Abstract
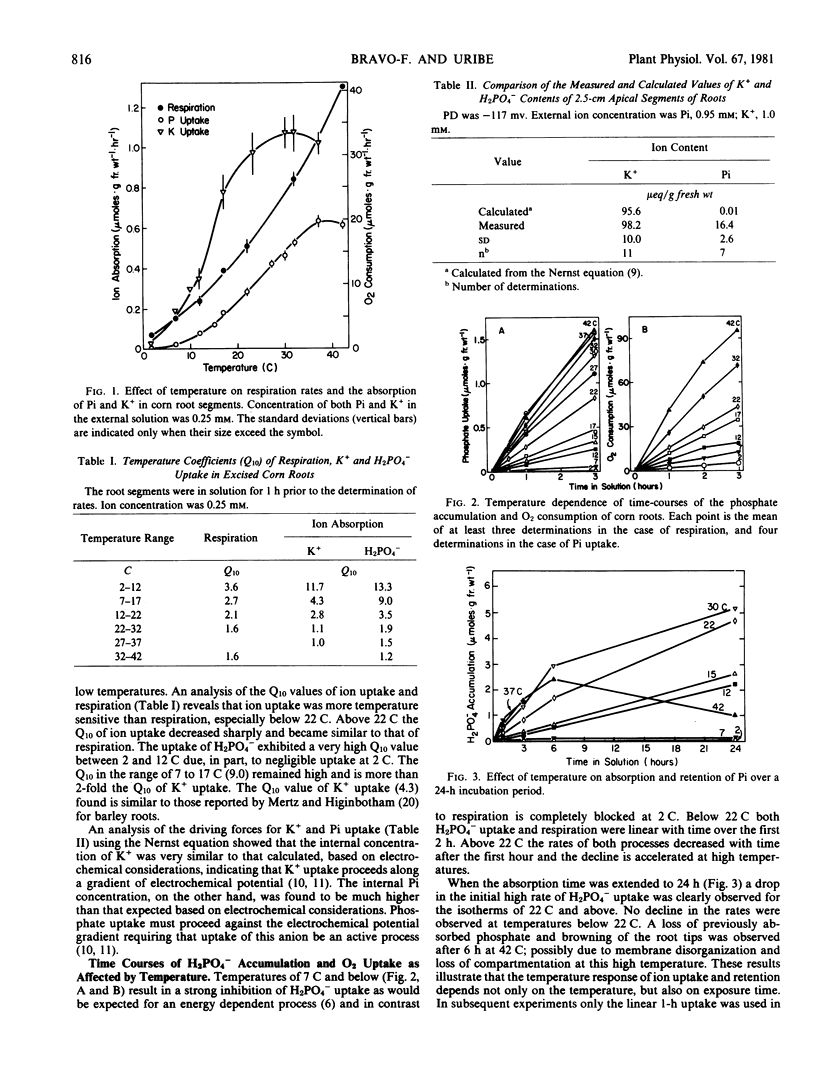
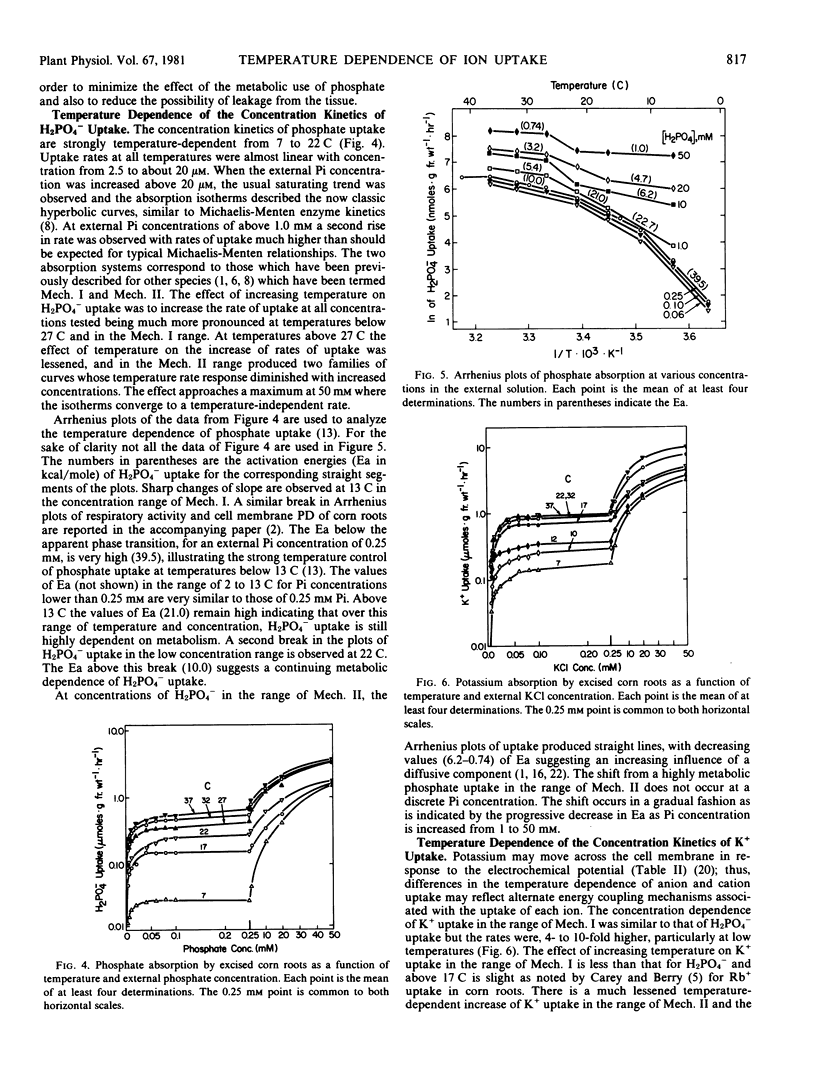
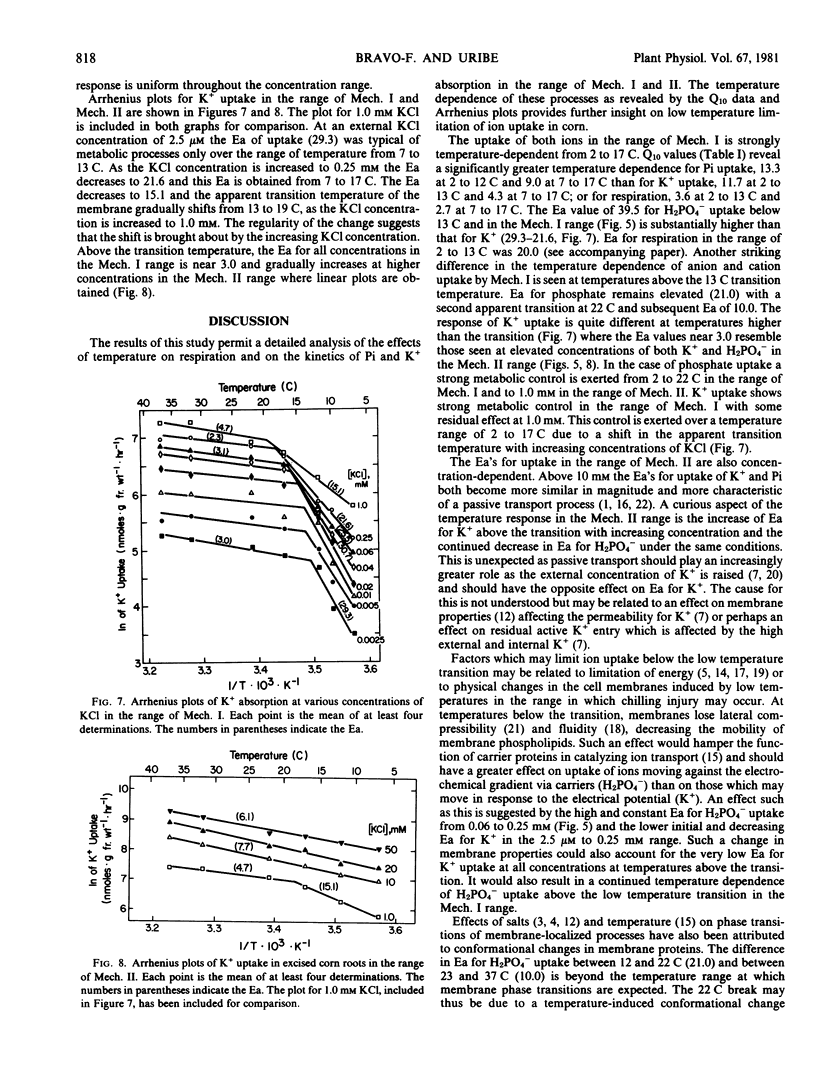
The effect of temperature on respiration and kinetics of H2PO4− and K+ uptake in corn roots was determined in the range of 2 to 42 C. The response of uptake to temperature, determined from Q10 and activation energy (Ea) data, for the anion and the cation differ significantly, especially in the range of uptake mechanism (Mech.) I. At 2.5 micromolar the Ea for K+ uptake below the 13 C transition is 29.3 kilocalories per mole. As the K+ concentration is increased, Ea declines and at 0.25 millimolar is 21.6 kilocalories per mole. Accompanying this change in Ea is a shifting of the apparent transition temperature from 13 to 17 C. Above the temperature transition the Ea's for K+ uptake in the Mech. I range are quite low (3.0) and this value is unchanged by increases of K+ concentration to 0.25 millimolar. In the range of Mech. II above 1 millimolar K+ the temperature transitions are not seen and plots become linear. The Ea's show an increasing trend from 4.7 at 1 millimolar to 6.1 at 50 millimolar. The uptake of H2PO4− is much more temperature sensitive having a constant Ea at concentrations in the Mech. I range below the 13 C temperature transition. The Arrhenius plots reveal a second transition at 22 C and the Ea for this segment is 21.0. Above the second transition the Ea remains high (10.0) and is constant in the range of Mech. I. In the range of Mech. II there is a concentration dependent decline in Ea for H2PO4− uptake (22.7 at 1.0 millimolar to 1.0 at 50 millimolar). There is no definable low temperature transition at these concentrations. Ion uptake is found to be much more sensitive to low temperature than respiration in this chill-sensitive species. The data suggest that the low temperature reduction of ion transport is more closely related to restriction of function of active transport systems than to either respiration or membrane permeability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo-F P., Uribe E. G. An Analysis of the Relationship between Respiration and the Transmembrane Potential in Corn Roots. Plant Physiol. 1981 Apr;67(4):809–814. doi: 10.1104/pp.67.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. W., Berry J. A. Effects of low temperature on respiration and uptake of rubidium ions by excised barley and corn roots. Plant Physiol. 1978 May;61(5):858–860. doi: 10.1104/pp.61.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter O. G., Lathwell D. J. Effects of temperature on orthophosphate absorption by excised corn roots. Plant Physiol. 1967 Oct;42(10):1407–1412. doi: 10.1104/pp.42.10.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Hanson J. B. Energy-linked Potassium Influx as Related to Cell Potential in Corn Roots. Plant Physiol. 1979 Nov;64(5):842–845. doi: 10.1104/pp.64.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J. C., Wong P. T., MacLennan D. H. Salt-dependent conformational changes in the cell membrane of Halobacterium salinarium. Biochem Biophys Res Commun. 1971 Apr 2;43(1):88–93. doi: 10.1016/s0006-291x(71)80090-6. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttge U., Laties G. G. Selective inhibition of absorption and long distance transport in relation to the dual mechanisms of ion absorption in maize seedlings. Plant Physiol. 1967 Feb;42(2):181–185. doi: 10.1104/pp.42.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E., Träuble H. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. I. Use of spin labels and optical probes as indicators of the phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4482–4491. doi: 10.1021/ja00768a013. [DOI] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


