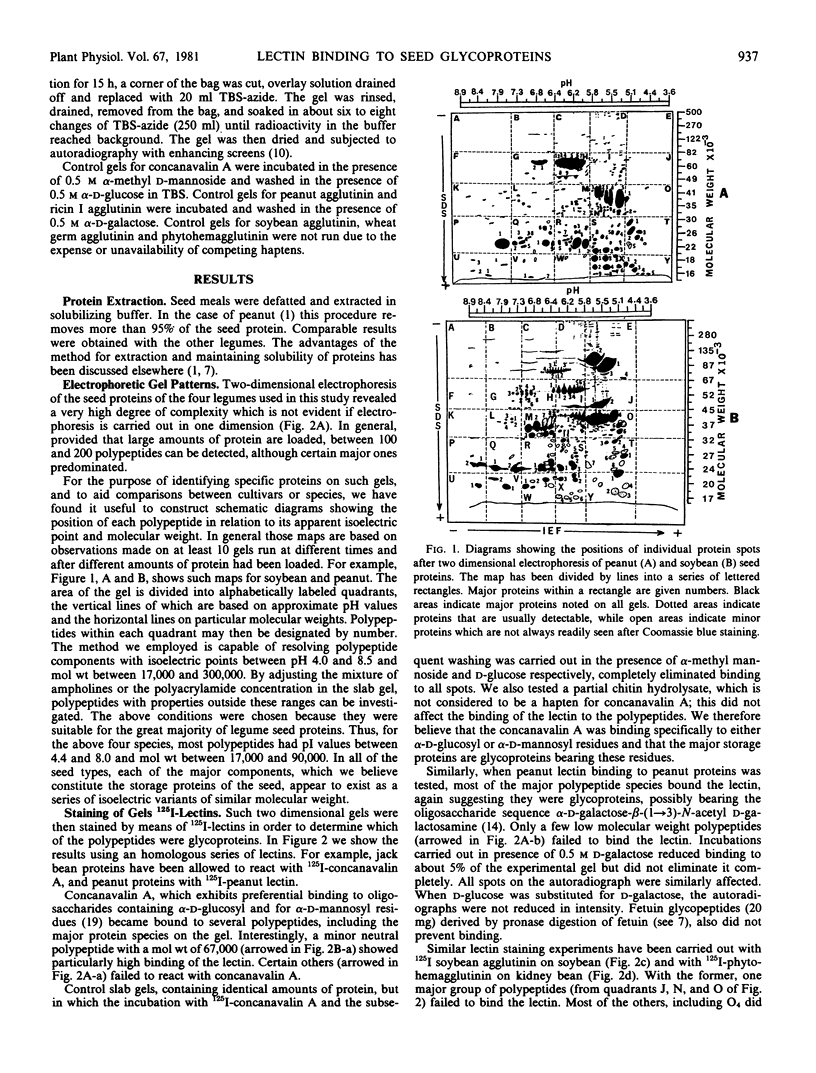
Abstract
Protein from the jack bean, peanut, soybean and kidney bean seeds were extracted with a solution containing 9.3 molar urea, 5 millimolar K2CO3, 0.5% dithiothreitol and 2% Nonidet P-40 and then subjected to two-dimensional gel electrophoresis. After electrophoresis, the slab gels were stained with a variety of 125I-labeled lectins and the lectin-binding proteins were identified after autoradiography. Incubation of slab gels of jack bean with concanavalin A, peanut with peanut agglutinin, soybean with soybean agglutinin, and kidney bean with phytohemagglutinin showed that the majority of the polypeptides in each seed type were able to bind to their homologous lectins. Control slab gels in which incubations were carried out with identical amounts of proteins, 125I-lectin and an appropriate sugar inhibitor showed little or no lectin binding to the polypeptides. Additionally, incubation of slab gels of peanut proteins with 125I-ricin, 125I-wheat germ agglutinin, 125I-concanavalin A, and 125I-soybean agglutinin each revealed a clearly distinct binding pattern compared to the one observed with the peanut agglutinin. The results demonstrate that a large number of legume seed polypeptides are glycoproteins and that the carbohydrate groups within a seed species are heterogeneous in structure, thus indicating the existence of complex glycosylating enzyme systems in legume seeds. It is suggested that the high degree of binding between seed proteins and their homologous lectins might have some functional significance in maintaining large aggregates of protein in compact, insoluble form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Al-Wandawi H. Composition, solubility, and gel electrophoretic properties of proteins isolated from Florunner (Arach is hypogaea L.) peanut seeds. J Agric Food Chem. 1976 Mar-Apr;24(2):359–365. doi: 10.1021/jf60204a058. [DOI] [PubMed] [Google Scholar]
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha S. M. Identification of Cultivar Differences in Seed Polypeptide Composition of Peanuts (Arachis hypogaea L.) by Two-Dimensional Polyacrylamide Gel Electrophoresis. Plant Physiol. 1979 Feb;63(2):301–306. doi: 10.1104/pp.63.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst M. N., Basha S. M., Baumbach G. A., Mansfield E. H., Roberts R. M. Alkaline urea solubilization, two-dimensional electrophoresis and lectin staining of mammalian cell plasma membrane and plant seed proteins. Anal Biochem. 1980 Mar 1;102(2):399–408. doi: 10.1016/0003-2697(80)90174-8. [DOI] [PubMed] [Google Scholar]
- Horst M. N., Baumbach G. A., Olympio M. A., Roberts R. M. Isolation of a domain of the plasma membrane in Chinese hamster ovary cells which contains iodinatable, acidic glycoproteins of high molecular weight. Biochim Biophys Acta. 1980 Jul 16;600(1):48–61. doi: 10.1016/0005-2736(80)90410-1. [DOI] [PubMed] [Google Scholar]
- Horst M. N., Baumbach G., Roberts R. M. Two-dimensional electrophoretic analysis of concanavalin-A binding components in the plasma membranes of Chinese hamster fibroblasts. FEBS Lett. 1979 Apr 15;100(2):385–388. doi: 10.1016/0014-5793(79)80376-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. The isolation of two proteins, glycoprotein I and a trypsin inhibitor, from the seeds of kidney bean (Phaseolus vulgaris). Biochem J. 1966 Nov;101(2):379–384. doi: 10.1042/bj1010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Shetty K. J., Rao M. S. Studies on groundnut proteins. III. Physicochemical properties of arachin prepared by different methods. Anal Biochem. 1974 Nov;62(1):108–120. doi: 10.1016/0003-2697(74)90372-8. [DOI] [PubMed] [Google Scholar]