A disproportionate number of incident diffuse large B-cell lymphoma (DLBCL) occurs in elderly patients. Evidence from this analysis suggests that chemoimmunotherapy effectiveness is similar for elderly patients in routine oncology practice and younger patients from clinical trial settings. Age alone should not discourage the use of guideline-recommended therapies for DLBCL.
Keywords: Diffuse large B-cell lymphoma, Elderly patients, Chemotherapy, Treatment, Survival
Abstract
Background.
The incidence of diffuse large B-cell lymphoma (DLBCL) occurs disproportionately in elderly patients. We evaluated real-world treatment patterns and outcomes in elderly DLBCL patients in the U.S.
Materials and Methods.
A retrospective cohort analysis of 9,333 DLBCL patients from the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database was conducted. Patients were diagnosed between January 1, 2000, and December 31, 2007; were aged >66 years, and were continuously enrolled in Medicare Part A and B in the year prior to diagnosis. Within 3 months of diagnosis, 4,565 (49%) received rituximab plus chemotherapy (R+chemo), 2,181 (23%) received chemotherapy only, and 467 (5%) received rituximab monotherapy (R-mono). Cox proportional hazards regression assessed overall survival between R+chemo versus chemotherapy only and R-mono versus no treatment.
Results.
Overall, 23% of patients received no treatment, and the proportion was higher among those aged >80 years (33%). Patients receiving R+chemo were younger and more likely white compared with those receiving chemotherapy only. Patients receiving R-mono were older and more likely female compared with those not treated. In multivariate analysis, patients receiving chemotherapy only had a twofold increased mortality risk versus R+chemo, and this was confirmed in a subanalysis of patients aged >80 years. A 91% higher mortality risk was noted with receipt of fewer than six cycles versus six cycles of chemotherapy or chemoimmunotherapy. Patients receiving R-mono had a 69% decreased mortality risk compared with patients who were not treated.
Conclusion.
This real-world analysis of elderly DLBCL patients confirmed that 23% do not receive treatment. Overall survival is higher for patients receiving R+chemo and R-mono relative to chemotherapy only and no treatment, respectively. Suboptimal durations of therapy with curative intent (fewer than six cycles) were associated with poorer outcomes.
Implications for Practice:
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoma that is curable with guideline-based chemoimmunotherapy; however, in real-world practice, very elderly patients are less likely to receive treatment. Evidence from this analysis suggests that chemoimmunotherapy effectiveness is generally similar between elderly patients in routine oncology practice and younger patients from clinical trial settings. This real-world comparative effectiveness study concludes that age alone should not discourage the use of guideline-recommended therapies for DLBCL, and in the absence of other reasons for withholding treatment, elderly patients should be given guideline-based treatment as often as nonelderly patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common histologic subtype of non-Hodgkin lymphoma (NHL) and accounts for approximately 25% of all lymphoid neoplasms in the developed world [1]. It is an aggressive form of NHL for which survival without treatment is measured in months. Like most other NHLs, there is a male predominance, with ∼55% of cases occurring in men [1]. Incidence increases with age, and the median age at presentation is 64 years [2]. Although outcomes for elderly patients with lymphoma are worse, very few differences have been described for morphology and clinical presentation between young and elderly patients with lymphoma [3, 4].
There is a paucity of data regarding the treatment of the very elderly with DLBCL, in part because of the lack of participation and/or inclusion of this population in clinical trials [5]. Very elderly patients have diverse attitudes toward cancer treatment. Although some desire aggressive treatment modalities, a large proportion declines therapies offered by oncologists [6]. In addition, clinicians are often concerned with the ability of elderly patients to tolerate intensive therapy, which can be complicated by the presence of comorbidity, diminished organ function, and altered drug metabolism [7].
R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab) is the standard treatment regimen for DLBCL, based on randomized trials that demonstrated significantly improved overall survival compared with CHOP alone in elderly patients with DLBCL [8–12]. R-CHOP is recommended as first-line therapy for most DLBCL patients across all disease stages [13]. Some reports suggest that patients aged 80 years or older may benefit from less intensive chemotherapy plus rituximab [14, 15]. Others have concluded that the best way to improve the survival of elderly patients with lymphoma who do not have significant comorbid illness is to treat them with an optimal chemotherapy regimen [16]. Given the increase in incidence of DLBCL related to the aging population, understanding the real-world treatment patterns and outcomes of guideline-recommended care is crucial to improving outcomes for this potentially curable disease.
Materials and Methods
Data Sources
Data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database were used for these analyses. Institutional review board approval was waived because there are no personal identifiers in the SEER-Medicare database. The SEER-Medicare database is a collaborative effort of the National Cancer Institute (NCI), the SEER registries, and the Centers for Medicare and Medicaid Services and provides information on Medicare patients included in SEER, a nationally representative collection of 18 population-based registries of all incident cancers from diverse geographic areas [17]. The linked database includes all incident cancer patients reported to the SEER registries and cross-matched with a master file of enrollees in Medicare [18], with approximately 97% of persons aged 65 years or older eligible for Medicare. Inpatient care, skilled nursing care, home health care, and hospice care are covered services under Medicare Part A, whereas Part B reimburses for physician and outpatient care, with about 95% of beneficiaries subscribing to Part B. The SEER-Medicare linkage includes all Medicare-eligible persons reported to SEER through 2007 and their Medicare claims for Part A (inpatient) and Part B (outpatient and physician services) through 2009.
Study Population
DLBCL was identified using two World Health Organization International Classification of Diseases for Oncology, third edition (ICD-O-3) histology codes in the SEER data set: code 9680 (malignant lymphoma, large B-cell, diffuse, centroblastic, not otherwise specified [NOS]) and code 9684 (malignant lymphoma, large B-cell, diffuse, immunoblastic, NOS). Eligibility criteria for the analysis included a diagnosis of untreated primary DLBCL from January 1, 2000, to December 31, 2007; age of 66 years or older; and continuous enrollment in both Medicare Parts A and B in the 12 months preceding the diagnosis. Patients were excluded if the cancer site code was for any part of the central nervous system, if diagnosis was made by death certificate or autopsy, or if patients were enrolled in a health maintenance organization at any time during the 12 months prior to diagnosis (because treatment and outcome data were not available). Of the 10,515 patients identified, 2,120 (20%) did not receive treatment with chemotherapy, immunotherapy, or radiation therapy. There were 686 patients (6.5%) who received radiation only and were excluded from this comparative effectiveness analysis. Of the remaining patients, 7,213 initiated chemotherapy with or without radiation within 3 months of diagnosis and were included in the final analytic cohort.
Study Variables
Patient age at diagnosis was used as a continuous variable and was stratified into five groups: 66–70, 71–75, 76–80, 81–85, and >85 years. Race and ethnicity were categorized into three mutually exclusive groups: white, nonwhite, and unknown. Socioeconomic information was not available for individual patients but was available as aggregate data at the ZIP code or census-tract level. We used quartiles of median annual household income and percentage of the adult population by education level as proxies for socioeconomic status. Education was categorized as percentages: without a high school diploma, with high school only, with some college, and with at least a college degree. The American Joint Committee on Cancer classification scheme was used to classify patients as early (stage I and II) or advanced (stage III and IV).
Data were abstracted from the following five merged SEER-Medicare files to identify claims for chemotherapy, immunotherapy, and/or radiation administration [19]: Medicare provider analysis and review, carrier claims, outpatient claims, durable medical equipment, and prescription drug event files. Each of these files provides calendar-year summaries of reimbursed services. Type of treatment was characterized and quantified using the ICD diagnosis codes, ICD procedural codes, Current Procedural Terminology codes, Healthcare Common Procedure Coding System codes, and revenue center codes. Chemotherapy claims were searched for specific drug codes to identify the type of chemotherapy administered to patients. The absence of chemotherapy, immunotherapy, or radiation claims was interpreted as evidence of no treatment. The first chemotherapy or immunotherapy claim within the first 3 months after diagnosis indicated the start of therapy. Patients receiving chemotherapy (with or without radiation) were classified into one of three treatment groups based on all chemotherapy and/or immunotherapy drugs administered during the first 60 days following initiation of treatment. The three groups included rituximab monotherapy (R-mono), rituximab and chemotherapy (R+chemo), and chemotherapy alone (chemotherapy only). The number of R+chemo and chemotherapy-only treatment cycles and the number of R-mono doses were defined as the number of consecutive claims per regimen, with no gaps >45 days beginning on the date of first regimen claim.
The NCI comorbidity index [20] was calculated for each patient using diagnosis and procedure codes in the year preceding diagnosis to identify the 15 noncancer comorbidities from the Charlson Comorbidity Index [21]. Specific conditions were required to appear on at least two different claims that were >30 days apart to ensure that “rule out” diagnoses were not counted as comorbid conditions. A weight was assigned to each condition based on its potential to influence 2-year mortality, and these weights are summed to obtain an index for each patient. The index accounts for the number and the severity of the conditions, with higher scores indicating a greater burden of comorbid disease.
Statistical Analysis
All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute Inc., Cary, NC, http://www.sas.com). Pairwise comparisons were carried out between R+chemo versus chemotherapy only and R-mono versus no treatment. Demographic and clinical characteristics were summarized descriptively and compared using the chi-square test for categorical variables and analysis of variance or t tests for continuous variables to determine differences between groups. A p value <.05 was considered statistically significant.
Two approaches were used for the multivariate survival analyses comparing R+chemo versus chemotherapy only and R-mono versus no treatment including Cox proportional hazards regression and propensity score-weighted analyses. The Cox regression was used to explore predictors of overall risk of death, adjusting for potential confounders that were selected from demographic and clinical characteristics. The fully adjusted model included all static variables of patient characteristics selected based on a priori beliefs that these factors are associated with receipt of treatment. The propensity score is the conditional probability of each patient receiving a specific treatment based on baseline characteristics. Multinomial logistic regression was used to calculate a propensity score for each patient. The effect of the propensity score weights was to balance the groups to reduce potential bias associated with treatment selection and to obtain better estimates of the treatment effect on survival. A propensity score-weighted Cox proportional hazards regression model was fitted to compare overall survival among treatment groups.
In the Cox models, follow-up was calculated beginning on the date of treatment initiation until the first occurrence of a censoring event: date of death, development of a second primary tumor, the last date for which Medicare claims were available, or the end of the follow-up period (December 31, 2009). The date of death was assigned using the Medicare date or SEER date of death if the Medicare date was missing. All other patients were assumed to be alive at the end of the follow-up period, although they may have been censored earlier for other reasons.
Results
Patient Characteristics
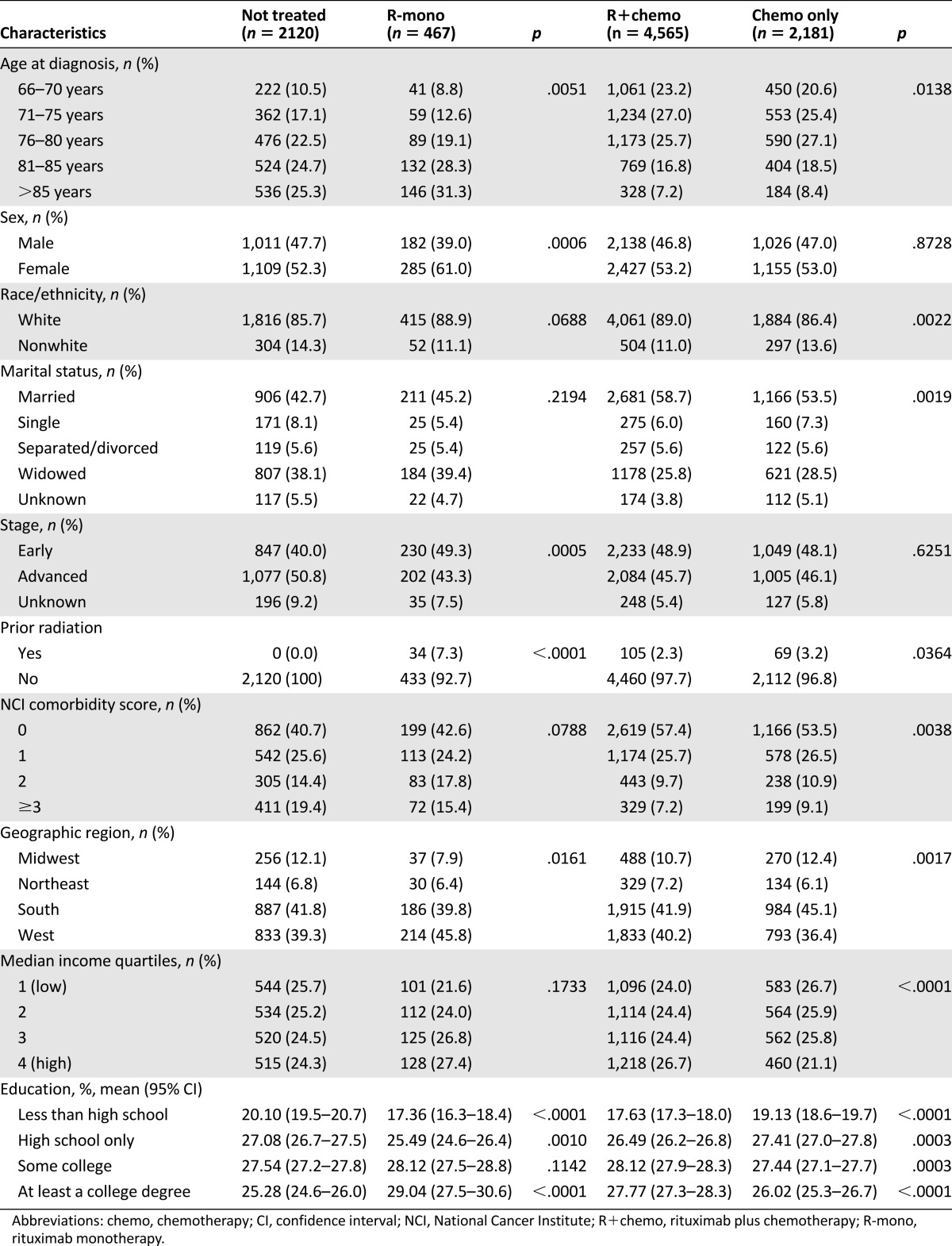
Table 1 shows the distribution of patient characteristics by treatment group. Patients receiving R-mono were the oldest at diagnosis, with a mean age of 82 years, followed by the group not treated (mean age: 80 years) and the R+chemo and chemotherapy-only groups (mean age: 76 years). Patients receiving R+chemo were slightly younger (≤75 years: 50% vs. 46%, p < .05), were more likely white (89% vs. 86%, p < .01) and married (59% vs. 54%, p < .01), and had a lower comorbidity burden (NCI score 0: 57% vs. 54%, p < .01) compared with those receiving chemotherapy only. Patients receiving R-mono were older (>80 years: 60% vs. 50%, p < .01), were more likely female (61% vs. 52%, p < .001), had earlier stage disease (49% vs. 40%, p < .001), and had a nonsignificantly lower comorbidity burden compared with those not treated. Among the treated groups, R-mono had the highest proportion of patients with prior radiation therapy (7%) compared with the chemotherapy-only group (3.2%) and the R+chemo group (2.3%).
Table 1.
Demographic and clinical characteristics at baseline
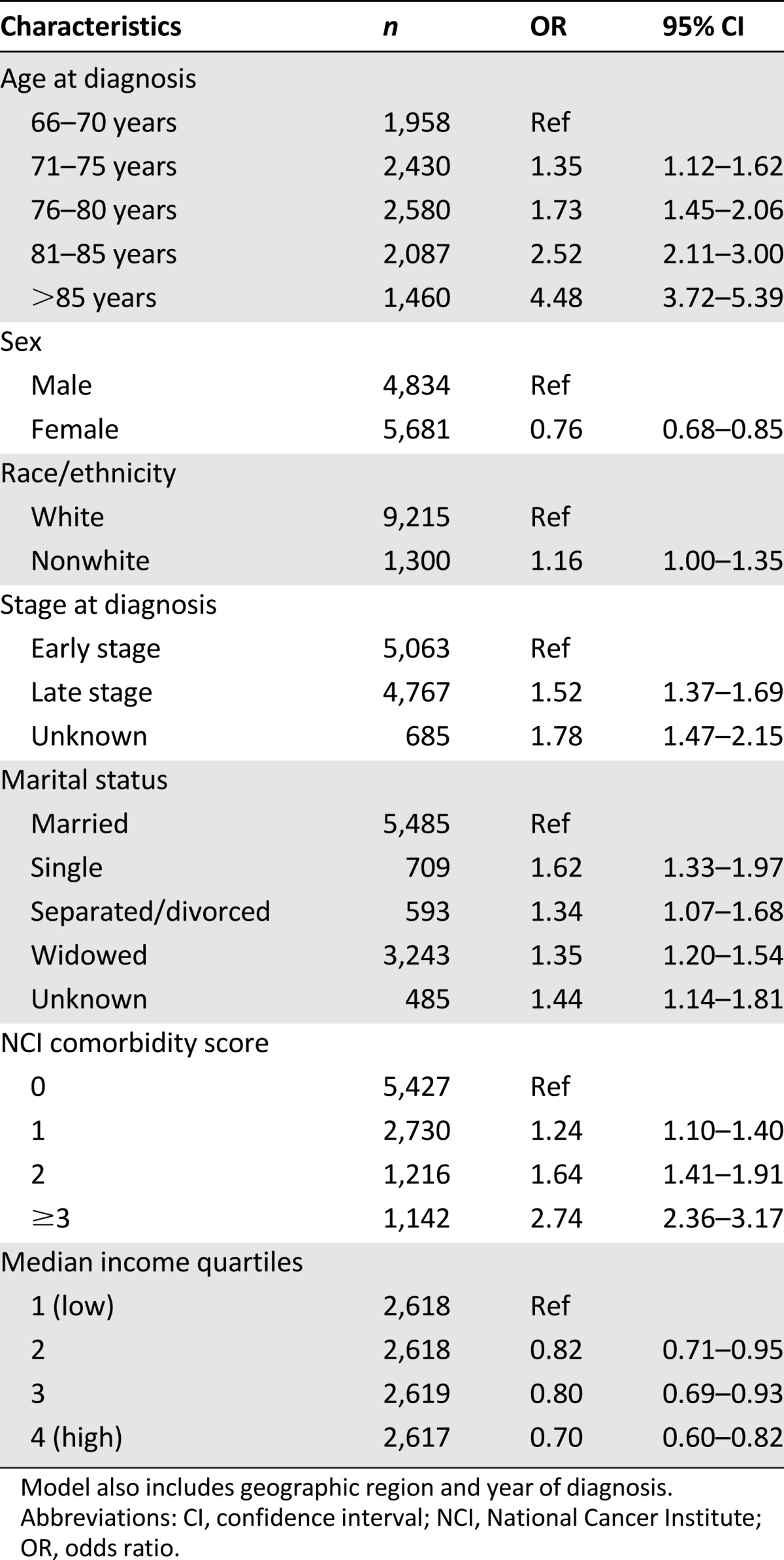
In the adjusted logistic regression model (Table 2), increasing age and higher NCI comorbidity score appeared to be the strongest predictors of receiving no treatment. Nonwhite race, male sex, unmarried status, and lower income were also predictive of receiving no treatment.
Table 2.
Factors associated with the odds of not receiving treatment
Treatment Patterns
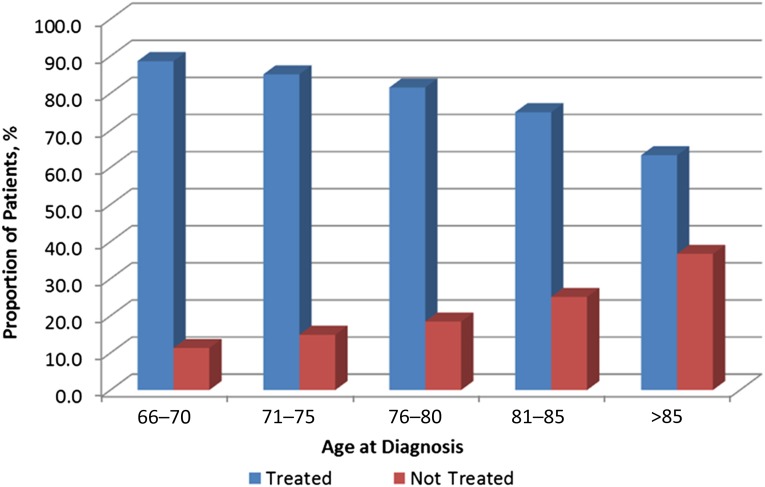
In this cohort of 9,333 Medicare patients, 23% did not receive any treatment with either chemotherapy and/or radiation therapy. As age increased, the treatment rate significantly decreased, especially among patients aged >80 years (Fig. 1). Among those aged >80 years, 33% were not treated.
Figure 1.
Age by treatment status.
Among patients who received treatment within 3 months after diagnosis, 4,565 (63%) received R+chemo, 2,181 (30%) received chemotherapy only, and 467 (6.5%) received R-mono. Use of R+chemo increased during the study time period, from 11% in 2000% to 76% in 2007, whereas use of chemotherapy only declined from 87% in 2000% to 17% in 2007 (supplemental online Fig. 1). R-mono use remained fairly consistent throughout the study time period.
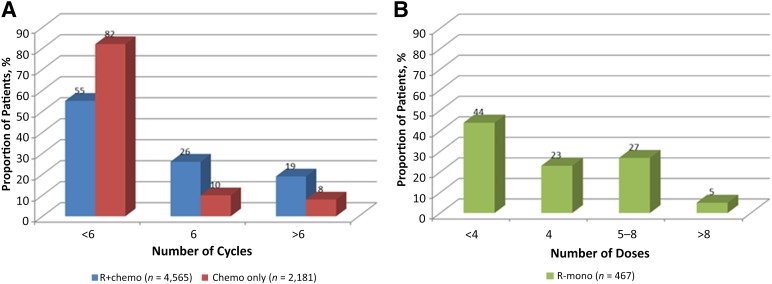
The median time to treatment initiation was 36 days from date of diagnosis. In general, most patients (64%) received fewer than six cycles of therapy. Within each treatment cohort, 55% and 82% of patients in the R+chemo and chemotherapy-only cohorts, respectively, received fewer than six cycles of therapy. Stratified by disease stage, 59% and 83% of early stage patients and 52% and 82% of advanced-stage patients received fewer than six cycles of R+chemo and chemotherapy only, respectively. More R+chemo cycles than chemotherapy-only cycles (mean cycles: 5 vs. 3, p < .0001) were delivered (Fig. 2). The median time interval between cycles of R+chemo or chemotherapy only was 22.3 days or 22.0 days, respectively, among early stage patients and 22.6 days or 22.0 days, respectively, among those with advanced-stage disease. Among those who received R-mono, 207 (44%) received <4 doses (Fig. 2). All patients who received fewer than four doses of R-mono also received radiation therapy. Stratified by stage, 38% of early stage patients and 53% of advanced-stage patients received <4 doses of R-mono. The median time interval between each dose of rituximab was 8.3 days among those with early stage disease and 9.0 days among those with advanced-stage disease.
Figure 2.
Number of treatment administrations of first-line therapy. (A): R+chemo versus chemotherapy only. (B): Rituximab monotherapy.
Abbreviations: chemo, chemotherapy; R+chemo, retuximab plus chemotherapy; R-mono, rituximab monotherapy.
Clinical Outcomes
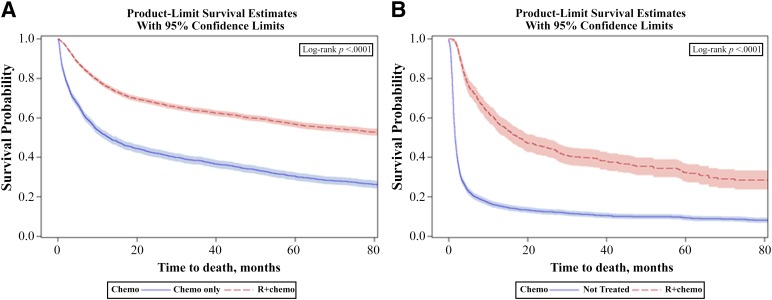
Figure 3 shows the unadjusted median overall survival of 96 months in the R+chemo group versus 13 months in the chemotherapy-only group (log-rank p < .0001). In the multivariate model (Table 3), patients receiving chemotherapy only had a twofold increased risk of mortality compared with R+chemo patients, and this was confirmed in the propensity score-weighted Cox regression model (hazard ratio [HR]: 2.11; 95% confidence interval [CI]: 1.98–2.26) (data not shown). In a subgroup analysis of patients aged ≥80 years, almost identical mortality risk reductions were observed with R+chemo relative to chemotherapy only as in the overall population. Supplemental online Figure 2 shows significant survival differences by stage among patients treated with R+chemo and chemotherapy only. Median overall survival was 77 months in the early stage group and 24 months in the advanced-stage group (log-rank p < .0001). We stratified the Cox regression models by stage (supplemental online Tables 1 and 2) and found similar mortality risks associated with receipt of chemotherapy only versus R+chemo among patients with early stage disease (HR: 1.94; 95% CI: 1.73–2.17) and advanced-stage disease (HR: 2.11; 95% CI: 1.90–2.34) compared with the overall population.
Figure 3.
Unadjusted overall survival. (A): R+chemo versus chemotherapy only. (B): Not treated versus R-mono.
Abbreviations: chemo, chemotherapy; R+chemo, retuximab plus chemotherapy; R-mono, rituximab monotherapy.
Table 3.
Multivariate analysis of overall survival, R+chemo versus chemo only
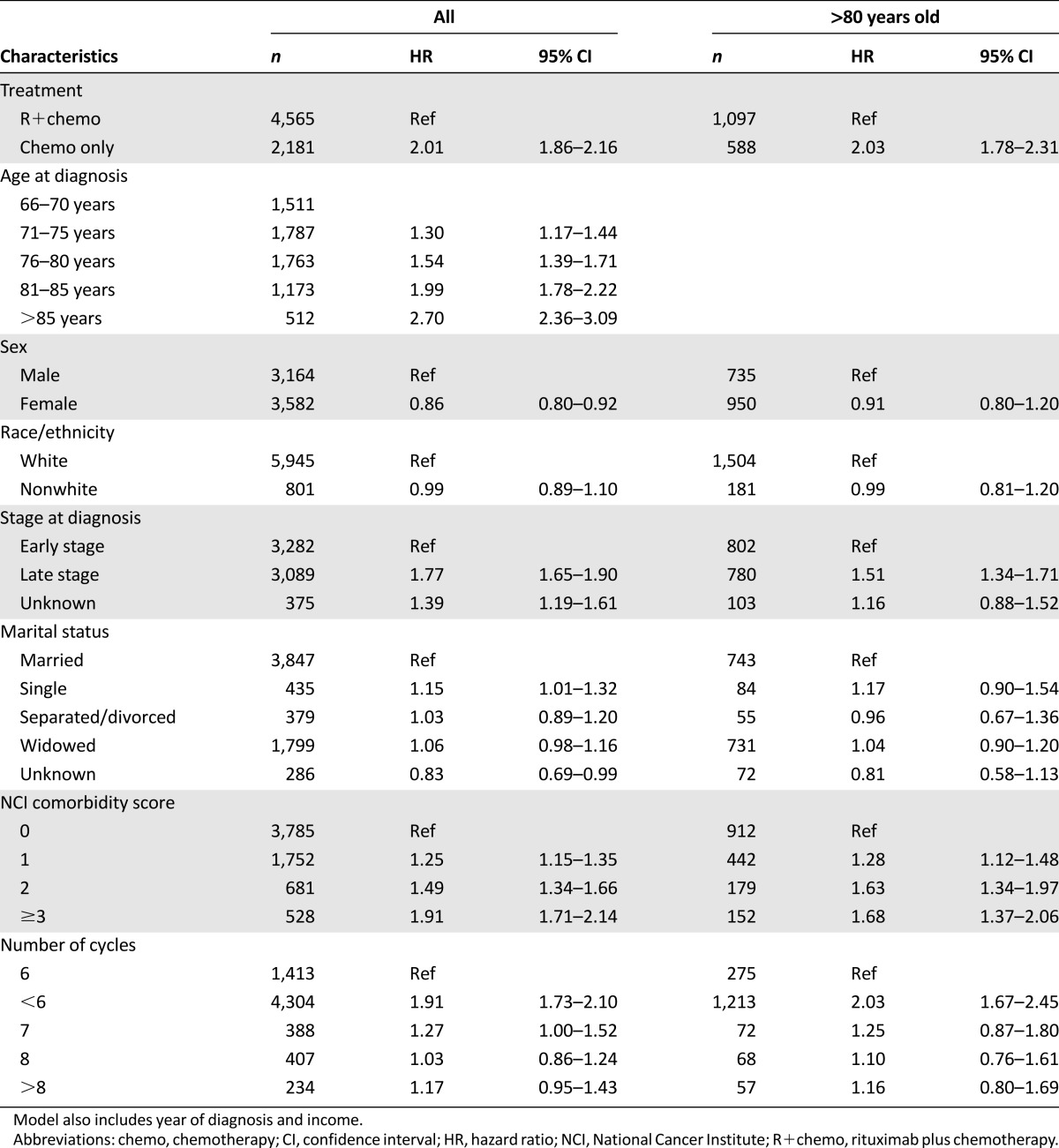
In general, increasing age, increasing comorbidity score, and advanced stage were associated with significant increases in mortality risk. Female sex exhibited a protective effect on mortality compared with male counterparts, and this was present in the rituximab-containing cohorts only. A 91% increased mortality risk was noted with receipt of fewer than six cycles compared with six cycles of chemotherapy and/or immunotherapy (Table 3). Among patients aged ≥80 years receiving <6 cycles of chemotherapy and/or immunotherapy, there was a 2-fold increased mortality risk compared with 6 cycles of therapy. Stratifying by stage (supplemental online Tables 1 and 2), patients with advanced disease (HR: 2.20; 95% CI: 1.92–2.52) had a higher mortality risk with receipt of <6 cycles compared with 6 cycles of therapy than patients with early stage disease (HR: 1.55; 95% CI: 1.33–1.80). In patients aged ≥80 years and diagnosed with early stage disease, receiving <6 cycles was associated with an 89% increased risk of death compared with receipt of 6 cycles of therapy. In patients aged ≥80 years and diagnosed as advanced stage, receiving <6 cycles of therapy was associated with a 2-fold increased risk of mortality compared with receipt of 6 cycles of therapy.
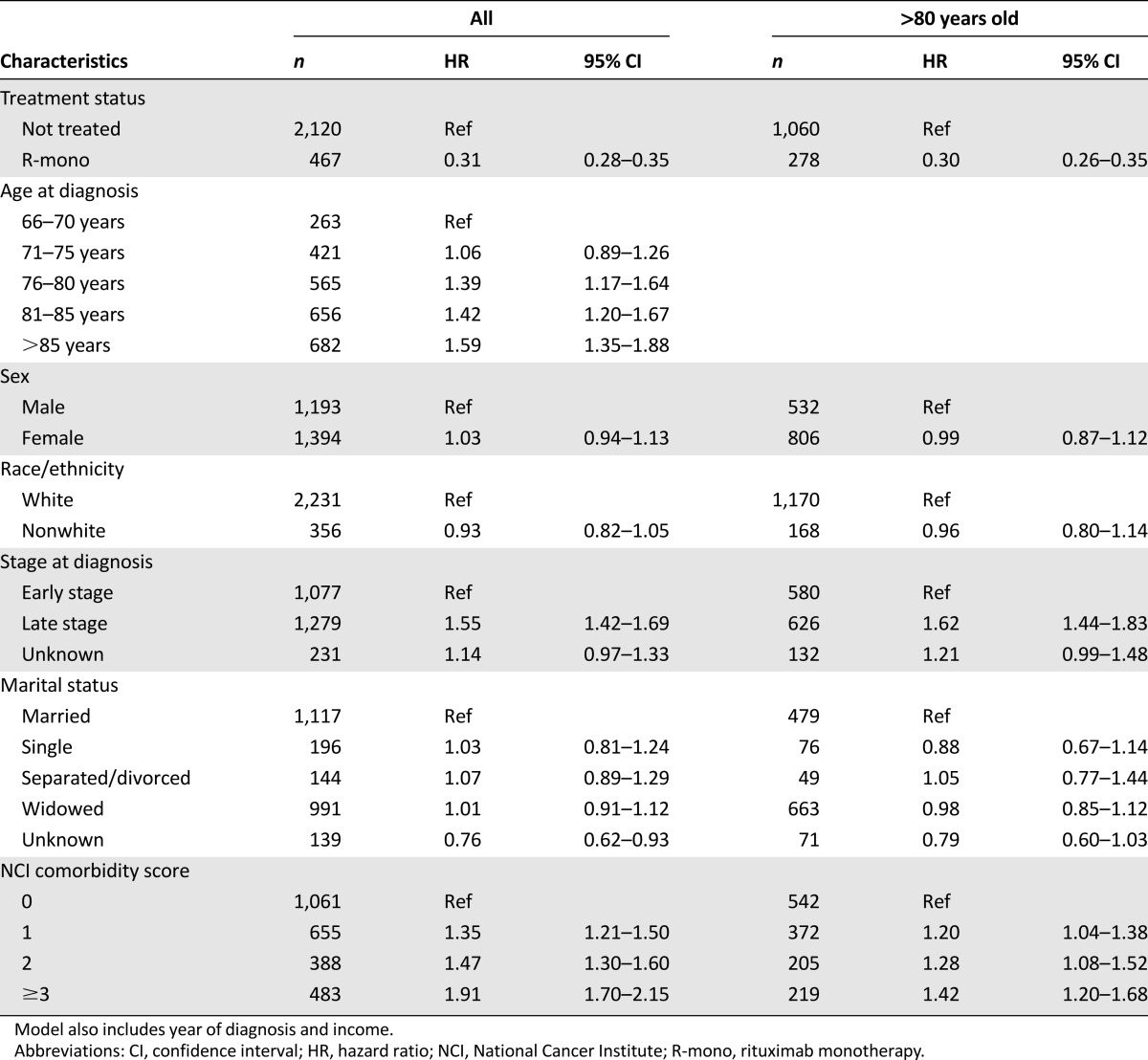
In Figure 3, the unadjusted median overall survival was 18 months in the R-mono group versus 2 months in the not-treated group (log-rank p < .0001). Table 4 shows the results of the multivariate analysis in which R-mono patients exhibit a 69% lower risk of death compared with patients not treated. The propensity-weighted model (data not shown) confirmed this significant risk reduction (HR: 0.38; 95% CI: 0.33–0.42). Increasing age and comorbidity score were associated with significant increases in mortality. A 55% increased mortality risk was noted among those diagnosed at advanced stage. In a subgroup analysis of patients aged ≥80 years, the benefit of R-mono relative to no treatment was similar to the overall population.
Table 4.
Multivariate analysis of overall survival, not treated versus R-mono
Discussion
This real-world analysis of elderly DLBCL patients in the SEER database affords us a unique ability to look at broad practice patterns and outcomes for older patients in the U.S., acknowledging there are inherent limitations with registry-based data. This is particularly important, given the increasing incidence of older adults diagnosed with DLBCL as a consequence of demographic shifts in the U.S. and worldwide. Randomized studies have clearly established the addition of rituximab to chemotherapy as the standard of care for older patients, although most clinical trials have few patients in the oldest (aged >80 years) categories [11, 12]. In this analysis, we have identified that 72% of older adults in this series received chemotherapy, either alone or with rituximab. Outcome analysis confirms a twofold reduction in mortality with the addition of rituximab in multivariate and propensity score-weighted Cox regression models. Importantly, this benefit was conferred across age categories, including those aged ≥80 years, with equal magnitude. Other population-based analyses have similarly confirmed the benefit of immunotherapy’s addition to chemotherapy [8] but with a smaller emphasis on older adults. This work also confirms an adverse impact on mortality for known factors such as age, comorbidity, and stage and a protective effect of female sex that has been suggested to potentially have a biological basis [22].
Although actual chemotherapy relative dose intensity could not be adequately assessed in this study, we found that suboptimal cycles of therapy with curative intent (fewer than six cycles) were frequently used and were associated with poorer survival outcomes. Decreasing dosage or duration of therapy can alleviate toxicity but, generally, at the compensatory cost of decreased effectiveness. Retrospective studies have shown that the single most important predictor of survival was dose intensity of scheduled chemotherapy [23, 24]. Others have suggested that older adults should be treated initially with the same dosing and schedule as younger adults, with adjustments made to future cycles according to tolerance [25–27]. We found no survival advantage with receipt of eight cycles compared with six cycles of chemotherapy and/or immunotherapy, and this was supported in a randomized study that recently reported similar overall survival with receipt of eight cycles compared with six cycles of R-CHOP [28].
At the other end of the spectrum, this analysis demonstrated that a full 23% of older DLBCL patients in the SEER database received no immunotherapy, chemotherapy, or radiation treatment for their disease. The decision to forgo therapy in the older adult often involves many factors, including patient and family preferences; physician concerns for poor tolerance of therapy; prohibitive comorbidity or frailty; and, at times, ageism. Interestingly, in this analysis, there was a significant benefit to immunotherapy alone over no therapy that persisted after adjusting for comorbidity burden and other factors in the multivariate survival analysis. Immunotherapy conferred a somewhat surprising survival benefit, with a 69% reduction in risk of death in multivariate analysis. Age, stage, and comorbidity remained associated with increased mortality in the R-mono versus no-treatment groups, but age >80 years did not change the magnitude of benefit. It is possible that the R-mono group represented a more “fit” population, with an increased percentage of female sex and early stage disease and a trend toward less comorbidity despite the older median age. Although rituximab monotherapy is generally associated with low toxicity, an analysis of this type cannot determine impact on quality of life.
Receipt of treatment varied by sex, race, income, and marital status, similar to patterns observed in prior oncology research [29, 30]. In the current study, nonwhite race, male sex, unmarried status, and lower income were predictive of not receiving treatment. Reducing the disparity of nonclinical factors on the receipt of cancer therapy may help improve outcomes among these patients. Further research is warranted to better quantify the full spectrum of nonclinical factors that contribute to receipt of cancer therapy to ensure appropriate cancer care for all patients.
Use of the SEER-Medicare data for this type of analysis has several strengths, including the large sample size from a population-based registry and the diverse geographic representation of DLBCL patients in the U.S. The database includes longitudinal data with claims for covered services from the time a person is eligible for Medicare until the date of death, regardless of residence or service area. The SEER-Medicare database does not provide information on performance status or lifestyle factors that could have influenced clinicians’ decisions to treat or the specific regimen to administer. Poor performance status may limit the administration of anthracyclines and may independently and adversely affect survival. Furthermore, classifying patients into early or advanced stage may be subject to interpretation because stage II patients could be considered either early or advanced stage depending on bulk and systemic symptoms. In such an analysis, we were also unable to investigate predictive factors such as disease biology (cell of origin) or clinical risk scores (age-adjusted International Prognostic Index). Information regarding treatment patterns and characteristics of patients enrolled in health maintenance organizations or fee-for-service plans were not available because these data are not collected by Medicare. Treatment patterns, prognosis, and complications may differ among these alternative health care plans and Medicare enrollees, and this would be a productive area for additional evaluation.
Conclusion
Overall survival of elderly DLBCL patients who were treated under real-world conditions was higher for patients receiving R+chemo and R-mono relative to chemotherapy only and no treatment, respectively. Furthermore, receipt of six cycles of chemotherapy and/or immunotherapy improved overall survival compared with receipt of fewer than six cycles. There was no demonstrable benefit with eight cycles over six cycles. These findings imply that age alone should not discourage the use of guideline-recommended therapies for DLBCL and should offer support for the use of treatments in elderly patients that are consistent to those administered to younger patients. Further investigation of appropriate treatment algorithms for very elderly DLBCL patients and of survival, quality-of-life outcomes, and attitudes toward treatment by both physicians and patients is essential for the future.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (Bethesda, MD); the Office of Information Services and the Office of Strategic Planning, Health Care Financing Administration (Baltimore, MD); Information Management Services, Inc. (Silver Spring, MD); and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. This study was funded by Genentech, Inc., through a contract with Q.D. Research, Inc. The interpretation and reporting of these data are the sole responsibility of the authors.
Author Contributions
Conception/Design: Paul A. Hamlin, Sacha Satram-Hoang, Carolina Reyes, Khang Q. Hoang, Sandra Skettino
Provision of study material or patients: Sacha Satram-Hoang, Sridhar R. Guduru
Collection and/or assembly of data: Sacha Satram-Hoang, Khang Q. Hoang, Sridhar R. Guduru
Data analysis and interpretation: Paul A. Hamlin, Sacha Satram-Hoang, Carolina Reyes, Khang Q. Hoang, Sridhar R. Guduru, Sandra Skettino
Manuscript writing: Paul A. Hamlin, Sacha Satram-Hoang, Carolina Reyes, Khang Q. Hoang, Sandra Skettino
Final approval of manuscript: Paul A. Hamlin, Sacha Satram-Hoang, Carolina Reyes, Khang Q. Hoang, Sridhar R. Guduru, Sandra Skettino
Disclosures
Sacha Satram-Hoang: Genentech, Inc. (RF); Paul A. Hamlin: Genentech, Gilead, Spectrum (C/A); Seattle Genetics, Pfizer, GlaxoSmithKline, Jansen&Jansen, Spectrum (RF); Sandra Skettino: Genentech, Inc. (E); Roche (OI); Carolina Reyes: Genentech, Inc. (E); Roche (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011;117:2530–2540. doi: 10.1002/cncr.25765. [DOI] [PubMed] [Google Scholar]
- 3.Effect of age on the characteristics and clinical behavior of non-Hodgkin’s lymphoma patients. The Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1997;8:973–978. [PubMed] [Google Scholar]
- 4.Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774–779. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 5.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 6.Oxnard GR, Fidias P, Muzikansky A, et al. Non-small cell lung cancer in octogenarians: Treatment practices and preferences. J Thorac Oncol. 2007;2:1029–1035. doi: 10.1097/JTO.0b013e318158d4a2. [DOI] [PubMed] [Google Scholar]
- 7.Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (≥80 years) non-Hodgkin lymphoma: Impact of functional status and co-morbidities on outcome. Br J Haematol. 2012;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- 8.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 9.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 10.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 11.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 12.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Hodgkin’s lymphomas: Version 3.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed August 18, 2014.
- 14.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 15.Weidmann E, Neumann A, Fauth F, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22:1839–1844. doi: 10.1093/annonc/mdq671. [DOI] [PubMed] [Google Scholar]
- 16.Kouroukis CT, Browman GP, Esmail R, et al. Chemotherapy for older patients with newly diagnosed, advanced-stage, aggressive-histology non-Hodgkin lymphoma: A systematic review. Ann Intern Med. 2002;136:144–152. doi: 10.7326/0003-4819-136-2-200201150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 19.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 23.Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 24.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87:429–430. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thieblemont C, Coiffier B. Lymphoma in older patients. J Clin Oncol. 2007;25:1916–1923. doi: 10.1200/JCO.2006.10.5957. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: A nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 27.Lee KW, Kim DY, Yun T, et al. Doxorubicin-based chemotherapy for diffuse large B-cell lymphoma in elderly patients: Comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer. 2003;98:2651–2656. doi: 10.1002/cncr.11846. [DOI] [PubMed] [Google Scholar]
- 28.Murawski N, Pfreundschuh M, Zeynalova S, et al. Optimization of rituximab for the treatment of DLBCL (I): Dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol. 2014;25:1800–1806. doi: 10.1093/annonc/mdu208. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Burau KD, Fang S, et al. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231–3241. doi: 10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.