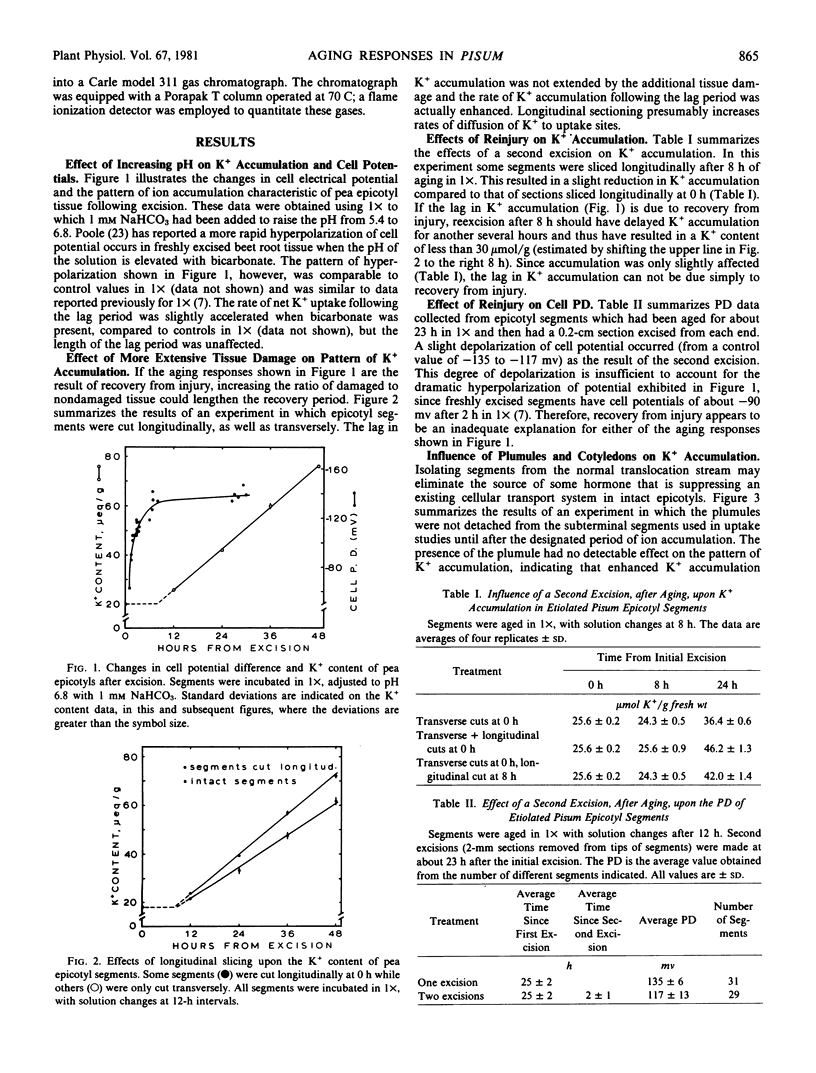
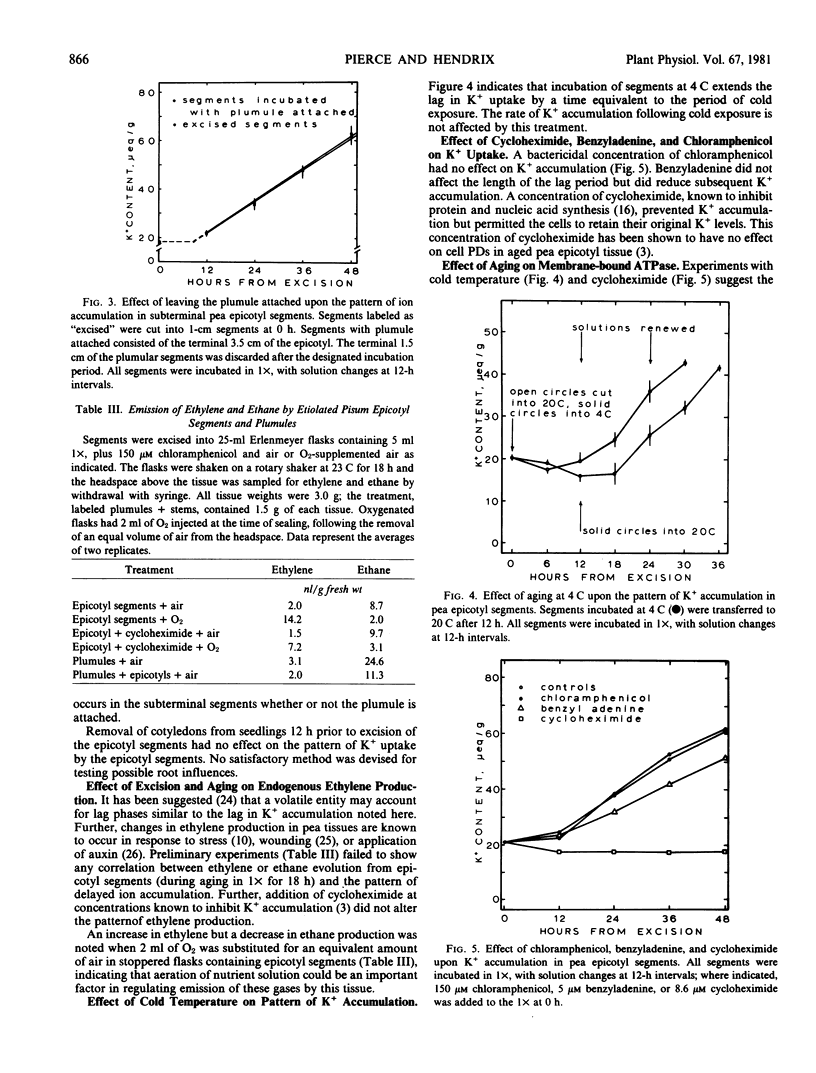
Abstract
Following excision, etiolated epicotyl segments of Pisum sativum L. cv. Alaska exhibit a marked hyperpolarization of membrane potential which is followed by a linear accumulation of K+ when segments are incubated in Higinbotham nutrient solution. Segments aged for several hours and then reexcised display only a slight depolarization of membrane potential and no delay in ion accumulation; thus, recovery from injury appears an unlikely explanation for these responses. Substances originating in either the plumule or the cotyledons do not seem to be directly involved in these “aging” responses. However, locally produced substances, such as ethylene, or substances originating in the roots have not been eliminated as causative factors. Cold temperatures and cycloheximide prolong the lag in K+ accumulation indicating a metabolic explanation for the induced K+ accumulation. However, similar specific activities of plasma membrane-bound ATPase were found in isolates from fresh and aged epicotyl segments. Reactivation of an ion transport mechanism, perhaps responsible for the osmotic control of growth in immature cells, is suggested as a possible explanation for the pattern of ion accumulation characteristic of excised pea epicotyl tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Active and passive transport of potassium in cells of excised pea epicotyls. Plant Physiol. 1970 Feb;45(2):133–138. doi: 10.1104/pp.45.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Electropotential in excised pea epicotyls. Plant Physiol. 1968 Jun;43(6):888–892. doi: 10.1104/pp.43.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976 Feb;57(2):123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrondo R. T., Smith R. C. Effect of removal of the root tip on the development of enhanced rb absorption by corn roots. Plant Physiol. 1976 Apr;57(4):607–611. doi: 10.1104/pp.57.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W. Sodium and potassium absorption by bean stem tissue. Plant Physiol. 1969 Apr;44(4):547–554. doi: 10.1104/pp.44.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit M. E., Dilley D. R. Studies of Rapidly Induced Wound Ethylene Synthesis by Excised Sections of Etiolated Pisum sativum L., cv. Alaska: IV. Requirement of a Water-soluble, Heat-stable Factor. Plant Physiol. 1979 Sep;64(3):417–420. doi: 10.1104/pp.64.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling N., Kende H. Methionine metabolism and ethylene formation in etiolated pea stem sections. Plant Physiol. 1979 Apr;63(4):639–642. doi: 10.1104/pp.63.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]


