Abstract
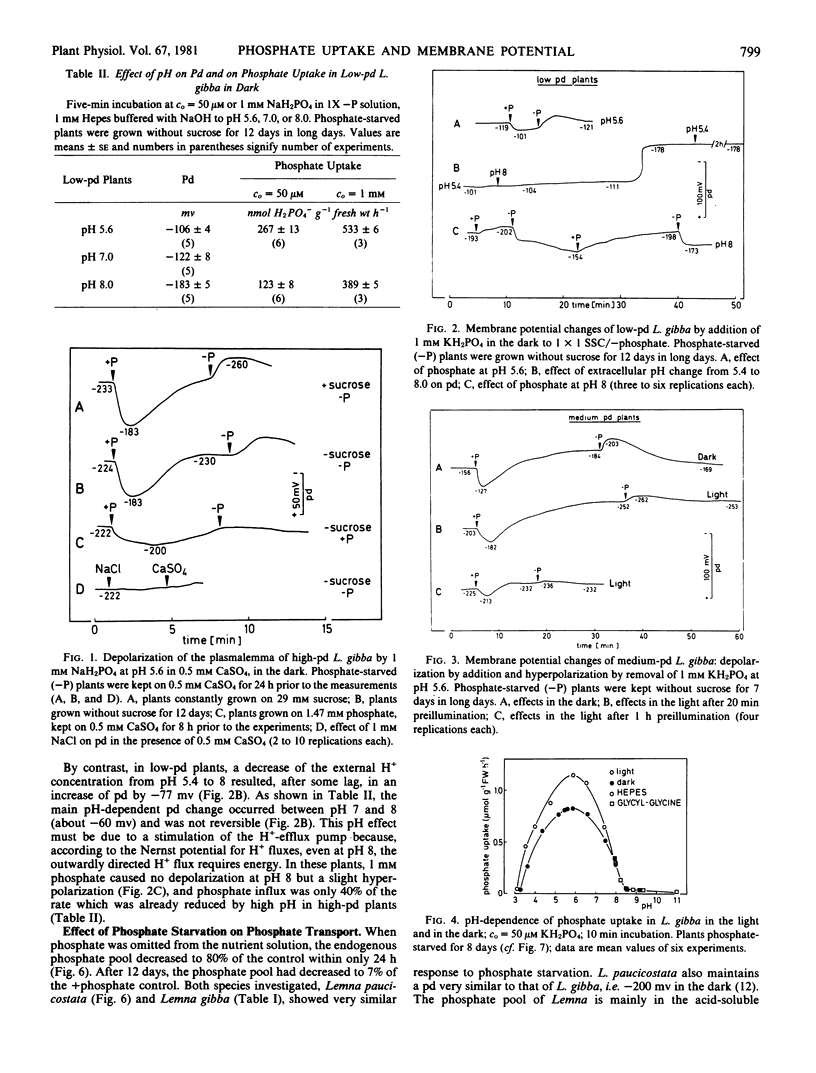
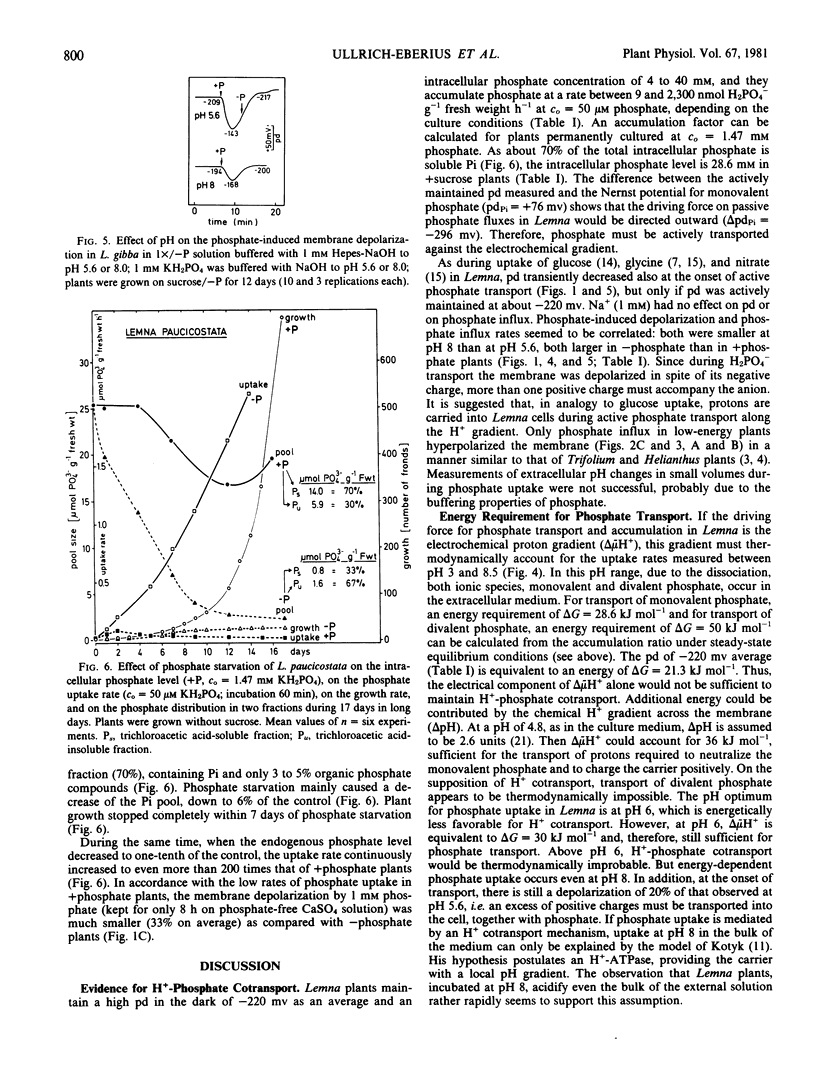
High rates of phosphate uptake into phosphate-starved Lemna gibba L. G1 were correlated with a high membrane potential (pd = −220 millivolts). In plants maintaining a low pd (−110 millivolts), the uptake rate was only 20% of that of high-pd plants. At the onset of phosphate transport, the membrane of high-pd plants was transiently depolarized. This effect was much smaller in low-pd plants. Light stimulated phosphate uptake and the repolarization upon phosphate-induced depolarization, especially in plants grown without sucrose. The phosphate uptake rate was optimal at pH 6 and decreased with increasing pH, corresponding to the phosphate-induced pd changes. Phosphate starvation stimulated the uptake and increased the phosphate-induced depolarization, thus indicating that phosphate uptake depends on the intracellular phosphate level. It is suggested that uptake of monovalent phosphate in Lemna gibba proceeds by an H+ cotransport dependent on the proton electrochemical potential difference and, hence, on the activity of an H+ -extrusion pump.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst-Pauwels G. W., Peters P. H. Effect of the medium pH and the cell pH upon the kinetical parameters of phosphate uptake by yeast. Biochim Biophys Acta. 1977 May 2;466(3):488–495. doi: 10.1016/0005-2736(77)90341-8. [DOI] [PubMed] [Google Scholar]
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETHERTON B., HIGINBOTHAM N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science. 1960 Feb 12;131(3398):409–410. doi: 10.1126/science.131.3398.409. [DOI] [PubMed] [Google Scholar]
- Fischer E., Lüttge U. Membrane Potential Changes Related to Active Transport of Glycine in Lemna gibba G1. Plant Physiol. 1980 May;65(5):1004–1008. doi: 10.1104/pp.65.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I. The effect of ionophores on phosphate and arsenate transport in Micrococcus lysodeikticus. FEBS Lett. 1977 Sep 15;81(2):264–266. doi: 10.1016/0014-5793(77)80531-0. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E. Proton-coupled hexose transport in Chlorella vulgaris. FEBS Lett. 1973 Dec 15;38(1):16–18. doi: 10.1016/0014-5793(73)80501-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Roomans G. M., Blasco F., Borst-Pauwels G. W. Cotransport of phosphate and sodium by yeast. Biochim Biophys Acta. 1977 May 16;467(1):65–71. doi: 10.1016/0005-2736(77)90242-5. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A., Belsky M. M., Goldstein S. Phosphate uptake in an obligately marine fungus: a specific requirement for sodium. Science. 1967 Jan 6;155(3758):93–94. doi: 10.1126/science.155.3758.93. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick R. M., Miller A. G. Measurement of the Cytoplasmic pH in Nitella translucens: Comparison of Values Obtained by Microelectrode and Weak Acid Methods. Plant Physiol. 1977 Apr;59(4):664–666. doi: 10.1104/pp.59.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]


