Abstract
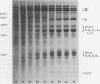
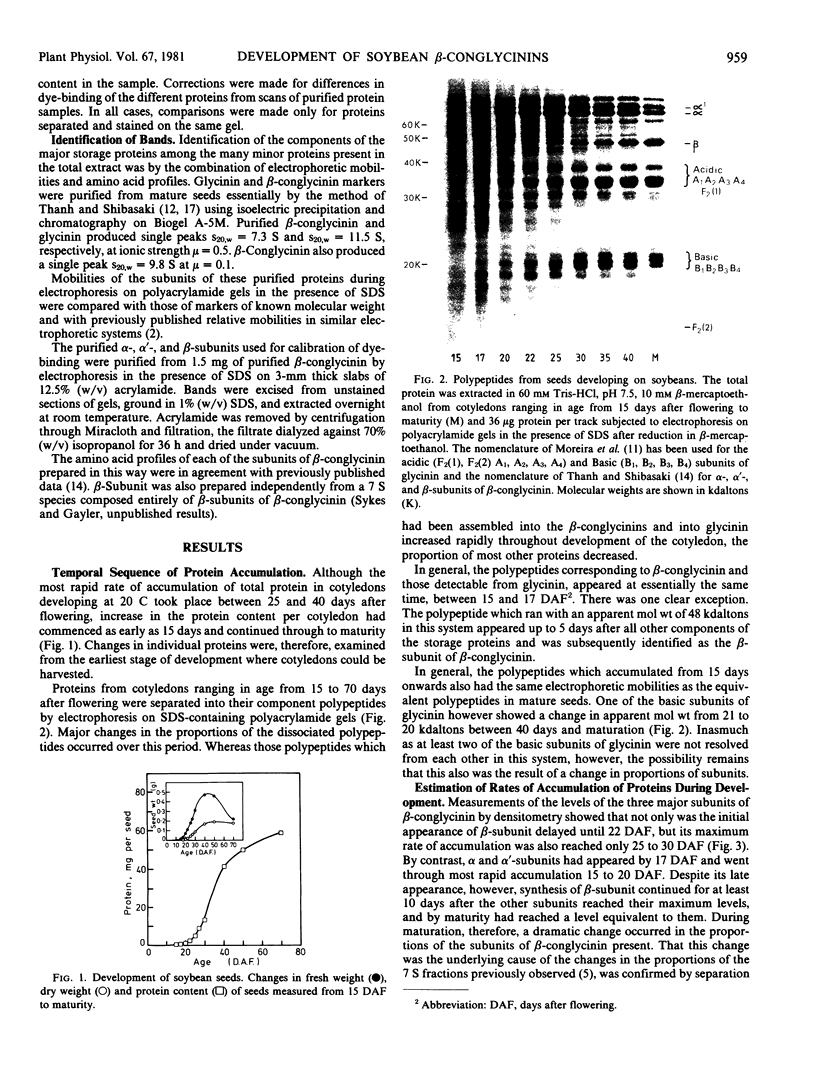
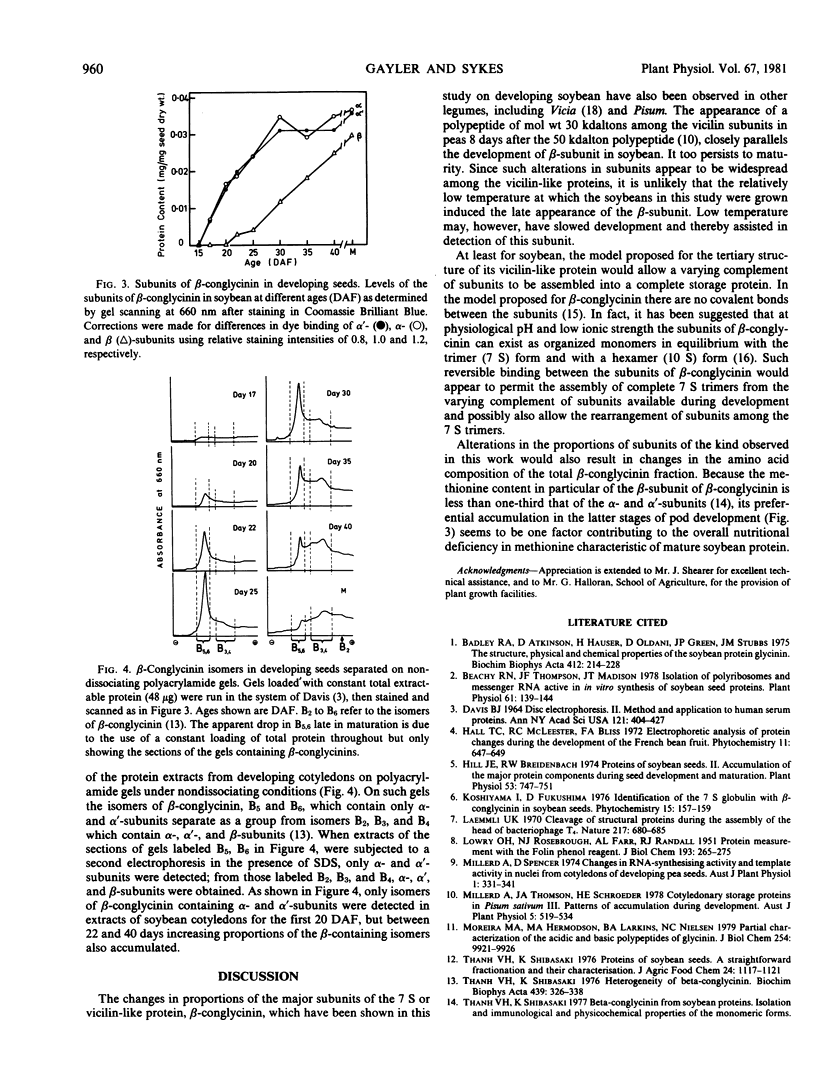
The temporal sequence of development of the major proteins of seeds of soybean (Merr.) has been studied during development of cotyledons from flowering to maturity. A well-defined difference occurred in the times of appearance and the periods of maximum accumulation of α, α′-, and β-subunits of betaconglycinin. Whereas α- and α′-subunits appeared 15 to 17 days after flowering, accumulation of β-subunit did not commence until 22 days after flowering. Such alterations in subunit composition infer that changes also occurred in the amino acid composition of betaconglycinin during maturation, particularly in the content of methionine which is low in the β-subunit.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Atkinson D., Hauser H., Oldani D., Green J. P., Stubb J. M. The structure, physical and chemical properties of the soy bean protein glycinin. Biochim Biophys Acta. 1975 Dec 15;412(2):214–228. doi: 10.1016/0005-2795(75)90036-7. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- Thanh V. H., Okubo K., Shibasaki K. Isolation and Characterization of the Multiple 7S Globulins of Soybean Proteins. Plant Physiol. 1975 Jul;56(1):19–22. doi: 10.1104/pp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Heterogeneity of beta-conglycinin. Biochim Biophys Acta. 1976 Aug 9;439(2):326–338. doi: 10.1016/0005-2795(76)90068-4. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Major proteins of soybean seeds. A straightforward fractionation and their characterization. J Agric Food Chem. 1976 Nov-Dec;24(6):1117–1121. doi: 10.1021/jf60208a030. [DOI] [PubMed] [Google Scholar]