Abstract
Background
Radiation treatment volumes in head and neck squamous cell carcinoma (HNSCC) are controversial. Here we report the outcomes, failures, and quality of life (QOL) of patients treated using intensity modulated radiation therapy (IMRT) that eliminated treatment of contralateral retropharyngeal lymph nodes (RPLN) in the clinically uninvolved neck.
Methods
A prospective institutional database identified patients with primary oral cavity, oropharynx, hypopharynx, larynx and unknown primary HNSCC treated using IMRT. There were three temporal groups (G1-3). G1 received comprehensive neck IMRT with parotid sparing, G2 eliminated the contralateral high level II (HLII) lymph nodes, and G3 further eliminated the contralateral RPLN in the clinically uninvolved neck. Patterns of failure and survival analyses were completed and QOL data measured by the MD Anderson Dysphagia Inventory (MDADI) was compared in a subset of patients from G1 and G3.
Results
There were 748 patients identified. Of the 488 patients treated in G2 or G3, 406 had a clinically uninvolved contralateral neck. There were no failures in the spared RPLNs (95% CI; 0-1.3%) or high contralateral neck (95% CI; 0-0.7%). QOL data was compared between 44 patients in G1 and 51 patients in G3. QOL improved both globally and in all domains assessed for G3 in which reduced radiotherapy volumes were used (p < 0.007).
Conclusions
For patients with locally advanced HNSCC, eliminating coverage to the contralateral HLII and contralateral RPLN in the clinically uninvolved side of the neck is associated with minimal risk of failure in these regions and significantly improved patient-reported QOL.
Keywords: IMRT, Head & Neck Squamous Cell Carcinoma, Treatment Volumes, Patterns of Failure, Quality of Life
INTRODUCTION
Intensity modulated radiotherapy (IMRT) for head and neck squamous Cell Carcinoma (HNSCC) has been adopted as the standard of care, supported with dosimetric and prospective randomized clinical evidence that reduced radiation dose reduces toxicity, especially in the parotid glands1. Early in the IMRT era, contouring guidelines advocated parotid sparing but otherwise maintained comprehensive nodal coverage2–5. Subsequent patterns of failure analyses have demonstrated that marginal recurrences are rare, thus reducing concerns of locoregional failure when IMRT is carefully applied2,6,7. The University of Michigan was the first to report data demonstrating the safety of eliminating elective radiotherapy to the high level II (HLII) lymph nodes in the contralateral clinically uninvolved side of the neck8. Subsequently these investigators showed there was an inverse dosimetric correlation with xerostomia9. More recent literature has focused on the importance of sparing swallowing structures to reduce dysphagia and further improve quality of life (QOL)10,11. Thus, it is important to determine the safe lower limits of elective radiation treatment volume in HNSCC in order to decrease dose to normal structures and in turn attempt to decrease morbidity.
At our institution three groups or generations of elective neck target delineation guidelines have been used, each generation eliminating radiotherapy to structures thought to be at low risk of recurrence. Generation 1 (G1) focused on parotid gland sparing, but otherwise maintained comprehensive bilateral elective neck coverage, consistent with institutional 2D treatment field coverage. Results with the first generation guidelines have been reported2,12. Generations 2 (G2) and 3 (G3) eliminated radiotherapy to the contralateral HLII and contralateral retropharyngeal lymph node (RPLN) regions, respectively, for patients with a clinically uninvolved contralateral neck. Since early data supporting the elimination of the high contralateral neck emerged many patterns of failure reviews have been reported, but no evidence based guidelines on radiotherapy volumes have been published and standard practice varies.13. The primary purpose of this study was to evaluate the safety and feasibility of eliminating coverage of the contralateral RPLNs. We had two hypotheses; first that eliminating coverage of this volume would not increase the risk of recurrence, and second that the reduced radiotherapy volumes in G3 would significantly decrease swallowing morbidity relative to G1. Thus, we expected to see improvements in patient reported QOL as measured by the physical, functional, and global domains of the MDADI.
MATERIALS AND METHODS
Patient data was collected using a prospective registry for head and neck cancer patients treated with IMRT approved by the institutional review board at Washington University in St. Louis. Eligibility criteria required the patient be treated with IMRT and have squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, larynx, or an unknown primary.
Staging
All patients had pretreatment staging workup including nasopharyngoscopy, CT with contrast and/or MRI, and/or FDG-PET/CT imaging. Patients were discussed at a multidisciplinary tumor board to determine the appropriate treatment course. If primary surgical treatment was chosen, unilateral or bilateral neck dissection for cervical adenopathy or nodal levels at risk of occult metastasis was completed at the time of primary tumor resection. Neck dissections usually included levels II-IV, but also extended to levels I and V when clinically indicated. Postoperatively, patients underwent adjuvant radiotherapy (RT) or chemoradiotherapy (CRT). Concurrent chemotherapy was typically platinum-based.
Targets and Dose Prescription
Gross tumor volumes (GTV) were contoured based on physical examination, nasopharyngoscopy, imaging studies, including CT, MRI, and/or FDG-PET/CT scans, as well as operative and pathology reports for surgically managed patients. The high-risk clinical target volume (CTV1) was defined as the primary tumor (pGTV + 1.5 - 2.0 cm) or tumor bed and any positive or suspicious lymph nodes (nGTV + 0.5 - 1.0 cm). The low-risk or elective clinical target volume (CTV2) was defined as the uninvolved elective neck. The nodal levels have been previously described by Grégoire et al.4. These volumes were then expanded by 0.5 cm to obtain a planning target volume (PTV).
Patients treated definitively (nonsurgical) received 70 Gy in 2.0 Gy fractions to CTV1 and 56 Gy in 1.6 Gy fractions to CTV2. Patients treated postoperatively received 60-66 Gy in 2.0 Gy fractions to the CTV1, and 50-56 Gy in 1.64-1.73 Gy fractions to the CTV2 depending on risk classification. All patients were treated using a simultaneous integrated boost.
Elective Neck Contouring Guidelines
In all generations, patients with well-lateralized T1/2 N0 tonsil, oral tongue, alveolar, buccal, retromolar trigone and soft palate primaries were treated using unilateral techniques. Selected patients with unknown primary cancers and unilateral neck disease were treated to the ipsilateral neck alone at our institution14. These patients were not excluded from any analysis.
Generation One (G1)
From June 1997 to July 2004, 260 patients were treated with first generation guidelines (G1) which have been reported.2,3 The focus was on parotid sparing but otherwise comprehensive IMRT was used. Bilateral level II lymph nodes to C1 and the bilateral RPLN to the base of skull were covered as was done in the 2D era. We attempted bilateral parotid sparing and used an anterior-posterior (AP) low neck matched field.
Generation Two (G2)
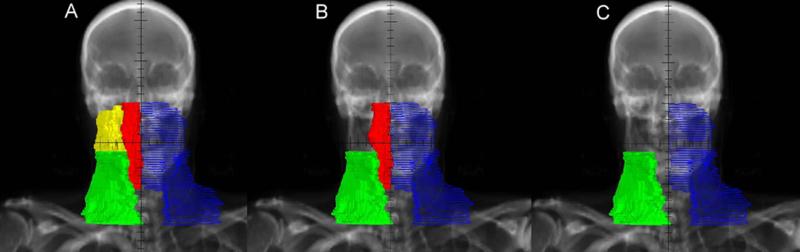
From July 2004 to June 2007, 205 patients were treated with second generation guidelines. G2 eliminated contralateral HLII coverage in the clinically uninvolved side of the neck but retained bilateral RPLN coverage. All treatment was IMRT (AP low neck match was eliminated). HLII was defined as the area of level II superior to the intersection of the posterior belly of the digastric muscle with the internal jugular vein.8 (Figure 1).
Figure 1.
AP radiograph demonstrating CTV elective nodal volume contours. In blue is the ipsilateral neck. Green represents the contralateral low neck, below level where the postierior belly of the digastric muscle crosses the internal jugular vein. Yellow indicates the contralateral high level II and red the contralateral retropharyngeal lymph nodes. (A) Generation 1. (B) Generation 2. (C) Generation 3.
Generation Three (G3)
From June 2007 to December 2010, 283 patients were treated with G3 guidelines. This generation eliminated coverage of the contralateral RPLN region in the clinically uninvolved side of the neck. Thus, in the contralateral neck, level II nodes were contoured up to the level where the posterior belly of the digastric muscle crosses the internal jugular vein and RPLNs were excluded (Figure 2). The ipsilateral RPLNs were treated to the base of skull.
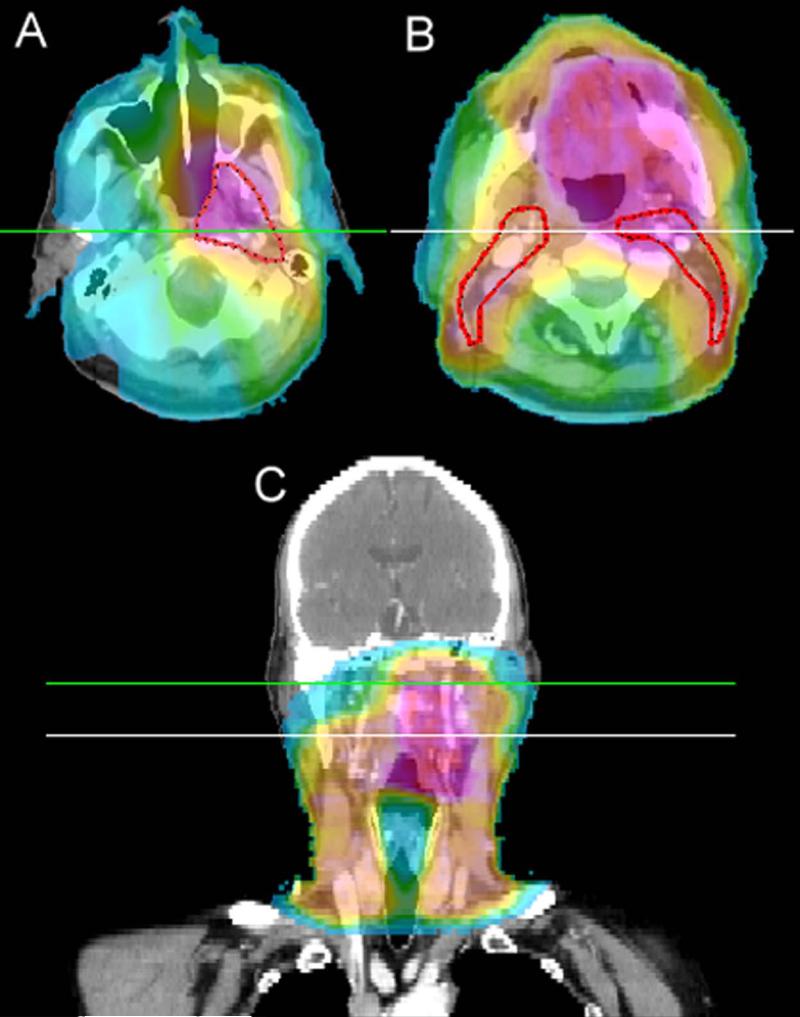
Figure 2.
Dose colorwash CT images of a patient with a T4AN0 oropharyngeal tumor treated in generation 3 which spared the contralateral retropharyngeal lymph nodes and the contralateral high level II lymph nodes. Colorwash range is from 5 Gy to 80 Gy. (A) Axial representation above the spared high level two with the elective CTV2 (56 Gy) volume shown in red. (B) Axial representation below the crossing of the digastric muscle and internal jugular vein also shows the elective CTV2 volume. (C) Coronal depiction shows the spared regions and indicates the axial slice levels.
Definition of Relapse
Recurrences were classified as local, regional or distant. Local failures were defined as failures that occurred at or near the site of primary disease. Regional failure pertains to nodal failure above the clavicle. The recurrent tumor volume (Vf) was defined for all patients with recurrent disease as previously described2,15. The Vf was compared or co-registered with the treatment planning CT data set using the Computational Environment for Radiation Research (CERR; Washington University). Recurrences were classified as “In-field,” defined as > 95% Vf receiving 95% prescription or “marginal,” 20-95% Vf receiving 95% prescription, or out of field, < 20% Vf receiving 95% prescription.
Quality of Life
The 20 question MDADI was used to collect patient reported QOL data16. The MDADI is a validated head and neck dysphagia instrument that has proven to be sensitive to swallowing outcomes for patients with HNSCC16. The questionnaire was chosen for clinical implementation at our institution due to its early validation in the IMRT era and widespread adoption. In 3/2008 the MDADI was mailed to all living patients in G1 with known addresses. Patients were requested to fill out the questionnaire and return it to clinic. During G3 QOL data was collected in a prospective longitudinal manner at the time of initial consultation and at each follow-up visit using a written questionnaire administered to the patient. All data was managed during and after collection by clinical trials data analysts. We compared late swallowing morbidity by comparing G1 patients with QOL data to G3 patients with QOL data after 30 months post treatment. Questionnaires were scaled from 0 to 100 with 0 being the worst and 100 the best QOL. Missing data was excluded. At validation, a score between groups of less than 10 points showed no difference and scores of 18 and greater showed significance. It is likely the minimally clinically significant difference lies between these values. Previous authors have suggested a difference of 16% of the instrument range was significant17. Using this as a guide, we hypothesized that a difference of 15% of the instrument range would result in a minimal clinically significant difference.
Statistical Analysis
Actuarial locoregional recurrence was calculated using the Kaplan-Meier method and was compared using the log-rank method18. Confidence intervals were created using a method derived from the Poisson distribution as described by Hanley and Lippman-Hand, 198319. Wilcoxon rank-sum test was used to compare the emotional, physical, functional, and global QOL domains between patients treated with the G1 and G3 guidelines. Fisher's exact test was used to test for association between clinical variables and generations.
RESULTS
Outcome Analysis
From June 1997 to December 2010, 748 patients with previously untreated HNSCC were treated consecutively using IMRT at Washington University in St. Louis. Favorable outcomes using the G1 guidelines for 260 patients were previously reported.2, 12 The remaining 488 patients were treated with G2 and G3 guidelines. Of these, 82 (17%) with bilateral (N2C) neck disease (33 G2; 49 G3) were excluded, leaving 406 patients with a clinically uninvolved side of the neck who were the subject of this report. There were 148 patients treated with IMRT in the definitive, and 258 in the postoperative setting. Of these 258, there were 200 (78%) that underwent primary tumor resection and ipsilateral neck dissection only. Overall, 61% (248/406) of the patients received chemotherapy (Table 1). Primary and nodal staging are shown in Table 2.
Table 1.
Patient and Tumor Characteristics
| Patients (n = 406) | Generation 2 (n = 172) | Generation 3 (n = 234) | |||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 319 | 137 | (80%) | 182 | (78%) |
| Female | 87 | 35 | (20%) | 52 | (22%) |
| Age | |||||
| Median | 57 | 57 | 57 | ||
| Range | 23-90 | 23-90 | 25-87 | ||
| Tumor Site* | |||||
| Oral Cavity | 64 | 25 | (15%) | 39 | (17%) |
| Oropharynx | 211 | 94 | (55%) | 117 | (50%) |
| Larynx | 86 | 35 | (20%) | 51 | (22%) |
| Hypopharynx | 27 | 10 | (6%) | 17 | (7%) |
| Unknown Primary | 25 | 10 | (6%) | 15 | (6%) |
| AJCC Tumor Stage† | |||||
| I | 7 | 3 | (2%) | 4 | (2%) |
| II | 26 | 12 | (7%) | 14 | (6%) |
| III | 82 | 31 | (18%) | 51 | (22%) |
| IVA | 234 | 97 | (56%) | 137 | (59%) |
| IVB | 28 | 18 | (10%) | 10 | (4%) |
| Chemotherapy | 248 | 95 | (55%) | 153 | (65%) |
| Radiotherapy | |||||
| Definitive | 148 | 61 | (35%) | 87 | (37%) |
| Postoperative | 258 | 111 | (65%) | 147 | (63%) |
Synchronous primary tumors occurred in 7 patients.
There were 4 patients with Oropharyngeal primary tumors with unstageable primary disease (Tx); 1 patient in Generation 2 and 3 patients in Generation 3. These were not included in the AJCC Tumor Stage. The 25 patients with neck Metastasis from unknown primary were also not included.
Table 2.
Distribution of AJCC Primary Tumor and Nodal Stages
| N0 | N1 | N2A | N2B | N2C | N3 | Total | % | |
|---|---|---|---|---|---|---|---|---|
| T0* | 0 | 2 | 9 | 8 | 0 | 6 | 25 | (5%) |
| Tx† | 0 | 0 | 0 | 2 | 1 | 1 | 4 | (1%) |
| T1 | 7 | 16 | 7 | 36 | 4 | 3 | 73 | (15%) |
| T2 | 25 | 18 | 9 | 68 | 15 | 9 | 144 | (29%) |
| T3 | 35 | 14 | 6 | 21 | 18 | 3 | 97 | (20%) |
| T4 | 33 | 24 | 5 | 31 | 44 | 8 | 145 | (30%) |
| Total (%) | 100 (21%) | 74 (15%) | 36 (7%) | 166 (34%) | 82 (17%) | 30 (6%) | 488 | |
Unknown Primary
Primary unable to be staged
Median follow up for living patients was 54 months in G2, 27 months in G3, and 37 months in the entire cohort. The actuarial local-regional recurrence (LRR) rate at 2 years was 19.2% in G2, 19.0% in G3 (p = 0.95). There was no difference in local-regional control between generations overall or when stratified by type of therapy. Of the 211 oropharyngeal carcinomas in the overall cohort, p16 status was known on 142 patients. In G2, 55 (80%) and in G3, 59 (81%) were p16 positive. Of the p16 positive patients, in G2 there were 3 (5%) LRR and 4 (7%) distant metastases (DM) and in G3 there were 4 (7%) LRR and 6 (10%) DMs. Of the p16 negative patients, in G2 there were 5 (36%) LRR and 1 (7%) DM and in G3 there were 6 (43%) LRR and 2 (14%) DMs.
There were 72 in-field failures and 3 out-of-field failures. None of the out-of-field failures occurred in the contralateral HLII neck or contralateral RPLNs. There were two patients who recurred in-field in the ipsilateral lateral RPLN region. Neither patient had involvement of the RPLNs at diagnosis. The first patient had a T0N2b unknown primary cancer recurred in-field in the CTV-2 after receiving the prescribed elective dose of 52 Gy. This patient was successfully salvaged with Gamma Knife Radiosurgery. The second patient had a T2N2b tonsillar cancer and recurred in-field in the CTV1 after receiving a dose of 66 Gy. The recurrence was in the high dose CTV due to the proximity to the primary tumor.
In G2 and G3, a total of 406 patients with a clinically uninvolved contralateral neck were treated with radiotherapy that eliminated coverage to the contralateral HLII. To date there have been no failures in the spared high contralateral neck (95% CI; 0-0.7%). In G3, 234 patients were treated with radiation sparing the contralateral RPLN and contralateral HLII of radiation with no failures in the spared region (95% CI; 0-1.3%).
QOL
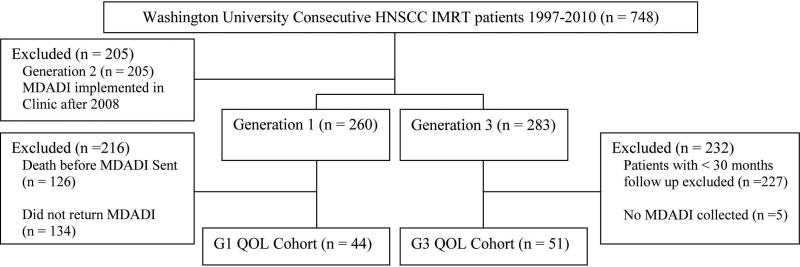
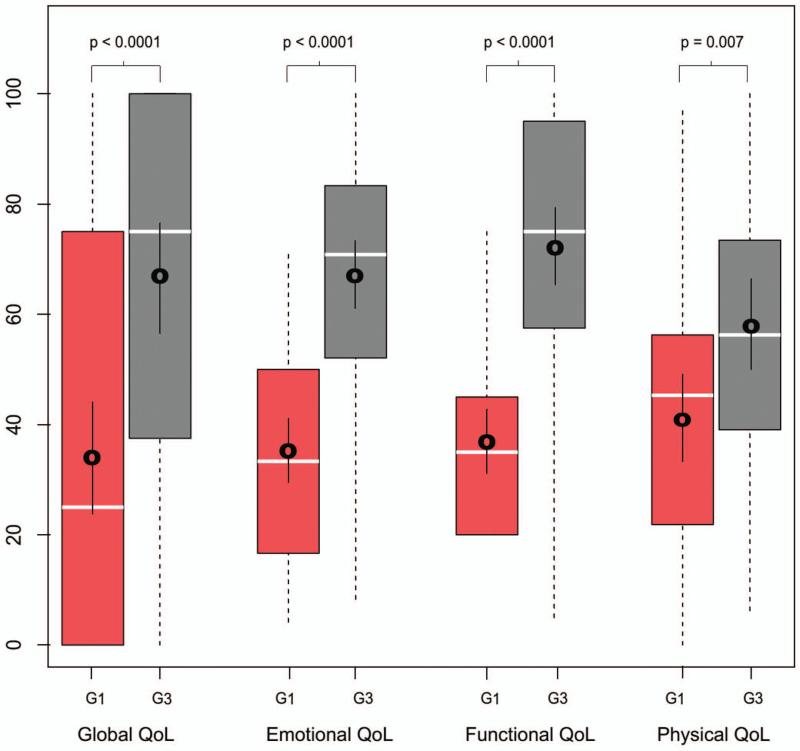
Long-term QOL data was analyzed for 44 patients from G1 and 51 patients from G3 with a mean time from therapy to completion of questionnaire of 5 years for G1 and 3 years for G3 (Figure 3). More patients in G3 received chemotherapy as compared to G1 (78% v. 39%; p < 0.001). This is because patients in G3 were treated after the publication of the seminal papers describing the indications for postoperative chemoradiation20–22. There were no other significant differences in the clinicopathologic characteristics between the groups (Table 3). The mean score on the physical domain of the MDADI was 41.9 (95% CI 34.0-49.9) in G1 and 57.5 (95% CI 50.5-64.4) in G3 (p <0.001) and in the global domain was 34.1 (95% CI 23.6-44.5) in G1 and 66.7 (95% CI 57.7-75.6) in G3 (p <0.001). Compared to G1, G3 was associated with superior patient reported QOL, both globally and in all domains tested with the MDADI (p < 0.001; Figure 4).
Figure 3.
Consort diagram demonstrating selection of QOL cohort
Table 3.
Baseline Characteristics for all patients by generation of treatment.
| Baseline Characteristics | Generation 1 | (%) | Generation 3 | (%) | p value |
|---|---|---|---|---|---|
| n = 44 | n = 51 | ||||
| Gender | NS | ||||
| Male | 35 | (80%) | 41 | (80%) | |
| Female | 9 | (20%) | 10 | (20%) | |
| Race | NS | ||||
| Caucasion | 40 | (91%) | 48 | (94%) | |
| African American | 4 | (9%) | 3 | (6%) | |
| Primary Site | NS | ||||
| Oral Cavity | 5 | (11%) | 1 | (2%) | |
| Oropharynx | 29 | (66%) | 30 | (59%) | |
| Hypopharynx | 3 | (7%) | 3 | (6%) | |
| Larynx | 6 | (14%) | 11 | (22%) | |
| Unknown Primary | 1 | (2%) | 6 | (12%) | |
| T Stage | NS | ||||
| 1 | 9 | (20%) | 10 | (20%) | |
| 2 | 16 | (36%) | 9 | (18%) | |
| 3 | 10 | (23%) | 16 | (31%) | |
| 4 | 8 | (18%) | 10 | (20%) | |
| N Stage | NS | ||||
| 0 | 8 | (18%) | 9 | (18%) | |
| 1 | 7 | (16%) | 12 | (24%) | |
| 2a | 5 | (11%) | 5 | (10%) | |
| 2b | 19 | (43%) | 17 | (33%) | |
| 2c | 4 | (9%) | 5 | (10%) | |
| 3 | 1 | (2%) | 2 | (4%) | |
| AJCC Stage Group | NS | ||||
| I | 0 | (0%) | 0 | (0%) | |
| II | 3 | (7%) | 0 | (0%) | |
| III | 8 | (18%) | 15 | (29%) | |
| IVa | 30 | (68%) | 29 | (57%) | |
| IVb | 2 | (5%) | 1 | (2%) | |
| Prior Surgery | NS | ||||
| Yes | 30 | (68%) | 29 | (57%) | |
| No | 14 | (32%) | 21 | (41%) | |
| Concurrent Chemotherapy | p<0.001 | ||||
| Yes | 17 | (39%) | 38 | (75%) | |
| No | 27 | (61%) | 11 | (22%) | |
| G-Tube Required | NS | ||||
| Yes | 23 | (52%) | 25 | (49%) | |
| No | 21 | (48%) | 25 | (49%) | |
| Smoking Status | NS | ||||
| Yes | 32 | (73%) | 34 | (67%) | |
| No | 10 | (23%) | 17 | (33%) | |
Figure 4.
Box plot demonstrating patient reported quality of life broken into the 4 domains; global, emotional, functional and physical. Generation 1 is in red and generation 3 in grey. Boxes indicate the 25-75 quartiles with dashed lines to the minimum and maximum; median is represented by white bar; mean with 95% CI bars is represented by black mark with bars surrounding. All p values were significant by Wilcoxon Rank Sum test.
DISCUSSION
This report provides evidence for the elimination of radiation to the RPLNs in the contralateral uninvolved neck for patients with oral cavity, oropharynx, hypopharynx, larynx, and unknown primary tumors. No recurrences were observed in the contralateral RPLN region when this volume was excluded in the 234 patients so treated. With a 95% confidence interval range of 0% to 1.3% one can be confident that there is a low risk of recurrence in properly staged patients after sparing this region. This is the first report to our knowledge that explores specifically the elimination of this nodal group. RPLN target delineation has been well-described4. It is important to recognize these findings were for the RPLN region in the clinically (and radiographically) uninvolved contralateral neck. Ipsilateral in-field RPLN failures did occur in this study, and have been reported by others8,15. In addition, it is important to note that although the RPLN's were not specifically targeted, they still received some radiation. We have previously reported that contralateral normal structure mean dose is significantly reduced when comparing bilateral vs ipsilateral treatment methods.14
The definition of the HLII neck was proposed by Eisbruch et al.8 and the safety of avoiding the contralateral HLII lymph nodes have been demonstrated. The present report corroborates the previous evidence for avoiding radiation to the HLII lymph nodes in the uninvolved neck with an additional 406 patients, yielding a 95% confidence interval of 0% to 0.7%.
The importance of dose to critical normal structures and correlation of dose with swallowing function and QOL have been explored10,23–25. Avoiding treatment of both the contralateral HLII and RPLN groups allows significant volume and dose reduction to the contralateral pharyngeal constrictor muscles, larynx, esophagus, and parotid gland, which have been implicated in xerostomia, swallowing dysfunction and QOL. We hypothesized that treatment volume reduction to these structures in G3 compared to G1 would be associated with significantly improved patient reported QOL. The QOL differences observed in all domains are large, statistically significant and likely clinically important. These results approximate those previously reported for both definitive and postoperative treatments using the MDADI.27–29 It is noteworthy that QOL scores improved despite the increased use of concurrent chemotherapy in G3 (78% G3 vs 39% G1), which is associated with greater risk of late toxicity and worse QOL29–31.
Given our hypothesis that reducing RT volumes would decrease swallowing morbidity we thought the largest impact would occur on the physical and global domains. We observed differences in the global, emotional and functional domains of greater than 30% of the instrument range. The physical domain only showed a difference of 16% of the instrument range, the smallest difference of all domains analyzed. We believe this improvement is still clinically relevant, but the etiology of this difference is unclear. A similar trend was seen by Gillespie et al. 2004.27 The physical domain asks specific quantifiable questions, whereas the emotional and functional refer to less quantifiable phenomena like being embarrassed about eating habits. One hypothesis could be that after an extended period patients have learned to cope with the physical morbidities of their treatment regardless of their extent, but the social (i.e. functional and emotional) stigma continues. Alternatively, it is possible that at three years post-treatment, the G3 cohort has not yet fully developed late swallowing dysfunction, whereas the G1 cohort by five years had sufficient time to fully develop this dysfunction. We feel this is unlikely as previous reports have shown dysphagia typically normalizes to a new baseline after one year and has been used as an endpoint in many reports.32–34 Another possibility is other non-measured factors like education level, socioeconomic status, access to care, and psychosocial support are biasing the QOL outcomes. We also feel this is unlikely as the other clinicopathologic characteristics between groups are not significantly different.
We believe the results reported here apply to a large proportion of patients with locally advanced HNSCC. In the original group of 488 patients treated to G2 or G3 guidelines, 87% had stage III/IV disease. After excluding patients with N2C disease, 85% of the remaining 406 patients remained stage III/IV. In the Quality of life analysis 89% had stage III/IV disease. In RTOG 0129 a total of 721 patients were enrolled on the trial and 24% had stage N2C neck disease.35 It follows that depending on referral patterns, approximately 75% of patients with locally advanced HNSCC may be eligible for the reduced volume treatment described in this manuscript.We recognize the large number of oropharyngeal cancer patients present in this study but feel it is representative of most institutions HNSCC population. We have captured p16 status on the majority of these patients. We found around 80% of our population tends to be p16 positive and this correlates with previous reports.35 Further, patterns of failure are consistent with the previously reported RTOG 9003 and 0129 trials, with LRR being significantly improved in p16 positive compared to p16 negative patients, with similar rates of DMs.36 These trials have shown the time to locoregional or distant recurrence predominately occurs in less than 2 years from diagnosis regardless of p16 status and thus we feel our follow up is adequate to capture the majority of recurrences.
Several important factors and practices which may limit the applicability of this data to other institutions should be considered. FDG-PET/CT imaging was used in the routine workup of most patients which aids in the accurate prediction of nodal stage37. At our institution primary surgical management of HNSCC is the preferred approach and favorable outcomes have been previously reported.38,39 Thus, we have a cohort of patients which is weighted in favor of postoperative radiotherapy. However, the majority of patients underwent ipsilateral neck dissections only. In these cases the contralateral neck was clinically staged using CT with IV contrast, FDG-PET/CT and clinical exam making staging applicable to most institutions. It should be noted that in patients with tumors that invade the posterior oropharyngeal wall or hypopharynx, primary drainage may be to the bilateral RPLNs. These cases were not excluded from our G3 treatment guidelines. However, sublocation of the primary tumor was not captured in our dataset and thus we cannot specifically address the patterns of failure for this subset. Therefore, special consideration should be taken when deciding to eliminate contralateral RPLN radiotherapy in this subset of patients.
Acknowledgments
Dr. Tanya Wildes’ research is supported by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH.
Footnotes
Conflicts of Interest and Source of Funding Statement: There are no conflicts of interest associated with this manuscript by any of the authors.
REFERENCES
- 1.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. doi:10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao KSC, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55(2):312–321. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 3.Chao KSC, Wippold FJ, Ozyigit G, Tran BN, Dempsey JF. Determination and delineation of nodal target volumes for head-and-neck cancer based on patterns of failure in patients receiving definitive and postoperative IMRT. Int J Radiat Oncol Biol Phys. 2002;53(5):1174–1184. doi: 10.1016/s0360-3016(02)02881-x. [DOI] [PubMed] [Google Scholar]
- 4.Grégoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56(2):135–150. doi: 10.1016/s0167-8140(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A, Foote RL, O'Sullivan B, Beitler JJ, Vikram B. Intensity-modulated radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol. 2002;12(3):238–249. doi: 10.1053/srao.2002.32435. doi:10.1053/srao.2002.32435. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Xia P, Fischbein NJ, Akazawa P, Akazawa C, Quivey JM. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57(1):49–60. doi: 10.1016/s0360-3016(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 7.Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma—the University of Iowa experience. International Journal of Radiation Oncology*Biology*Physics. 2005;63(2):410–421. doi: 10.1016/j.ijrobp.2005.02.025. doi:10.1016/j.ijrobp.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59(1):28–42. doi: 10.1016/j.ijrobp.2003.10.032. doi:10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. International Journal of Radiation Oncology*Biology*Physics. 2001;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. doi:10.1016/S0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 10.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28(16):2732–2738. doi: 10.1200/JCO.2009.24.6199. doi:10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81(3):e93–99. doi: 10.1016/j.ijrobp.2010.12.067. doi:10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorstad W, Hong S, Hope AJ, et al. Patterns of Failure in Patients Receiving Intensity Modulated Radiation Therapy (IMRT) for Head and Neck Cancer. International Journal of Radiation Oncology*Biology*Physics. 2005;63(Supplement 1):S74. doi:10.1016/j.ijrobp.2005.07.127. [Google Scholar]
- 13.Hong TS, Tomé WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiotherapy and Oncology. 2012;103(1):92–98. doi: 10.1016/j.radonc.2012.02.010. doi:10.1016/j.radonc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins SM, Spencer CR, Chernock RD, et al. Radiotherapeutic management of cervical lymph node metastases from an unknown primary site. Arch Otolaryngol Head Neck Surg. 2012;138(7):656–661. doi: 10.1001/archoto.2012.1110. doi:10.1001/archoto.2012.1110. [DOI] [PubMed] [Google Scholar]
- 15.Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46(5):1117–1126. doi: 10.1016/s0360-3016(99)00550-7. [DOI] [PubMed] [Google Scholar]
- 16.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 17.Thomas L, Moore EJ, Olsen KD, Kasperbauer JL. Long-term quality of life in young adults treated for oral cavity squamous cell cancer. Ann Otol Rhinol Laryngol. 2012;121(6):395–401. doi: 10.1177/000348941212100606. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 19.Hanley JAL-HA. If nothing goes wrong, is everything all right?: Interpreting zero numerators. JAMA. 1983;249(13):1743–1745. doi:10.1001/jama.1983.03330370053031. [PubMed] [Google Scholar]
- 20.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head & Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. doi:10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 21.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. doi:10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. doi:10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 23.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiotherapy and Oncology. 2007;85(1):64–73. doi: 10.1016/j.radonc.2007.07.009. doi:10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85(1):74–82. doi: 10.1016/j.radonc.2007.06.004. doi:10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen HR, Jensen K, Aksglæde K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiother Oncol. 2013;107(3):288–294. doi: 10.1016/j.radonc.2013.06.001. doi:10.1016/j.radonc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero Urbano MT, Clark CH, Kong C, et al. Target volume definition for head and neck intensity modulated radiotherapy: pre-clinical evaluation of PARSPORT trial guidelines. Clin Oncol (R Coll Radiol) 2007;19(8):604–613. doi: 10.1016/j.clon.2007.07.001. doi:10.1016/j.clon.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Gillespie MB, Brodsky MB, Day TA, Lee F-S, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114(8):1362–1367. doi: 10.1097/00005537-200408000-00008. doi:10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 28.More YI, Tsue TT, Girod DA, et al. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. JAMA Otolaryngol Head Neck Surg. 2013;139(1):43–48. doi: 10.1001/jamaoto.2013.1074. doi:10.1001/jamaoto.2013.1074. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg. 2011;145(5):767–771. doi: 10.1177/0194599811414506. doi:10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 30.Al-Mamgani A, Mehilal R, van Rooij PH, Tans L, Sewnaik A, Levendag PC. Toxicity, quality of life, and functional outcomes of 176 hypopharyngeal cancer patients treated by (chemo)radiation: the impact of treatment modality and radiation technique. Laryngoscope. 2012;122(8):1789–1795. doi: 10.1002/lary.23387. doi:10.1002/lary.23387. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mamgani A, Tans L, van Rooij P, Levendag PC. A single-institutional experience of 15 years of treating T3 laryngeal cancer with primary radiotherapy, with or without chemotherapy. Int J Radiat Oncol Biol Phys. 2012;83(3):1000–1006. doi: 10.1016/j.ijrobp.2011.07.045. doi:10.1016/j.ijrobp.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus CL, Logemann JA, Pauloski BR, et al. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res. 2000;43(4):1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- 33.Pauloski BR, Rademaker AW, Logemann JA, et al. Pretreatment swallowing function in patients with head and neck cancer. Head Neck. 2000;22(5):474–482. doi: 10.1002/1097-0347(200008)22:5<474::aid-hed6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Pauloski BR, Logemann JA, Rademaker AW, et al. Speech and swallowing function after oral and oropharyngeal resections: one-year follow-up. Head Neck. 1994;16(4):313–322. doi: 10.1002/hed.2880160404. [DOI] [PubMed] [Google Scholar]
- 35.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. doi:10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. doi: 10.1200/JCO.2011.38.4099. doi:10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg MK, Glanzman J, Kalnicki S. The evolving role of positron emission tomography-computed tomography in organ-preserving treatment of head and neck cancer. Semin Nucl Med. 2012;42(5):320–327. doi: 10.1053/j.semnuclmed.2012.04.005. doi:10.1053/j.semnuclmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A united states multicenter study. Head & Neck. 2011;33(12):1683–1694. doi: 10.1002/hed.21669. doi:10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 39.Rich JT, Milov S, Lewis JS, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) ± adjuvant therapy for advanced stage oropharyngeal cancer. The Laryngoscope. 2009;119(9):1709–1719. doi: 10.1002/lary.20552. doi:10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]