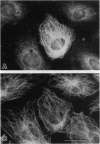
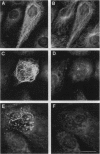
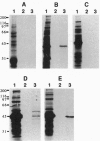
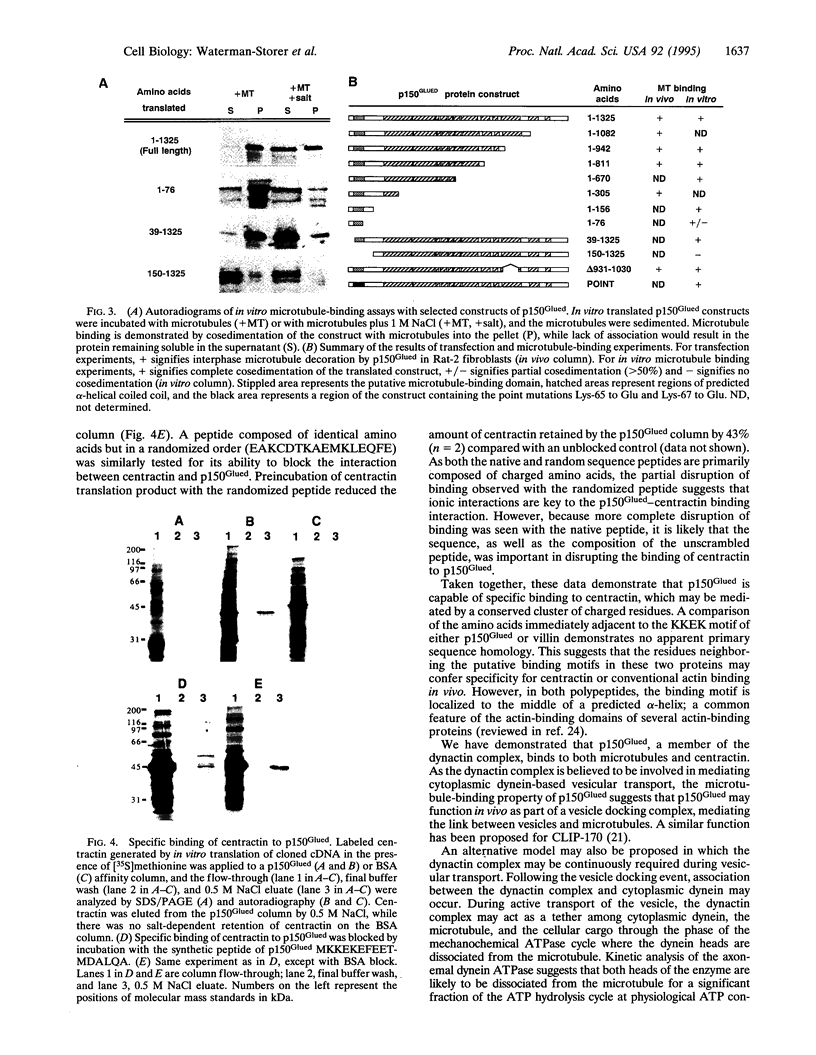
Abstract
p150Glued was first identified as a polypeptide that copurifies with cytoplasmic dynein, the minus-end-directed microtubule-based motor protein, and has more recently been shown to be present as a member of the oligomeric dynactin complex, which includes the actin-related protein centractin (Arp-1). Dynactin is thought to mediate dynein-driven vesicle motility, as well as nuclear transport, in lower eukaryotes. The mechanism by which dynactin may function in these cellular processes is unknown. To examine the role of the dynactin complex in vivo, we overexpressed the rat cDNA encoding p150Glued in Rat-2 fibroblasts. Overexpression of full-length, as well as C-terminal deletion, constructs resulted in the decoration of microtubules with the p150Glued polypeptides. This cellular evidence for microtubule association was corroborated by in vitro microtubule-binding assays. Amino acids 39-150 of p150Glued were determined to be sufficient for microtubule association. We also tested for a direct interaction between p150Glued and centractin. In vitro translated centractin was specifically retained by a p150Glued affinity column, and this interaction was blocked by a synthetic peptide which corresponds to a highly conserved motif from the C terminus of p150Glued. These results demonstrate that p150Glued, a protein implicated in cytoplasmic dynein-based microtubule motility, is capable of direct binding to both microtubules and centractin.
Full text
PDF

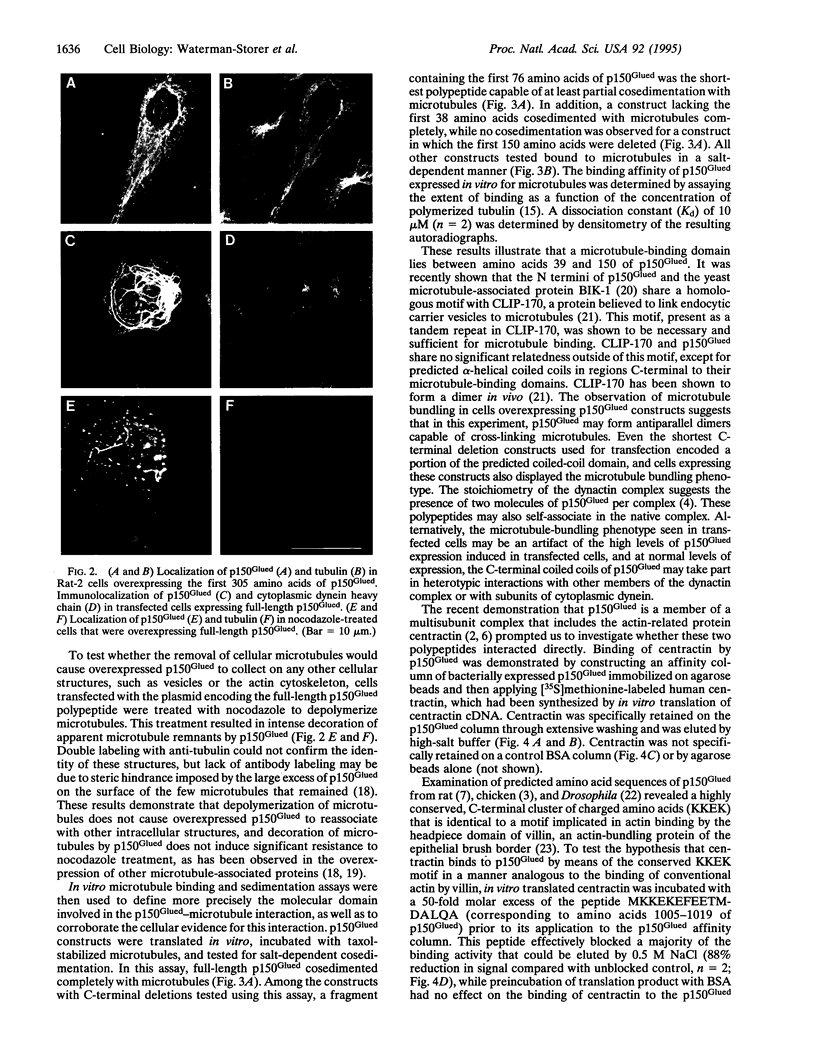

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kelley J. M., Gocayne J. D., Dubnick M., Polymeropoulos M. H., Xiao H., Merril C. R., Wu A., Olde B., Moreno R. F. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991 Jun 21;252(5013):1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- Berlin V., Styles C. A., Fink G. R. BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J Cell Biol. 1990 Dec;111(6 Pt 1):2573–2586. doi: 10.1083/jcb.111.6.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. ACT3: a putative centractin homologue in S. cerevisiae is required for proper orientation of the mitotic spindle. J Cell Biol. 1994 Oct;127(1):129–138. doi: 10.1083/jcb.127.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. Centractin is an actin homologue associated with the centrosome. Nature. 1992 Sep 17;359(6392):246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- Collins C. A., Vallee R. B. Preparation of microtubules from rat liver and testis: cytoplasmic dynein is a major microtubule associated protein. Cell Motil Cytoskeleton. 1989;14(4):491–500. doi: 10.1002/cm.970140407. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L. A., Vissers S., Jauniaux J. C., van Vliet-Reedijk J. C., Planta R. J., Gibbons I. R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Vancompernolle K., Huet C., Goethals M., Finidori J., Vandekerckhove J., Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992 Jul 10;70(1):81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Gill S. R., Schroer T. A., Szilak I., Steuer E. R., Sheetz M. P., Cleveland D. W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991 Dec;115(6):1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode B. L., Feinstein S. C. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994 Mar;124(5):769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte P. J., Kankel D. R. Genetic analysis of mutations at the Glued locus and interacting loci in Drosophila melanogaster. Genetics. 1982 Jul-Aug;101(3-4):477–501. doi: 10.1093/genetics/101.3-4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Hammarback J. A., Paschal B. M., Kravit N. G., Pfister K. K., Vallee R. B. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 1991 Jun 13;351(6327):579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Johnson K. A. Microtubules accelerate ADP release by dynein. Biochemistry. 1989 Aug 22;28(17):7010–7016. doi: 10.1021/bi00443a034. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M., Schroer T. A. A vertebrate actin-related protein is a component of a multisubunit complex involved in microtubule-based vesicle motility. Nature. 1992 Sep 17;359(6392):244–246. doi: 10.1038/359244a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Ivanov I. E., Lee G. H., Cowan N. J. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989 Nov 30;342(6249):498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Kreis T. E. Identification and molecular characterization of E-MAP-115, a novel microtubule-associated protein predominantly expressed in epithelial cells. J Cell Biol. 1993 Oct;123(2):357–371. doi: 10.1083/jcb.123.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Vainberg I. E., Chow R. L., Cowan N. J. Chaperonin-mediated folding of vertebrate actin-related protein and gamma-tubulin. J Cell Biol. 1993 Sep;122(6):1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhua L., Karpova T. S., Cooper J. A. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994 Aug 26;78(4):669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Paschal B. M., Holzbaur E. L., Pfister K. K., Clark S., Meyer D. I., Vallee R. B. Characterization of a 50-kDa polypeptide in cytoplasmic dynein preparations reveals a complex with p150GLUED and a novel actin. J Biol Chem. 1993 Jul 15;268(20):15318–15323. [PubMed] [Google Scholar]
- Pierre P., Scheel J., Rickard J. E., Kreis T. E. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992 Sep 18;70(6):887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994 Oct;127(1):139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Gill S. R., Cooper J. A., Heuser J. E., Schroer T. A. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994 Jul;126(2):403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E. R., Wordeman L., Schroer T. A., Sheetz M. P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990 May 17;345(6272):266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Swaroop A., Swaroop M., Garen A. Sequence analysis of the complete cDNA and encoded polypeptide for the Glued gene of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6501–6505. doi: 10.1073/pnas.84.18.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Vancompernolle K. Structural relationships of actin-binding proteins. Curr Opin Cell Biol. 1992 Feb;4(1):36–42. doi: 10.1016/0955-0674(92)90056-i. [DOI] [PubMed] [Google Scholar]