Abstract
Rickettsia parkeri, a newly recognized tick-borne pathogen of humans in the Americas, is a confirmed cause of spotted fever group rickettsiosis in Argentina. Until recently, almost all cases of R. parkeri rickettsiosis in Argentina have originated from the Paraná River Delta, where entomological surveys have identified populations of R. parkeri-infected Amblyomma triste ticks. In this report, we describe confirmed cases of R. parkeri rickettsiosis from Córdoba and La Rioja provinces, which are located several hundred kilometers inland, and in a more arid ecological region, where A. triste ticks do not occur. Additionally, we identified questing A. tigrinum ticks naturally infected with R. parkeri in Córdoba province. These data provide evidence that another human-biting tick species serves as a potential vector of R. parkeri in Argentina and possibly, other countries of South America.
Introduction
Rickettsia parkeri, a newly recognized spotted fever group (SFG) rickettsial pathogen of humans in the Americas, was first confirmed as a cause of disease in Argentina in 2011, when several cases were identified in patients who were bitten by ticks in the Paraná River Delta.1 This large wetland expanse, located approximately 130 km northwest of the city of Buenos Aires, is characterized by a temperate climate with abundant annual rainfall and contains an intricate network of tidal channels, marshes, and islands.2 Almost all cases of R. parkeri rickettsiosis in Argentina have originated from the Paraná River Delta,1,3 and entomological surveys have identified populations of R. parkeri-infected Amblyomma triste ticks from this region4; nonetheless, a few suspected cases have been recognized recently in areas far beyond the boundaries of Paraná River Delta, including the Bahía Samborombón area in the province of Buenos Aires and the Traslasierra Valley in the province of Córdoba.1,5
Herein, we present four confirmed cases of R. parkeri rickettsiosis, including three cases acquired in an ecological region considerably different from the recognized habitat of A. triste6 and located several hundred kilometers inland from where R. parkeri rickettsiosis is considered endemic in Argentina. Additionally, we identify another human-biting tick species in this region that represents a second potential vector of R. parkeri in Argentina and possibly, other countries of South America.
Materials and Methods
Serological and molecular analysis of patient specimens.
Four patients were referred to the Zoonosis Service at the F. J. Muñiz Hospital, Buenos Aires, Argentina, during 2011–2013 for evaluation of illnesses characterized by fever, rash, and one or more inoculation eschars that followed a tick bite. Patients were bitten by ticks in widely separated geographical regions in the provinces of Buenos Aires, Córdoba, and La Rioja. From each patient, serum and skin biopsy specimens were obtained for serological and molecular analysis. Biopsy specimens of inoculation eschar lesions were obtained by using a 6-mm skin punch. DNA was extracted from these specimens by using a QIAamp DNA Mini Kit, (QIAGEN, Valencia, CA) and 2.5 μL of each extract was tested by using a real-time polymerase chain reaction (PCR) assay targeting a 96-bp segment of the R. parkeri ompB gene and a 111-bp segment of a hypothetical gene of R. rickettsii.7 A Ct (threshold cycle) value < 40 was considered positive. Serum samples were collected during the acute and convalescent phases of the patients' illnesses and tested for immunoglobulin G (IgG) antibodies reactive with antigens of R. parkeri and R. rickettsii by using an indirect immunofluorescence antibody (IFA) assay8 starting at an initial dilution of 1:32. Antibody titers were expressed as the reciprocal of the last dilution exhibiting specific fluorescence. An antibody titer ≥ 64 to one or both antigens was considered evidence of exposure to an SFG Rickettsia species. A fourfold or greater change in titer between specimens collected separately was considered evidence of a recent infection with an SFG Rickettsia species.
Tick collection and molecular analysis.
During September of 2011, 20 free-living, adult-stage A. triste ticks were collected from vegetation in Punta Indio by using cloth drags. Specimens were stored at −20°C and later processed as two pools of 10 ticks each. DNA was extracted using a NucleoSpin Tissue DNA Kit (Macherey-Nagel Gmb H and Co. KG, Düren, Germany) according to the manufacturer's instructions and eluted in a final volume of 100 μL. During October of 2013, 102 free-living, adult-stage A. tigrinum ticks were collected by dragging vegetation in the Traslasierra Valley. Specimens were preserved in 96% ethanol and identified to species using accepted morphological features.9 DNA was extracted individually from 81 specimens using the AxyPrep Multisource Genomic DNA Miniprep Kit (Axygen Biosciences Inc., Union City, CA), and each sample was eluted in a final volume of 100 μL. DNA specimens were screened for Rickettsia species using a PCR assay with primers RCK/23-5-F and RCK/23-5-R that amplify a segment of the 23S-5S intergenic spacer.10 Positive samples were subsequently tested by a PCR assay with primers Rr190.70p and Rr190.602n to amplify a segment of the 190-kDa outer-membrane protein gene ompA.11 All amplicons were gel-purified and sequenced. Sequences were manually edited using the BioEdit Sequence Alignment Editor12 with the CLUSTAL W program.13 Sequences of ompA gene were compared with those available in GenBank using the program MEGA, version 5.0.14
Results
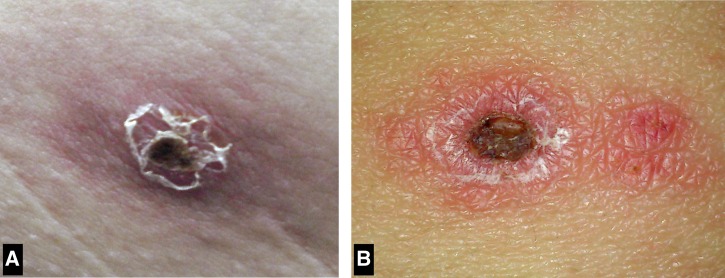
Patient 1 was a 47-year-old man from Punta Indio in Buenos Aires province (35°16′ S, 57°14′ W) who presented in November of 2011 with fevers as high as 40°C, headaches, myalgia, and a pruritic rash (Table 1). Five days before the onset of fever, he had removed a tick attached near his ear. Physical examination revealed three painless inoculation eschars on the left arm, abdomen, and back that measured 1.0–1.5 cm (Figure 1A ) and a sparse vesicular rash involving predominantly the trunk and upper extremities. There was no lymphadenopathy. His serum chemistries and blood cell counts were within normal range. Doxycycline was administered, and he became afebrile within 48 hours. No antibodies reactive with R. rickettsii or R. parkeri antigens were detected in the serum sample obtained on the 5th day of illness; however, the convalescent serum specimen, collected 15 days after illness onset, revealed antibody titers to R. rickettsii and R parkeri antigens of 128 and 512, respectively. Real-time PCR analysis of DNA extracted from an inoculation eschar biopsy specimen was positive for R. parkeri and negative for R. rickettsii.
Table 1.
Selected epidemiological, clinical, and laboratory features of four Argentina patients with R. parkeri rickettsiosis
| Feature | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (years)/gender | 47/male | 57/female | 47/male | 28/male |
| Province of exposure | Buenos Aires | Córdoba | Córdoba | La Rioja |
| Month of exposure | November | December | November | November |
| IFA titer(s)* | ||||
| R. rickettsii | < 32, 128 | 32 | 1,024 | 128, 512 |
| R. parkeri | < 32, 512 | 256 | 1,024 | 256, 512 |
Acute- and convalescent-phase IgG antibody titers are represented as the reciprocal of the last serum dilution exhibiting a specific pattern of fluorescence when reacted with the antigen.
Figure 1.
Appearance of inoculation eschars of R. parkeri rickettsiosis. (A) An eschar on the abdomen of patient 1 from the Bahía Samborombón region of Buenos Aires province. (B) An eschar on the abdomen of patient 2 from the Traslasierra Valley of Córdoba province.
Patient 2 was a 57-year-old woman from the town of Nono in Córdoba province (31°46′ S, 64°59′ W) who presented in December of 2012 with 7 days of fever and generalized rash. Three days before the onset of symptoms, she noted a tick attached to her abdomen while working in her yard. She was initially evaluated at a community clinic, where she was diagnosed with cellulitis. She was treated with amoxicillin-clavulanate for 5 days without improvement and subsequently referred for additional evaluation. Physical examination revealed a temperature of 39°C, a 1.5-cm painless eschar on her abdomen (Figure 1B), and a sparse maculopapular and vesicular rash involving her trunk and limbs. There was no lymphadenopathy. Except for a prolonged erythrocyte sedimentation rate of 50 mm/hour (normal = 1–20 mm/hour), her serum chemistries and blood cell counts were normal. Empirical treatment with doxycycline was initiated, and her fever resolved within 48 hours. A serum specimen obtained on day 8 of her illness revealed antibodies reactive with R. rickettsii and R. parkeri antigens at titers of 32 and 256, respectively. PCR evaluation of the inoculation eschar biopsy specimen was positive for infection with R. parkeri and negative for R. rickettsii.
Patient 3 was a 47-year-old man from Valle de Calamuchita (31°59′ S, 64°22′ W) in Córdoba province who presented in November of 2013 after 10 days of fever and rash. Five days before the start of fever, he removed a tick from behind his right knee. Physical examination revealed a temperature of 38°C, a tender 1-cm eschar in his popliteal fossa, and a sparse maculopapular and vesicular rash involving his trunk and limbs that spared his palms and soles. There was no lymphadenopathy. His serum chemistries and blood cell counts were normal. He was started on doxycycline, and his symptoms resolved in 48 hours. A biopsy specimen of the eschar was obtained on day 10 of fever, and a serum sample was collected 30 days later. His serum contained antibodies reactive with R. rickettsii and R. parkeri at titers of 1,024 to each antigen. Real-time PCR evaluation of the eschar biopsy specimen was positive for infection with R. parkeri and negative for R. rickettsii.
Patient 4 was a 28-year-old man who was bitten by a tick in November of 2013 while camping in Malanzán (30°48′ S, 66°36′ W) in La Rioja province. Approximately 1 week later, he developed fever, myalgia, and a lesion on his neck. A non-pruritic rash developed on his body approximately 5 days later. Physical examination revealed a temperature of 40°C, a small tender eschar on his posterior cervical region, and a maculopapular exanthem involving his trunk, limbs, and face that spared his palms and soles. There was no lymphadenopathy. His serum chemistries and blood cell counts were normal. Empirical treatment with doxycycline was started, and his symptoms resolved in 72 hours. A biopsy specimen of the eschar and a serum sample were obtained on day 10 of fever, and a convalescent-phase serum sample was collected 30 days later. His acute- and convalescent-phase serum samples reacted with R. rickettsii antigens at titers of 128 and 512, respectively, and R. parkeri at titers of 256 and 512, respectively. PCR evaluation of the eschar biopsy specimen was positive for infection with R. parkeri and negative for R. rickettsii.
DNA of R. parkeri was detected by PCR in one pool of A. triste ticks from Punta Indio, representing a minimum infection rate of 5%. DNA of R. parkeri was amplified from 2 (2.5%) of 81 questing A. tigrinum ticks collected from Traslasierra Valley. Both ompA sequences were identical with each other (GenBank accession number KM099395) and those ompA sequences of R. parkeri from A. triste ticks from Buenos Aires province4,15 and A. maculatum ticks from the United States.16
Discussion
Since 1999, three pathogenic SFG Rickettsia species, namely R. rickettsii, R. massiliae, and R. parkeri, have been identified as causes of tick-borne illnesses of humans in Argentina.1,3,5,17–19 Rickettsioses are diagnosed infrequently in most South American countries, despite growing recognition that the tick fauna of this continent contains a diverse group of rickettsial pathogens.20 A contributing factor is the coexistence and sympatric distribution of other clinically similar and highly prevalent infectious diseases, such as dengue and leptospirosis.1,18 Clinicians are better positioned to correctly diagnose rickettsioses in locations where pathogenic Rickettsia species have been identified previously in human and arthropod hosts. This study and others provide evidence that R. parkeri is endemic to and infects humans in at least four provinces of Argentina.
The clinical characteristics shared by these patients included moderate fever, one or more inoculation eschars, and macuolopapular or vesicular rash. These features are consistent with those described previously in larger series of R. parkeri-infected patients from the Paraná River Delta1,3 and the United States.8,21,22 Regional lymphadenopathy was not identified in any of the patients described in this series, and it has been documented in fewer than 30% of cases reported from Argentina and the United States. For reasons currently unknown, regional lymphadenopathy is reported with much greater frequency among R. parkeri-infected patients in Uruguay.23,24
Molecular confirmation of R. parkeri infection in a patient and a pool of A. triste ticks from Punta Indio (approximately 160 km southeast of the city of Buenos Aires) corroborates a previous investigation that identified patients with an eschar-associated SFG rickettsiosis from Verónica and General Lavalle in Buenos Aires province.1 All of these locations share environmental features with the Paraná River Delta, including grasslands, freshwater lagoons, intertidal mudflats, and marshes, and are included within the Delta e Islas del Paraná ecoregion.25 In addition, recent entomological investigations have identified populations of R. parkeri-infected A. triste ticks from these same areas.4,15 In Argentina, A. triste is found predominantly in wetland environments in the provinces of Buenos Aires, Corrientes, Entre Ríos, and Formosa, where the immature stages parasitize a broad range of sigmodontine and caviid rodents and the adult stages parasitize many domesticated and wild large mammals.6 A. triste is an efficient host and vector of R. parkeri,26 and natural infections of this tick also occur in areas of Uruguay and Brazil, where R. parkeri rickettsiosis is endemic.27–31
Córdoba province, located in the central aspect of Argentina, is the second most populous province in the country and includes many scenic areas that are visited frequently by tourists. The Traslasierra Valley is one of these regions, and it is situated between the Sierra de Córdoba and Sierra de San Luis mountain ranges (approximately 600 km inland from the Paraná River Delta). Malanzán is located in the southern aspect of La Rioja province (approximately 850 km from the Paraná River Delta). This semiarid region is also visited by many tourists. These areas comprise several striking contrasts from the alluvial wetland ecosystems of the coastal provinces from where all previously confirmed cases of R. parkeri rickettsiosis in Argentina have originated. The Traslasierra Valley and Malanzán are situated in the Chaco Seco ecoregion,25 and populations of A. triste do not occur in this part of Argentina; however, A. tigrinum is collected commonly throughout Córdoba province32 and from various sites in La Rioja province (Nava S, unpublished data).
R. parkeri has been detected previously in A. tigrinum ticks attached to dogs in Bolivia and Uruguay,33,34 but this is the first report to describe R. parkeri infection in questing A. tigrinum ticks and document natural infections in specimens of A. tigrinum collected from an area where confirmed human cases of R. parkeri rickettsiosis were also identified. Additional surveys are needed to more fully assess the infection prevalence of R. parkeri in A. tigrinum; nonetheless, the prevalence in specimens collected from the Traslasierra Valley was slightly lower than the percentages of R. parkeri-infected A. triste reported from Argentina (approximately 7–8%)4,15 and other countries in South America (approximately 3–10%)27,28,31 and much lower than the percentage of infected A. maculatum ticks (approximately 10–56%) in the United States.16,35 The phylogenetic relationships among these three vector species are very close, and the morphological differences are subtle9; for many years, specimens of A. tigrinum and A. triste (Figure 2) were misidentified frequently as A. maculatum.36
Figure 2.
A. tigrinum (A) male and (B) female compared with A. triste (C) male and (D) female.
A. tigrinum shows a remarkable ability to adapt to different ecosystems with contrasting climatic conditions.37 In Argentina, small rodents and birds are the principal hosts for larvae and nymphs of A. tigrinum. Adults are commonly found on wild and domestic carnivores and occasionally, other large mammals, including humans.38–41 A. tigrinum occurs in all of the phytogeographical domains of Argentina north of 40° S,37,41 which suggests that the geographical distribution of R. parkeri rickettsiosis in Argentina is more extensive than previously recognized. Additionally, there are records of attachment of A. tigrinum to humans in Brazil, Chile, French Guiana, Paraguay, and Uruguay,40 which suggest that its role in the transmission of R. parkeri to humans may extend to other countries in South America. For example, R. parkeri rickettsiosis is well-recognized in Uruguay, where parasitism of humans by coastal populations of A. triste and inland populations of A. trigrinum occur in a manner similar to Argentina.42
The recent discoveries of diverse tick-borne agents in Argentine ticks that now include various Rickettsia, Coxiella, and Ehrlichia species4,15,43,44 reflect the increasing awareness of tick-associated diseases throughout Latin America. In this context, enhanced efforts are needed to accurately diagnose and better characterize the scope and magnitude of these complex zoonoses.45
ACKNOWLEDGMENTS
The authors thank James Gathany (Centers for Disease Control and Prevention) for providing the photographs of A. tigrinum and A. triste used in Figure 2.
Disclaimer: The findings and conclusions are those of the authors and do not necessarily represent the official position of the US Department of Health and Human Services.
Footnotes
Financial support: This work was funded by the US Department of Health and Human Services.
Authors' addresses: Yamila Romer, Infectious Diseases, Hospital F. J. Muñiz, Buenos Aires, Argentina, E-mail: yromer@hotmail.com. Santiago Nava, Instituto Nacional de Tecnologia Agropecuaria, Laboratorio de Parasitología e Inmunología Rafaela, Santa Fe, Argentina, E-mail: nava.santiago@inta.gob.ar. Francisco Govedic, Infectious Diseases, Sanatorio Allende, Córdoba, Argentina, E-mail: frangovedic@hotmail.com. Gabriel Cicuttin, Zoonoses, Instituto de Zoonosis Luis Pasteur, Buenos Aires, Argentina, E-mail: gcicuttin@gmail.com. Amy M. Denison, Infectious Diseases Pathology Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: crk6@cdc.gov. Joseph Singleton, Cecilia Y. Kato, and Christopher D. Paddock, Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jsingleton@cdc.gov, hex0@cdc.gov, and cdp9@cdc.gov. Aubree J. Kelly, Bacterial Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: hrr6@cdc.gov.
References
- 1.Romer Y, Seijo AC, Crudo F, Nicholson WL, Varela-Stokes A, Lash RR, Paddock CD. Rickettsia parkeri rickettsiosis, Argentina. Emerg Infect Dis. 2011;17:1169–1173. doi: 10.3201/eid1707.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violante RA, Cavallotto JL, Kandus P. Río de la Plata y Delta del Paraná. In: Ardolino A, Lema H, editors. Sitios de Interés Geológicos de la República Argentina. Servicio Geológico Minero Argentino. Buenos Aires, Argentina: Buenos Aires Instituto de Geología y Recursos Minerales Anales; 2008. pp. 461–475. [Google Scholar]
- 3.Seijo A, Picollo M, Nicholson WL, Paddock CD. Fiebre manchada por rickettsias en el Delta del Paraná: una enfermedad emergente. Medicina (B Aires) 2007;67:723–726. [PubMed] [Google Scholar]
- 4.Nava S, Elshenawy Y, Eremeeva ME, Sumner JW, Mastropaolo M, Paddock CD. Rickettsia parkeri in Argentina. Emerg Infect Dis. 2008;14:1894–1897. doi: 10.3201/eid1412.080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romer Y, Seijo A. Fiebres manchadas por rickettsias en la Mesopotamia y Pampa Húmeda. In: Basualdo J, Cacchione R, Durlach R, Martino P, Seijo A, editors. Temas de Zoonosis V. Buenos Aires, Argentina: Asociación Argentina de Zoonosis; 2011. pp. 147–151. [Google Scholar]
- 6.Guglielmone AA, Nava S, Mastropaolo M, Mangold AJ. Distribution and genetic variation of Amblyomma triste (Acari: Ixodidae) in Argentina. Ticks Tick Borne Dis. 2013;4:386–390. doi: 10.1016/j.ttbdis.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Denison AM, Amin BJ, Nicholson WL, Paddock CD. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis. 2014;59:635–642. doi: 10.1093/cid/ciu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremmeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 9.Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 2005;60:99–112. doi: 10.1007/s11230-004-1382-9. [DOI] [PubMed] [Google Scholar]
- 10.Jado I, Escudero R, Gil H, Jiménez-Alonso MI, Sousa R, García-Pérez AL, Rodríguez-Vargas M, Lobo B, Anda P. Molecular method for identification of Rickettsia species in clinical and environmental samples. J Clin Microbiol. 2006;44:4572–4576. doi: 10.1128/JCM.01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall TA. BioEdit: a user friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 13.Thompson JD, Higgins D, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalities and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicuttin G, Nava S. Molecular identification of Rickettsia parkeri infecting Amblyomma triste ticks in an area of Argentina where cases of rickettsiosis were diagnosed. Mem Inst Oswaldo Cruz. 2013;108:123–125. doi: 10.1590/S0074-02762013000100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ripoll CM, Remondegui CE, Ordoñez G, Arazamendi R, Fusaro H, Hyman MJ, Paddock CD, Zaki SR, Olson JG, Santos-Buch CA. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–354. doi: 10.4269/ajtmh.1999.61.350. [DOI] [PubMed] [Google Scholar]
- 18.Paddock CD, Fernandez S, Echenique GA, Sumner JW, Reeves WK, Zaki SR, Remondegui CE. Rocky Mountain spotted fever in Argentina. Am J Trop Med Hyg. 2008;78:687–692. [PubMed] [Google Scholar]
- 19.Garciá-Garciá JC, Portillo A, Nuñez MJ, Santibáñez S, Castro B, Oteo JA. Case report: a patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg. 2010;82:691–692. doi: 10.4269/ajtmh.2010.09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labruna MB. Ecology of Rickettsia in South America. Ann N Y Acad Sci. 2009;1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- 21.Cragun WC, Bartlett BL, Ellis MW, Hoover AZ, Tyring SK, Mendoza N, Vento TJ, Nicholson WL, Eremeeva ME, Olano JP, Rapini RP, Paddock CD. The expanding spectrum of eschar associated rickettsioses in the United States. Arch Dermatol. 2010;146:641–648. doi: 10.1001/archdermatol.2010.48. [DOI] [PubMed] [Google Scholar]
- 22.Myers T, Lalani T, Dent M, Jiang J, Daly PL, Maguire JD, Richards AL. Detecting Rickettsia parkeri infection from eschar swab specimens. Emerg Infect Dis. 2013;19:778–780. doi: 10.3201/eid1905.120622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti-Díaz I, Rubio I, Somma Moreira RE, Pérez Bórmida G. Lymphatic cutaneous rickettsiosis caused by Rickettsia conorii in Uruguay. Rev Inst Med Trop São Paulo. 1990;32:313–318. doi: 10.1590/s0036-46651990000500001. [DOI] [PubMed] [Google Scholar]
- 24.Conti-Díaz IA, Moraes-Filho J, Pacheco RC, Labruna MB. Serological evidence of Rickettsia parkeri as the etiological agent of rickettsiosis in Uruguay. Rev Inst Med Trop Sao Paulo. 2009;51:337–339. doi: 10.1590/s0036-46652009000600005. [DOI] [PubMed] [Google Scholar]
- 25.Burkart R, Bárbaro O, Sánchez RO, Gómez DA. Eco-regiones de la Argentina. Buenos Aires, Argentina: Administración de Parques Nacionales, Secretaría de Recursos Naturales y Desarrollo Sustentables; 1999. [Google Scholar]
- 26.Nieri-Bastros FA, Szabó MPJ, Pacheco RC, Soares JF, Soares HS, Moraes-Filho J, Dias RA, Labruna MB. Comparative evaluation of infected and noninfected Amblyomma triste ticks with Rickettsia parkeri, the agent of an emerging rickettsiosis in the New World. Biomed Res Int. 2013;2013(402737) doi: 10.1155/2013/402737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venzal JM, Portillo A, Estrada-Peña A, Castro O, Cabrera P, Oteo JA. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg Infect Dis. 2004;10:1493–1495. doi: 10.3201/eid1008.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco RC, Venzal JM, Richtzenhain LJ, Labruna MB. Rickettsia parkeri in Uruguay. Emerg Infect Dis. 2006;12:1804–1805. doi: 10.3201/eid1211.060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venzal JM, Estrada-Peña A, Portillo A, Mangold AJ, Castro O, de Souza CG, Félix ML, Pérez-Martinez L, Santibáñez S, Oteo JA. Rickettsia parkeri: a rickettsial pathogen transmitted by ticks in endemic areas for spotted fever rickettsiosis in southern Uruguay. Rev Inst Med Trop Sao Paulo. 2012;54:131–134. doi: 10.1590/s0036-46652012000300003. [DOI] [PubMed] [Google Scholar]
- 30.Portillo A, García-García C, Sanz MM, Santibáñez S, Venzal JM, Oteo JA. Case report: a confirmed case of Rickettsia parkeri infection in a traveler from Uruguay. Am J Trop Med Hyg. 2013;89:1203–1205. doi: 10.4269/ajtmh.13-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labruna M, Silveira I, Pacheco R, Szabó M, Ramos H. Rickettsia parkeri in Brazil. Emerg Infect Dis. 2007;13:1111–1113. doi: 10.3201/eid1307.061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nava S, Caparros JA, Mangold AJ, Guglielmone AA. Ticks (Acari: Ixodida: Argasidae, Ixodidae) infesting humans in northwestern Córdoba Province, Argentina. Medicina (B Aires) 2006;66:225–228. [PubMed] [Google Scholar]
- 33.Tomassone L, Conte V, Parrilla G, De Meneghi D. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum ticks, Cochabamba Department, Bolivia. Vector Borne Zoonotic Dis. 2010;10:953–958. doi: 10.1089/vbz.2009.0126. [DOI] [PubMed] [Google Scholar]
- 34.Lado P, Castro O, Labruna MB, Venzal JM. First molecular detection of Rickettsia parkeri in Amblyomma tigrinum and Amblyomma dubitatum ticks in Uruguay. Ticks Tick Borne Dis. 2014;5:660–662. doi: 10.1016/j.ttbdis.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Varela-Stokes AS, Paddock CD, Engber B, Toliver M. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg Infect Dis. 2011;17:2350–2353. doi: 10.3201/eid1712.110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohls GM. Concerning the identity of Amblyomma maculatum, A. tigrinum, A. triste, and A. ovatum of Koch, 1944 (Acarina, Ixodidae) Proc Entomol Soc Wash. 1956;58:143–147. [Google Scholar]
- 37.Guglielmone AA, Mangold AJ, Luciani CE, Viñabal AE. Amblyomma tigrinum (Acari: Ixodidae) in relation to phytogeography of central-northern Argentina with notes on hosts and seasonal distribution. Exp Appl Acarol. 2000;24:983–989. doi: 10.1023/a:1010775528628. [DOI] [PubMed] [Google Scholar]
- 38.Nava S, Mangold AJ, Guglielmone AA. The natural hosts of larvae and nymphs of Amblyomma tigrinum Koch, 1844 (Acari: Ixodidae) Vet Parasitol. 2006;140:124–132. doi: 10.1016/j.vetpar.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Guglielmone AA, Nava S. Las garrapatas del género Amblyomma como parásitos de humanos en la Argentina. In: Cacchione R, Durlach R, Larghi O, Martino P, editors. Temas de Zoonosis III. Buenos Aires, Argentina: Asociación Argentina de Zoonosis; 2006. pp. 269–277. [Google Scholar]
- 40.Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Mangold AJ, Szabó MPJ, Martins JR, González-Acuña D, Estrada-Peña A. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 2006;40:83–100. doi: 10.1007/s10493-006-9027-0. [DOI] [PubMed] [Google Scholar]
- 41.Guglielmone AA, Nava S. Las garrapatas Argentinas del género Amblyomma (Acari: Ixodidae): distribution y hospedadores. RIA. 2006;35:133–153. [Google Scholar]
- 42.Venzal JM, Guglielmone AA, Estrada Peña A, Cabrera PA, Castro O. Ticks (Ixodida: Ixodiae) parasitizing humans in Uruguay. Ann Trop Med Parasitol. 2003;97:769–772. doi: 10.1179/000349803225002327. [DOI] [PubMed] [Google Scholar]
- 43.Pacheco RC, Echaide IE, Alves RN, Beletti ME, Nava S, Labruna MB. Coxiella burnetii in ticks, Argentina. Emerg Infect Dis. 2013;19:344–346. doi: 10.3201/eid1902.120362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomassone L, Nuñez P, Gürtler RE, Ceballos LA, Orozco MM, Kitron UD, Farber M. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks, Argentina. Emerg Infect Dis. 2008;14:1953–1955. doi: 10.3201/eid1412.080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker DH. The challenges of rickettsial diagnosis, research, and awareness in Latin America. Acta Med Costarric. 2013;55((Suppl 1)):4–10. [Google Scholar]