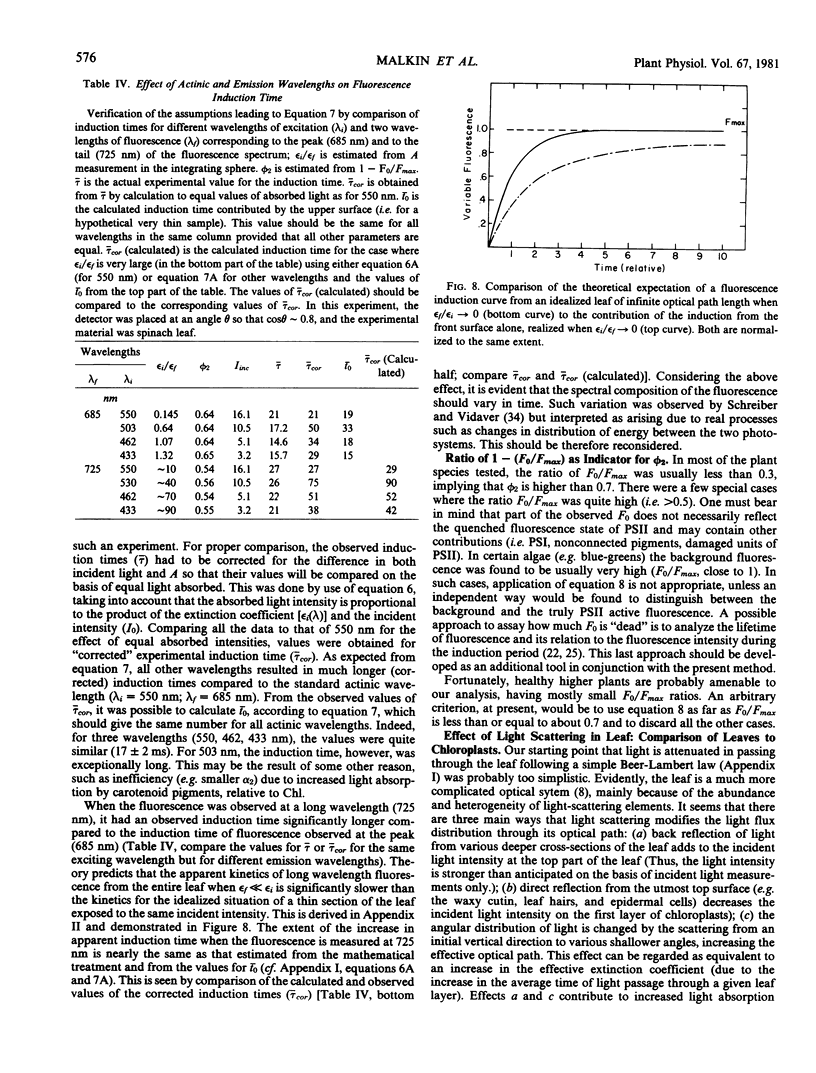
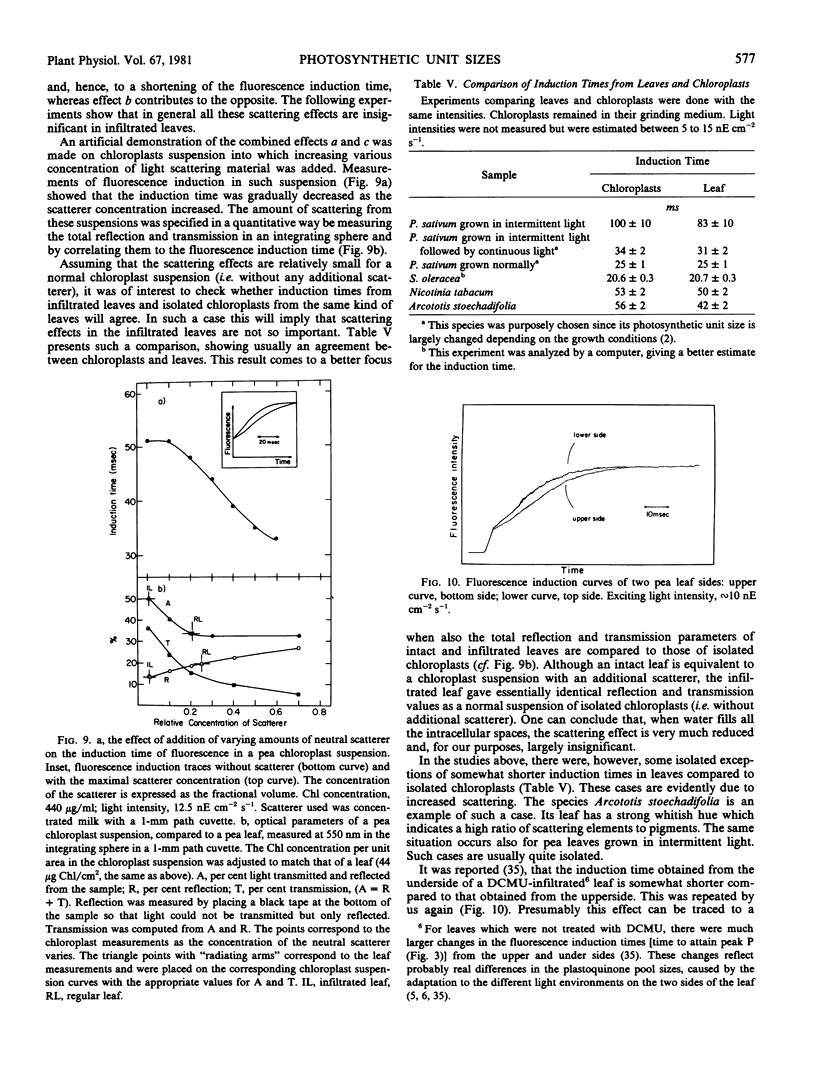


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Hesketh J. D., Hofstra G., Thornber J. P., Naylor A. W., Bernard R. L., Brim C., Endrizzi J., Kohel R. J. Composition and activity of the photosynthetic apparatus in temperature-sensitive mutants of higher plants. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2414–2418. doi: 10.1073/pnas.71.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J., Briantais J. M., Vernotte C. Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch Biochem Biophys. 1976 Jul;175(1):54–63. doi: 10.1016/0003-9861(76)90484-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahen D., Malkin S. Development of Photosystem II Complex during Greening of Chlamydomonas reinhardi y-1. Plant Physiol. 1976 Sep;58(3):257–267. doi: 10.1104/pp.58.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubertret G., Joliot P. Structure and organization of system II photosynthetic units during the greening of a dark-grown Chlorella mutant. Biochim Biophys Acta. 1974 Sep 20;357(3):399–411. doi: 10.1016/0005-2728(74)90030-9. [DOI] [PubMed] [Google Scholar]
- Ehleringer J., Björkman O. Quantum Yields for CO(2) Uptake in C(3) and C(4) Plants: Dependence on Temperature, CO(2), and O(2) Concentration. Plant Physiol. 1977 Jan;59(1):86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R., Arnold W. THE PHOTOCHEMICAL REACTION IN PHOTOSYNTHESIS. J Gen Physiol. 1932 Nov 20;16(2):191–205. doi: 10.1085/jgp.16.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel W. The ratio of the two light reactions and their coupling in chloroplasts. Biochim Biophys Acta. 1976 Mar 12;423(3):499–509. doi: 10.1016/0005-2728(76)90203-6. [DOI] [PubMed] [Google Scholar]
- KOK B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim Biophys Acta. 1956 Nov;22(2):399–401. doi: 10.1016/0006-3002(56)90172-x. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Malkin S., Siderer Y. The effect of salt concentration on the fluorescence parameters of isolated chloroplasts. Biochim Biophys Acta. 1974 Dec 19;368(3):422–431. doi: 10.1016/0005-2728(74)90187-x. [DOI] [PubMed] [Google Scholar]
- Moya I., Govindjee, Vernotte C., Briantais J. M. Antagonistic effect of mono- and divalent-cations on lifetime (tau) and quantum yield of fluorescence (phi) in isolated chloroplasts. FEBS Lett. 1977 Mar 15;75(1):13–18. doi: 10.1016/0014-5793(77)80042-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Ogawa T., Shibata K. Chlorophyll and peptide compositions in the two photosystems of marine green algae. Biochim Biophys Acta. 1976 Feb 16;423(2):227–237. doi: 10.1016/0005-2728(76)90181-x. [DOI] [PubMed] [Google Scholar]
- Okabe K., Schmid G. H., Straub J. Genetic characterization and high efficiency photosynthesis of an aurea mutant of tobacco. Plant Physiol. 1977 Jul;60(1):150–156. doi: 10.1104/pp.60.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. T., Bunce J. A., Alberte R. S., Van Volkenburgh E. Photosynthesis in relation to leaf characteristics of cotton from controlled and field environments. Plant Physiol. 1977 Mar;59(3):384–387. doi: 10.1104/pp.59.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G. H., Gaffron H. Photosynthetic units. J Gen Physiol. 1968 Aug;52(2):212–239. doi: 10.1085/jgp.52.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Teeri J. A., Patterson D. T., Alberte R. S., Castleberry R. M. Changes in the photosynthetic apparatus of maize in response to simulated natural temperature fluctuations. Plant Physiol. 1977 Sep;60(3):370–373. doi: 10.1104/pp.60.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]


