Abstract
Backgroud
Acute decompensated heart failure (ADHF) is a common and highly morbid cardiovascular disorder. Diuresis is a major therapy for the reduction of congestive symptoms. However, most diuretics cause hyponatremia, which is a worsening factor of ADHF patients prognosis. The purpose of this study was to examine the efficacy and safety of tolvaptan, which is a selective vasopressin V2 receptor antagonist and produces water excretion without changes in sodium excretion, compared with carperitide.
Methods and Results
One hundred and nine hospitalized ADHF patients were enrolled and randomly assigned to tolvaptan or carperitide treatment groups. Subjective symptoms and plasma BNP level were similarly improved by treatment in both groups. Urine volume was significantly higher in the tolvaptan group (P < .05), but volume of water intake was also higher in the tolvaptan group (P < .05). Blood pressure was significantly lower in the carperitide group than in the tolvaptan group after treatment (P < .05). Less adverse events such as worsening heart failure and hypotension requiring drug discontinuation were observed in the tolvaptan group (P = .027). The average drug cost of tolvaptan was lower than that of carperitide (P < .001).
Conclusions
Tolvaptan might be a novel promising agent for ADHF in terms of efficacy and safety compared to carperitide.
Keywords: acute decompensated heart failure, volume control, tolvaptan, carperitide
Acute decompensated heart failure (ADHF) has emerged over the past several decades as a common medical problem associated with major morbidity and mortality.1–3 ADHF is a complex syndrome that is usually recognized by typical findings of low cardiac output associated with signs of pulmonary and systemic congestion. Because patients hospitalized with ADHF almost always have symptoms of congestion,4 removing excess fluid is required to help them feel better. Therapy to reduce volume overload during hospitalization for ADHF leads to marked improvement in signs and symptoms of elevated left ventricular filling pressure.5 In many cases of treatment of ADHF, diuretics are the first line for reducing congestion. The Acute Decompensated Heart Failure National Registry (ADHERE) demonstrates that approximately 90% of patients hospitalized with ADHF receive intravenous loop diuretics during hospitalization.6 However, there are several concerns regarding safety and efficacy of loop diuretics.7 One of the most important and fatal disadvantages for diuretic therapy of ADHF patients are electrolyte abnormalities (e.g., hypokalemia, hyponatremia, and hypomagnesemia). A large-scale OPTIMIZE-HF study (Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Heart Failure) revealed that hyponatremia in hospitalized ADHF patients was relatively common and was associated with longer hospital stays and higher in-hospital and early post-discharge mortality.8 Moreover, loop diuretics induce renal dysfunction and activate the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system, both of which are known to play a fundamental role in heart failure progression.9–12 Several reports revealed that loop diuretics worsen renal function and the prognosis of heart failure patients in a dose-dependent manner.12–14
Intravenous administration of carperitide has been used as an acute phase therapy for ADHF due to natriuretic and vasodilation effects. Several reports revealed the efficacy and safety in acute phase treatment and improvement of long-term prognosis of carperitide,15,16 but the evidence of this drug is insufficient. Tolvaptan is a selective vasopressin V2 receptor antagonist that produces water excretion without changes in renal hemodynamics or sodium and potassium excretion.17 In ADHF patients, oral tolvaptan in addition to standard therapy, including conventional diuretics, improved heart failure signs and symptoms without serious adverse events.18 Then we designed the Acute heart failure Volume Control Multicenter rAndomized (AVCMA) trial, and the purpose of the AVCMA trial was to compare the efficacy and safety of carperitide and tolvaptan in ADHF patients.
Methods
Study Design
AVCMA trial was a multicenter, randomized study to determine the efficacy and safety of tolvaptan compared to carperitide in ADHF patients. This study was registered in University Hospital Medical Information Network (ID, 000006258). The first patient was enrolled in January 2011, and a total of 111 patients were randomized by May 2012. No patients were discontinued, but two subjects were excluded before randomization by physician's decision (Figure 1). Written informed consent was obtained from all study subjects. The study protocol was approved by the ethical committee at Fukushima Medical University and each participating institution in compliance with the Declaration of Helsinki.
Figure 1.
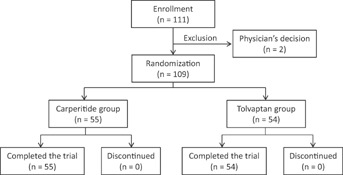
Participant's flow chart in this study.
Sample Size Estimation
Matsuzaki et al. reported that tolvaptan increased urine volume from baseline approximately 700 mL per day.19 On the other hand, Izumi et al. reported that carperitide increased urine volume approximately 100 mL per hour.20 Our hypothesis is that change in urine volume is 300 mL per day from baseline in the carperitide group, contrarily 700 mL per day in the tolvaptan group. Sample size was calculated based on this hypothesis. A two-sided test, with 0.05 significance level and 90% power (α = 0.05, β = 0.10), would require 53 subjects per group. A minimum of 53 subjects per group would be required to obtain statistical power for this study.
Study Participants
Patients were eligible for enrollment if they had presented volume fluid retention with ADHF or acute exacerbation of chronic heart failure (CHF), diagnosed on the basis of the presence of at least one subjective symptom (dyspnea, orthopnea, or leg edema) and one sign (rales, peripheral edema, ascites, or pulmonary vascular congestion on chest X-ray) of heart failure. Patients with acute myocardial infarction, severe hypotension (cardiogenic shock), anuria, hypernatremia (Na > 147 mEq/L), and who did not feel thirsty or have difficult water intake, were excluded.
These ADHF patients underwent the standard initial treatment, including conventional loop diuretic administration, and were randomly assigned into two groups, oral administration of tolvaptan or continuous intravenous infusion of carperitide. Tolvaptan was orally administered at 3.75–15 mg per day, and carperitide was administered at 0.0125–0.025 µg/kg depending on the pathological condition of the patient. Study subjects were investigated for the etiology of heart failure, New York Heart Association (NYHA) functional class, and the presence or absence of hypertension, dyslipidemia, and diabetes mellitus. Electrocardiography, chest X-ray, and echocardiography were performed before the administration of tolvaptan or carperitide. Echocardiography was performed to assess intra-ventricular septal wall thickness (IVS), left ventricular end-diastolic diameter (LVEDd), left ventricular ejection fraction (LVEF), and dimension of inferior vena cava (IVC). Heart rate, blood pressure, body weight, daily urine volume, daily volume of water intake and infusion solution, levels of plasma B-type natriuretic peptide (BNP), serum sodium, potassium, blood urea nitrogen (BUN), creatinine, estimated glomerular filtration rate (eGFR), and plasma osmolarity were measured before administration of tolvaptan or carperitide for baseline, and at 2, 3, 4, 7, and 14 days after administration even if these drugs are discontinued <14 days. GFR was estimated from the modification of diet in renal disease formula for Japanese. The severity of subjective symptoms such as dyspnea and leg edema were quantified by modified Borg Scale.21 Objective findings of ADHF including the degree of cardiothoracic ratio (CTR) and pulmonary congestion in chest X-ray, and the dilation of jugular vein were assessed before and after administration of tolvaptan or carperitide. Primary endpoint of this study was the increase in urine volume. Secondary endpoints were the improvement in modified Borg Scale and plasma BNP level.
Definition of Adverse Events
We checked all adverse events of study subjects during hospitalization. Adverse events were determined as the unfavorable situation, which needed the change or discontinuation of tolvaptan or carperitide. Drug change or discontinuation was decided by the attending physician. Cardiovascular events were defined as worsening heart failure and hypotension. Definition of hypotension was systolic blood pressure of <90 mmHg or recognition of symptoms caused by low blood pressure (dizziness, general fatigue, shiver, etc.).
Comparison of Drug Costs
We compared the total cost of tolvaptan or carperitide during hospitalization. Costs of each drug were calculated based on the dose and length of drugs used, which were collected from each attending physician. We calculated tolvaptan as 2525.7 yen per 15 mg, and carperitide as 2,270 yen per 1,000 µg.
Statistical Analysis
Results are expressed as mean ± standard deviation (SD), and skewed variables are presented as median and inter-quartile range. A P value of 0.05 was considered statistically significant, but we did not analyze by multiplicity control. Significance between the two groups was determined by unpaired Student's t test for continuous variables and by chi-square test for discrete variables. The changes of blood pressure, heart rate, subjective symptoms quantified by modified Borg Scale, daily urine volume, daily volume of water intake and infusion solution, blood samples data, and echocardiographic data from baseline in same group were determined by paired t test. If data were not distributed normally, the Mann–Whitney U test was used. Missing data were excluded from the analysis. We used Fisher's exact probability test for the evaluation of adverse events. Statistical analysis was performed with a standard statistical program package (JMP9, SAS Institute, Cary, NC).
Results
Comparisons of Baseline Clinical Characteristics Between Tolvaptan and Carperitide Groups
The comparison of baseline clinical characteristics, including vital sign, laboratory data, and echocardiographic data between tolvaptan and carperitide groups, are shown in Table 1. There was no significant difference in baseline clinical characteristics between the two groups. The mean administration duration of the respective drug (10 ± 8 days in the tolvaptan group and 8 ± 5 days in the carperitide group, P = .123) and the mean length of hospitalization showed no significant difference (30 ± 13 days in the tolvaptan group and 29 ± 18 days in the carperitide group, P = .894). Concomitant medications including loop diuretics, thiazide diuretics, spironolactone, β-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and inotorpic agents were not significant difference between these two groups (Table 1).
Comparisons of Clinical Characteristics Between Tolvaptan and Carperitide Groups
| Tolvaptan (n = 54) | Carperitide (n = 55) | |
|---|---|---|
| Age (years) | 74 ± 12 | 75 ± 11 |
| Gender (male/female) | 29/25 | 33/22 |
| NYHA functional class (II/III and IV) | 11/43 | 11/44 |
| Body weight (kg) | 58.2 ± 13.1 | 56.3 ± 11.3 |
| Current or past smoker, n | 22 | 20 |
| Hypertension, n | 29 | 33 |
| Diabetes mellitus, n | 17 | 18 |
| Hyperlipidemia, n | 12 | 13 |
| Atrial fibrillation, n | 30 | 24 |
| Etiology of chronic heart failure, n | ||
| Dilated cardiomyopathy | 22 | 24 |
| Ischemic heart disease | 13 | 13 |
| Valvular heart disease | 6 | 5 |
| Hypertensive heart disease | 6 | 6 |
| Other | 7 | 7 |
| Heart rate (/min) | 91 ± 27 | 87 ± 26 |
| Systolic blood pressure (mmHg) | 130 ± 26 | 129 ± 27 |
| Diastolic blood pressure (mmHg) | 76 ± 16 | 74 ± 19 |
| Echocardiography | ||
| Left ventricular end diastolic diameter (mm) | 52 ± 12 | 53 ± 9 |
| Left ventricular ejection fraction (%) | 47 ± 18 | 44 ± 14 |
| Inferior vena cava (mm) | 20 ± 6 | 19 ± 6 |
| B-type natriuretic peptide* (pg/mL) | 544.3 (421.1) | 599.0 (397.1) |
| Blood urea nitrogen (mg/dL) | 24.5 ± 15.2 | 24.9 ± 12.7 |
| Serum creatinine (mg/dL) | 1.18 ± 0.76 | 1.24 ± 0.76 |
| Estimated GFR (mL/min/1.73m2) | 52.7 ± 22.5 | 50.0 ± 21.9 |
| Serum sodium (mEq/L) | 139 ± 6 | 140 ± 4 |
| Serum potassium (mEq/L) | 4.2 ± 0.6 | 4.1 ± 0.6 |
| Concomitant medication, n | ||
| Loop diuretics | 48 | 51 |
| Thiazide diuretics | 4 | 5 |
| Spironolactone | 30 | 33 |
| β-blocker | 29 | 35 |
| ACE inhibitors or ARBs | 23 | 31 |
| Mean administration duration (days) | 10 ± 8 | 8 ± 5 |
| Mean length of hospitalization (days) | 30 ± 22 | 29 ± 18 |
NYHA, New York Heart Association; GFR, glomerular filtration rate; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Skewed data are reported as median (inter-quartile range).
Comparisons of Volume of Water Intake and Urine, Symptoms, Hemodynamic, Laboratory, and Echocardiographic Data
As shown in Figure 2, urine volume was significantly higher in the tolvaptan group on the 2nd and 3rd day (P < .01), however, the volume of water intake was also greater in the tolvaptan group than in the carperitide group. The total intake volume including the infusion solution was significantly higher in the tolvaptan group from the 1st day to the 4th day (P < .01). The body weight decrease tended to be higher in the tolvaptan group than in the carperitide group, but did not show a statistically significant difference (data not shown).
Figure 2.
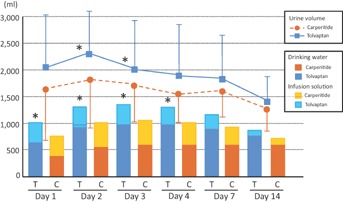
Comparisons of trends in urine volume and total volume of water intake (drinking water and infusion solution) between tolvaptan (n = 53) and carperitide groups (n = 53). *P < .01 versus carperitide group at the same day. T, tolvaptan group; C, carperitide group.
Subjective symptoms such as leg edema and dyspnea were estimated by modified Borg Scale and were assessed at baseline and day 7, and compared between the two groups (Figure 3A). The mean modified Borg Scale of leg edema at baseline were 3.9 in the tolvaptan group and 3.7 in the carperitide group (P = N.S.). The mean values on the 7th day after treatment were similarly decreased to 1.2 in the tolvaptan group and 1.0 in the carperitide group (P < .001 from baseline, respectively), and there was no significant difference between the two groups. The mean modified Borg Scale of dyspnea also improved by treatment in both groups (4.8–0.9 in the tolvaptan group, P < .001; 5.0–1.2 in the carperitide group, P < .001), however, this improvement was not significantly different between the two groups (Figure 3A).
Figure 3.
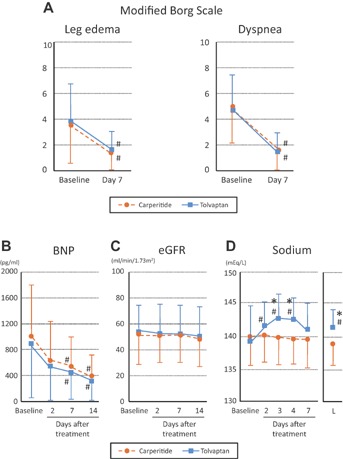
Comparisons of trends in Borg Scale (A), plasma B-type natriuretic peptide (BNP) (B), estimated glomerular filtration rate (eGFR) (C), and serum sodium level (D) between tolvaptan (n = 54) and carperitide (n = 55) groups. L, Last day of drug administration: *P < .05 versus carperitide group at the same day. #P < .001 versus baseline of same group.
Plasma BNP levels were similarly decreased after treatment in both groups (P < .001, Figure 3B). CTR decreased at the 14th day in both groups (64–59% in tolvaptan group, P < .05; 63–59% in carperitide group, P < .05), and the degree of jugular venous dilation was improved at the 14th day compared to baseline (improvement rate from baseline to the 14th day: 92.6% in tolvaptan and 86.2% in carperitide group). Improvements in these parameters were not significantly different between groups. Renal function assessed by eGFR was not influenced by either drug (Figure 3C). The serum sodium level in tolvaptan group was increased at day 2 (139 ± 6 mEq/L at baseline vs. 142 ± 5 mEq/L at day 2, P < .001). However, the serum sodium level in the carperitide group was unchanged during the study period. This level was significantly higher in the tolvaptan group than in the carperitide group at 3, 4, and 7 days after treatment (P < .05, respectively; Figure 3D). In tolvaptan group, at day 7, serum sodium level returned to the same level as baseline, although sodium level was higher in tolvaptan group than in carperitide group at the last day of drug administration (139 ± 5 mEq/L vs. 141 ± 7 mEq/L, P < .05) as shown in Figure 3D. Plasma osmolarity level did not show significant difference between these two groups (data not shown).
The changes in heart rate and blood pressure between baseline and after administration of tolvaptan or carperitide are shown in Figure 4. As shown in Table 1, the systolic and diastolic blood pressure showed no significant difference between the two groups at baseline. These were significantly decreased at day 2 in both groups (systolic blood pressure: 123 ± 23 mmHg in the tolvaptan group, P < .01; 114 ± 21 mmHg in the carperitide group, P < .01; diastolic blood pressure: 71 ± 13 mmHg in the tolvaptan group, P < .05; 63 ± 12 mmHg in the carperitide group, P < .01) as shown in Figure 4A. These decreases were greater in the carperitide group than in the tolvaptan group, and systolic and diastolic blood pressures were significantly lower in the carperitide group than in the tolvaptan group at day 2 (P < .05, respectively). At day 3 and 4, the diastolic blood pressure was significantly lower in the carperitide group than in the tolvaptan group (P < .05, respectively). Although the heart rate was decreased after treatment in both groups, there was no significant difference between these two groups (Figure 4B).
Figure 4.
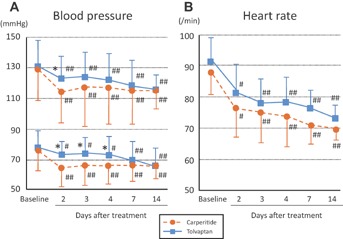
Comparisons of trends in blood pressure (A) and heart rate (B) during the treatment period between tolvaptan (n = 54) and carperitide (n = 55) groups. *P < .05 versus carperitide group at the same day. #P < .05, ##P < .01 versus baseline of same group.
In echocardiographic data, although LVEDd was not significantly changed after treatment in both groups, LVEF and IVC were significantly improved at the 14th day in both groups (mean LVEF: 47% to 50% in the tolvaptan group, P = .011; 44% to 47% in the carperitide group, P = .015; mean IVC: 20 mm to 15 mm in the tolvaptan group; 19 mm to 16 mm in the carperitide group, P < .001, respectively).
Comparison of Adverse Events Between Tolvaptan and Carperitide Group
In the present study, eight adverse events requiring drug discontinuation occurred during the study period (Table 2). In the tolvaptan group, there was only one event of hypernatremia, however, there were seven adverse events in the carperitide group. A case of hypernatremia in tolvaptan group was that initial serum sodium concentration was 142 mEq/L and increased to 147 mEq/L at 4 days after tolvaptan started. On the other hand, most of adverse events in carperitide group were hypotension. One patient occurred hypotension immediately, and the others were at 2–7 days after the start of carperitide. Although there was no statistically significant difference when considering all adverse events (12.7% vs. 1.9%, P = .060), cardiovascular events such as worsening heart failure and hypotension were significantly higher in the carperitide group than in the tolvaptan group (10.9% vs. 0%, P = .027).
Table 2.
Summary of Patients With Serious Adverse Events Requiring Drug Discontinuation in This Study
| Patient number | Adverse events | The day of drug discontinuation | |
|---|---|---|---|
| Tolvaptan group | |||
| Pt. #82 | Hypernatremia | Na 147 mEq/L | 4th day |
| Carperitide group | |||
| Pt. #3 | Worsening heart failure | Worsening pulmonary congestion | 5th day |
| Pt. #28 | Hypotension | SBP < 80 mmHg | 1st day |
| Pt. #32 | Hypotension | SBP < 80 mmHg | 3rd day |
| Pt. #43 | Liver dysfunction | AST 77 IU/L, ALT 179 IU/L | 20th day |
| Pt. #60 | Hypotension | SBP < 90 mmHg with general fatigue | 2nd day |
| Pt. #73 | Hypotension | SBP < 70 mmHg | 2nd day |
| Pt. #96 | Hypotension | SBP < 80 mmHg with dizziness | 7th day |
SBP, systolic blood pressure; AST, aspartate amino transferase; ALT, alanine transaminase.
Comparison of Drug Costs Between Tolvaptan and Carperitide
We compared the drug costs of tolvaptan and carperitide. Figure S1 demonstrates cumulative drug costs distribution of carperitide and tolvaptan. The average drug cost per patient was significantly higher in the carperitide group than in the tolvaptan group (39,778 yen vs. 15,062 yen, P < .001) as in Figure S1.
Discussion
In the present study, we have shown for the first time that the new water diuretic, tolvaptan, was as effective as, and safer than, the intravenous natriuretic peptide, carperitide, for the treatment of ADHF. Diuretics are major therapeutic drugs in ADHF. In the ADHERE registry, it was reported that intravenous loop diuretics remain the first-line therapy for ADHF and are currently prescribed for >90% of hospitalized ADHF patients.22 Loop diuretics inhibit the Na+/2Cl−/K+ cotransporter in the thick ascending loop of Henle, and thiazides inhibit the Na+/Cl− cotransporter in the distal tubule, resulting in decreased urine sodium and chloride reabsorption with natriuresis and diuresis. These mechanisms of conventional diuretics often lead to electrolyte abnormalities, among which hyponatremia is the most important. Some clinical trials demonstrated that hyponatremia was an independent predictor of mortality, longer hospitalization, and death or rehospitalization despite clinical and hemodynamic improvements that were similar to those in patients without hyponatremia.8,23 Therefore, normalization of serum sodium level has been considered an important therapeutic target for the treatment of ADHF.
Tolvaptan is an orally active vasopressin V2 receptor antagonist that promotes aquaresis—excretion of electrolyte-free water—and might be of benefit in hyponatremia. The effect of tolvaptan in hyponatremia was proven by the increasing serum sodium concentration regardless of the severity of hyponatremia in SALT-1 and 2 studies.24 In the present study, the serum sodium level was also increasing by administration of tolvaptan, and only one patient showed an increases as an adverse event (Table 2). Several large-scale placebo-controlled multicenter randomized trials on heart failure patients reported that the incidence of hypernatremia as adverse event by tolvaptan was much less frequent.25–28 Only one patient of hypernatremia in our study did not show a fairly rapid increase (0.5 mEq/L increase per hour) sufficient to meet the criteria of drug discontinuation, however, the basal serum sodium level of this patient was comparatively high (142 mEq/L), and this level increased to 147 mEq/L on the 4th day after administration. Therefore, it is considered that hypernatremia may not be a severe issue if it is strictly observed in the early stage of tolvaptan administration. On the other hand, hyponatremia, which may be associated with the natriuretic effect of carperitide, would be important for treatment of ADHF. In terms of these points, tolvaptan might be more effective for treatment of ADHF compared to carperitide.
Previously described SALT-1 and 2 studies demonstrated that serum sodium levels declined after discontinuation of tolvaptan, to the same level as the placebo group.24 As with these SALT-1 and -2 studies, our present study demonstrated that the serum sodium level returned to the same level as baseline after drug discontinuation in the tolvaptan group, although sodium level was significantly higher in the tolvaptan group than in carperitide group during the drug administration period. In order to prevent hyponatremia, long-term administration of tolvaptan might be necessary in patients with very low sodium level at baseline, and this point might need further study in the future.
In SALT studies, the dose of tolvaptan was increased to 60 mg daily, if necessary, on the basis of serum sodium concentrations, and the average dose of tolvaptan in SALT studies was larger than that in this study.24 However, the physique of Japanese is smaller than that of Westerner, actually, the average body weight in this study (54.2 kg) was smaller than that in SALT studies (73–78 kg). Moreover, the standard dose of tolvaptan in Japan is 15 mg, which is smaller than that in Western countries, we considered that the dose of tolvaptan was not small for treatment of ADHF in this study.
Renal dysfunction is a frequent finding in patients with ADHF and is a powerful independent prognostic factor for prolonged length of hospital stay, increased in-hospital mortality, and higher rates of rehospitalization and death post-discharge.11,29,30 Several reports revealed that the use of a high dose of diuretics was a prognostic factor of worsening renal function and worsening prognosis of ADHF patients.11,13,31 Therefore inhibiting worsening renal function is an important issue for ADHF patients. In the present study, tolvaptan did not adversely affect renal function in ADHF patients.
Carperitide, which develops vasodilation and natriuretic effects, is used frequently in Japan. ADHF patients with hypertension have a good indication for carperitide due to its vasodilation effect. A large-scale multicenter cohort study of >4,842 registered in Japan (ADHF syndrome; ATTEND registry) revealed that although patient characteristics did not differ from those reported in Western countries, there was a unique finding, a high rate of carperitide use (69.4%) for ADHF in Japan.32 Therefore, we selected carperitide as a competitor of tolvaptan to compare diuretic effect. The efficacy and safety of carperitide in the acute phase treatment of ADHF were reported by the COMPASS trial in 2008, and the incidence of hypotension was only 3.55% in this trial.15 The incidence of hypotension was 9.09% (5/55) in our present study, however, we could not simply compare these results due to the difference in terms of blood pressure of study subjects. In the COMPASS trial, patients over 120 mmHg in systolic blood pressure were enrolled, however, there were no strict exclusion criteria in our study. In fact, the mean systolic blood pressure in the COMPASS trial and our present study were 151.1 and 128.4 mmHg at baseline, respectively. Another report in the “real world” of carperitide therapy revealed that the incidence of hypotension was 9.45%.33 Therefore, the incidence of hypotension in the carperitide group was not high in the present study. The long-term prognostic examination associated with carperitide reported a significant reduction of cardiac events compared to standard therapy without carperitide.16 We would compare the long-term prognostic effect of tolvaptan compared to carperitide in a future report.
Study Limitations
First, tolvaptan is different from carperitide in terms of administration routes (tolvaptan is orally administered and carperitide is intravenous). In the present study, enrollment eligible patients did not have any severe hemodynamic abnormality requiring an auxiliary circulation system such as intra-aortic balloon pump or percutaneous cardiopulmonary support, because these patients often have difficulty with sufficient oral intake and complaint of thirst. There were also no patients who needed ventilation. Second, decision of study drugs was randomly assigned, but not blinded, in this trial. Therefore, it is possible that any results of this study including adverse events might be influenced due to the lack of blinding. Third, the numbers of study subjects were small. Third, we compared merely the cost of subjected two drugs, however, we did not compare the total cost in this study. The cost offset model of tolvaptan revealed that the reduction of length of hospital stay and total cost saving of $265 per admission in heart failure patients with hyponatremia in the United States,34 however, cost effectiveness of carperitide has not been previously reported. In future research, a larger scale study will be necessary to validate the clinical usefulness and cost effectiveness of tolvaptan in our country.
Declaration of Conflicting Interests
None.
Funding
This study was supported in part by a grant from the Japanese Heart Foundation (No. 12100026). No additional external funding received for this study.
Appendix
In addition to the authors, the following investigators participated in the AVCMA trial: Takamasa Sato, Takashi Owada, Yuichi Nakamura, Hiroyuki Yamauchi, Minoru Nodera, Atsuro Masuda, Tetsuro Yokokawa, Fukushima Medical University; Shigebumi Suzuki, Kazuyuki Yoshinari, Yasuyuki Watanabe, Hideaki Dairaku, Fukushima Accident Hospital; Kazuaki Tamagawa, Fukushima Prefecture Aizu General Hospital; Kenichi Watanabe, Takayuki Sakamoto, Fukushima Red Cross Hospital; Tomoyuki Watanabe, Health Co-op Watari Hospital; Hiroshi Ohtani, Iwase General Hospital; Nobuo Komatsu, Hiroto Takeda, Ohta Nishinouchi Hospital; Masahiko Sato, Katuya Ando, Public Soma General Hospital; Hideki Ohtake, Hirofumi Machii, Saiseikai Fukushima General Hospital; Masahiro Ono, Mitsuru Muto, Taku Ohsugi, Keiichi Kawamura, Wakako Naganuma, Southern Tohoku General Hospital; Tetsu Watanabe, Takuya Miyamoto, Takehiko Miyashita, Takanori Arimoto, Mitsunori Ishino, Hyuma Daidouji, Yoshinori Yashiro, Shintaro Sasaki, Daisuke Ishigaki, Yuki Honda, Yamagata University School of Medicine; Tomoyasu Yahagi, Yamagata Prefectural Central Hospital; Shigeo Sugawara, Kazuyoshi Kaneko, Toshiki Sasaki, Nihonkai General Hospital.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
(A) Comparison of drug cost between tolvaptan and carperitide illustrated by cumulative distribution plots. (B) Comparison of mean drug cost per patient between tolvaptan and carperitide groups.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 3.Redfield MM. Heart failure—an epidemic of uncertain proportions. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Binanay C, Califf RM, Hasselblad V, et al. ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 6.Peacock WF, Costanzo MR, De Marco T, et al. ADHERE Scientific Advisory Committee and Investigators. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, O'Connor CM, Braunwald E. Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil. Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Abraham WT, Albert NM, et al. OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration, clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 9.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left-ventricular dysfunction with and without congestive-heart-failure—A substudy of the studies of left-ventricular dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 10.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart-failure—activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Metra M, Nodari S, Parrinello G, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62:187–193. doi: 10.1016/S0009-9236(97)90067-9. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Qadir HM, Tu JV, Yun L, et al. Diuretic dose, long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J. 2010;160:264–271. doi: 10.1016/j.ahj.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 15.Nomura F, Kurobe N, Mori Y, et al. Multicenter prospective investigation on efficacy, safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: carperitide effects observed through monitoring dyspnea in acute decompensated heart failure study. Circ J. 2008;72:1777–1786. doi: 10.1253/circj.cj-07-0760. [DOI] [PubMed] [Google Scholar]
- 16.Hata N, Seino Y, Tsutamoto T, et al. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J. 2008;72:1787–1793. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 17.Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–F278. doi: 10.1152/ajprenal.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki M, Hori M, Izumi T, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study) Cardiovasc Drugs Ther. 2011;25:S33–S45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 20.Izumi K, Eishi K, Yamachika S, et al. The efficacy of human atrial natriuretic peptide in patients with renal dysfunction undergoing cardiac surgery. Ann Thorac Cardiovasc Surg. 2008;14:294–302. [PubMed] [Google Scholar]
- 21.Carvalho VO, Bocchi EA, Guimarães GV. The Borg scale as an important tool of self-monitoring and self-regulation of exercise prescription in heart failure patients during hydrotherapy. A randomized blinded controlled trial. Circ J. 2009;73:1871–1876. doi: 10.1253/circj.cj-09-0333. [DOI] [PubMed] [Google Scholar]
- 22.Adams KF, Jr, Fonarow GC, Emerman CL, et al. ADHERE Scientific Advisory Committee, Investigators. Characteristics, outcomes of patients hospitalized for heart failure in the United States: rationale, design, preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007;167:1998–2005. doi: 10.1001/archinte.167.18.1998. [DOI] [PubMed] [Google Scholar]
- 24.Schrier RW, Gross P, Gheorghiade M, et al. SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 25.Udelson JE, Orlandi C, Ouyang J, et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:1540–1545. doi: 10.1016/j.jacc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Udelson JE, McGrew FA, Flores E, et al. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol. 2007;49:2151–2159. doi: 10.1016/j.jacc.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 27.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 29.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Cowie MR, Komajda M, Murray-Thomas T, et al. Prevalence, impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 31.Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment, development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Sato N, Kajimoto K, Asai K, et al. ATTEND Investigators. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, preliminary data. Am Heart J. 2010;159:949–955. doi: 10.1016/j.ahj.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Suwa M, Seino Y, Nomachi Y, et al. Multicenter prospective investigation on efficacy, safety of carperitide for acute heart failure in the “real world” of therapy. Circ J. 2005;69:283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 34.Chiong JR, Kim S, Lin J, et al. Evaluation of costs associated with tolvaptan-mediated length-of-stay reduction among heart failure patients with hyponatremia in the US, based on the EVEREST trial. J Med Econ. 2012;15:276–284. doi: 10.3111/13696998.2011.643329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Comparison of drug cost between tolvaptan and carperitide illustrated by cumulative distribution plots. (B) Comparison of mean drug cost per patient between tolvaptan and carperitide groups.


