Abstract
Horizontal gene transfer (HGT) is widespread amongst prokaryotes, but eukaryotes tend to be far less promiscuous with their genetic information. However, several examples of HGT from pathogens into eukaryotic cells have been discovered and mimicked to improve non-viral gene delivery techniques. For example, several viral proteins and DNA sequences have been used to significantly increase cytoplasmic and nuclear gene delivery. Plant genetic engineering is routinely performed with the pathogenic bacterium Agrobacterium tumefaciens and similar pathogens (e.g. Bartonella henselae) may also be able to transform human cells. Intracellular parasites like Trypanosoma cruzi may also provide new insights into overcoming cellular barriers to gene delivery. Finally, intercellular nucleic acid transfer between host cells will also be briefly discussed. This article will review the unique characteristics of several different viruses and microbes and discuss how their traits have been successfully applied to improve non-viral gene delivery techniques. Consequently, pathogenic traits that originally caused diseases may eventually be used to treat many genetic diseases.
Keywords: Mimicry, Horizontal gene transfer, Agrobacterium tumefaciens, Trypanosoma cruzi, Bartonella henselae, Non-viral gene delivery
1. Introduction
Horizontal gene transfer (HGT) is defined as the exchange of genetic material between different species. HGT occurs frequently between prokaryotes, allowing them to quickly adapt to environmental changes by sharing genes for antibiotic resistance [1] or metabolic enzymes [2,3]. This phenomenon revolutionized the field of biotechnology by allowing genetic engineers to transform bacteria with valuable eukaryotic genes for industrial production (e.g. insulin [4] and various antibodies [5]).
In contrast to the genetic promiscuity of prokaryotes, eukaryotes are much more resistant to HGT. Eukaryotic cells possess several barriers that repel foreign DNA, including a nuclear membrane and DNase enzymes in the cytosol [6]. However, several significant HGT events have been discovered in multicellular eukaryotes. For example, the red color of some aphids and spider mites has been attributed to the HGT of fungal genes for carotenoid biosynthesis [7,8]. The coffee berry borer beetle (Hypothenemus hampei) also expresses a mannanase gene of bacterial origin which allows the beetle to digest galactomannan, the major polysaccharide in coffee berries [9]. The most stunning example of eukaryotic HGT may be the photosynthetic sea slug, Elysia chlorotica, which is able to harvest and support algal plastids for several months by expressing plastid maintenance genes of algal origin [10].
While the previous examples are highly random and isolated events, there are other examples of eukaryotic HGT which are more frequent. For example, viruses are highly efficient HGT vectors that transfer viral genes and even some host genes between cells [11,12]. The bacterium Agrobacterium tumefaciens infects plant tissues by transferring oncogenes to plant cells to induce tumor formation [13]. Finally, Trypanosoma cruzi is an intracellular eukaryotic parasite which infects human cells and is responsible for adverse HGT events which may cause chronic Chagas Disease [14]. The purpose of this review is to highlight the mechanisms that these pathogens use to transfer genetic material and show how those mechanisms have been applied to improve modern gene delivery techniques. In addition, the natural transfer of nucleic acids between host cells via plasmodesmata, nanotubes, vesicles, and carrier proteins will also be discussed.
2. Highly evolved HGT: Viruses
Viruses have evolved over millennia into highly efficient gene delivery vehicles. Their efficiency is highlighted by the success of many clinical trials with viral gene therapy [15]. For example, recombinant viruses have been used to successfully treat Leber's congenital amaurosis (LCA, a type of blindness) [16] and Severe Combined Immunodeficiency (SCID) [17]. Unfortunately, the clinical progress of viral gene therapy has been hindered by severe side effects, including immune responses [18,19], inflammation [20], and even oncogenesis [21]. Additional concerns associated with viral gene therapy include restrictions on gene size (<5–40 kb, depending on the virus) [15] and the relative difficulty of manufacturing viruses. Therefore, interest in non-viral gene delivery has grown significantly over the past few decades. Many non-viral gene delivery techniques have been developed (cationic polymers, lipids, dendrimers, peptides, etc.), but these techniques are typically much less efficient than viral gene delivery. This section will focus on the unique characteristics of viruses that have been used to increase the efficiency of non-viral gene delivery techniques, including methods of DNA protection/transport, cell invasion, endosomal escape, nuclear transport, and transgene expression/maintenance (see Fig. 1 for overview).
Fig. 1.
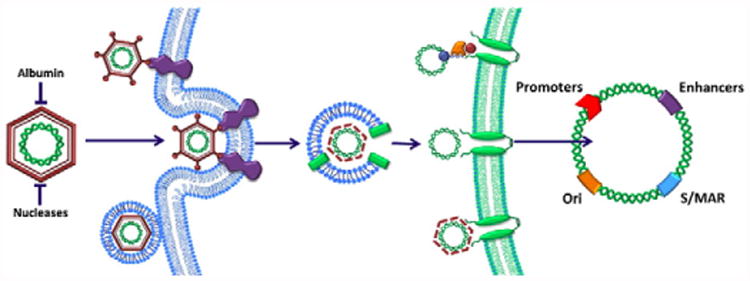
Useful traits of viral gene delivery and expression that have been used to enhance non-viral gene delivery. From left to right: in the extracellular space, viruses protect their nucleic acid cargo from plasma scavengers and nucleases with protein capsids and/or lipid membranes (envelopes). Antigens on the capsid or envelope surface allow viruses to bind one or more receptors on specific cell types and directly fuse with the cell membrane (enveloped viruses) or induce endocytosis. As pH decreases within the maturing endosome, capsid proteins change conformation and destabilize the endosomal membrane to release viral nucleic acids, with or without the capsid. Nuclear import of viral nucleic acids is then facilitated either by binding to host transcription factors or viral proteins with nuclear localization signals that interact with nuclear pore complexes. Finally, viral gene expression within the nucleus is enhanced by highly efficient promoters and enhancers while origins of replication (Ori) and/or scaffold/matrix attachment regions (S/MAR) ensure plasmid replication and sustained gene expression.
2.1. Nucleic acid protection: Capsids & envelopes
One of the simplest ways viruses enhance gene delivery is by storing their nucleic acids within protein capsules (capsids), which may also be surrounded by a lipid membrane or “envelope” from the previous host cell [22,23]. Capsids protect their nucleic acid cargo during intercellular transport from degradation by plasma nucleases [24] and scavenging by albumin [25]. In addition, the size and shape of viral particles directly influence their circulation half life, since filamentous capsids have been shown to persist 10 times longer (∼1 week) in the circulation than spherical capsids [26]. Specialized capsid proteins also play key roles in cell binding and invasion. However, capsid proteins have also been shown to initiate immune responses, thereby significantly reducing the effectiveness of some viral gene therapies after the initial treatment [27]. Some viral capsids may also cause inflammation and even apoptosis in certain cells [28].
Many non-viral gene delivery vehicles have aimed to mimic the beneficial/protective properties of capsids while avoiding the immune and inflammatory effects of capsids [29–31]. For example, cationic polymers and peptides readily bind to anionic plasmid DNA to form polyplexes, thereby condensing the DNA and protecting it from serum nucleases [32,33]. Cationic polymers (PEI and poly-lysine) have also been used to coat non-infectious viruses to create polymer–virus hybrids that are able to transduce a wide variety of cells and sustain gene expression for a considerable period (up to 40 days) [34] with much lower doses of hybrid than native virus [35].
Development of artificial capsids for gene delivery has also been the focus of much research, but controlling the crucial factors of size and shape while packaging bulky plasmid DNA has proven to be a considerable challenge. Nonetheless, Lim et al. were able to synthesize a self-assembling filamentous capsid containing siRNA by using self-assembling β-sheet peptides with poly-lysine sequences for DNA binding and covalently attached glucose ligands for cell-specific receptor binding. This synthetic capsid was able to deliver siRNA and silence GFP expression in HeLa cells just as well as lipofectamine (∼70% reduction in GFP expression) [36]. Malay et al. also showed that gold nanoparticles could be used to catalyze formation of capsids with cysteine rich trp RNA-binding attenuation protein (TRAP) monomers. However, the diameter of these synthetic capsids was quite small (15–22 nm) and they did not contain any nucleic acids [37]. It is also worth mentioning that polyplexes of plasmid DNA and a cationic peptide from the HIV protein Vpr (aa 52–93) were shown to have transfection efficiencies 100–1000 fold higher than poly-lysine (but roughly equivalent to PEI) [38].
There have also been significant efforts to mimic enveloped viruses. For example, Muller et al. synthesized a PEI–lipid–RGD peptide conjugate that formed artificial virus-like envelopes (AVEs) loaded with plasmid DNA and presenting RGD peptides for cell-specific binding to HUVEC cells [39]. These micelles were able to transfect nearly 100% of HUVEC cells in vitro, while non-RGD micelles and PEI polyplexes transfected only 50% and 5% of cells, respectively [39]. Similar AVEs consisting of PEI–lipid–PEG monomers were also able to effectively transfect murine tumor cells in vivo, while avoiding accumulation in the reticuloendothelial system (RES) [40]. Finally, micelles with multiple lipid bilayers (Multifunctional Enveloped Nano-Devices or MENDs) have been shown to sequentially fuse with cell, endosome, and nuclear membranes to facilitate transfection at 10-fold higher levels than lipofectamine in vitro [41,42].
2.2. Cellular invasion – Receptor targeting by protein antigens & antibodies
Many viruses rely on the unique biochemical machinery within specific cell types to successfully replicate. Consequently, these viruses have developed highly effective cell targeting mechanisms that might also be used to enhance the specificity of non-viral gene delivery techniques. Viruses target specific cell types by binding to one or more cell surface receptors (e.g. Adenovirus = CAR receptor, HIV = CD4 + CCR5, HepC = CD81 + Claudin + SR−Ba + Occludin) [43,44]. Aside from allowing viruses to selectively bind to target cells, receptor binding can also accelerate cell membrane fusion or induce biochemical pathways that enhance viral transduction. For example, the enveloped Epstein–Barr Virus (EBV) directly fuses to the cell membrane after it binds to integrins [45], while receptor binding by non-enveloped viruses triggers key host cell pathways (including activation of PI3K [46], ERK [47], and PKC [48] kinases) which directly increase the rate of endocytosis [49] and influence endosomal sorting [50].
The benefits of cell-specific targeting may be easily adapted to enhance non-viral gene delivery by polymers and lipids. As previously mentioned, simply adding the RGD tripeptide to target integrins significantly enhances non-viral gene delivery to HUVEC [39] and other cell types up to 50-fold [51–53]. Conjugation of small molecules like folate has also been shown to enhance the transfection of PEI–PEG hybrids while reducing serum scavenging and toxicity [54]. Interestingly, conjugation of epidermal growth factor (EGF) to PEI for EGFR targeting also enhanced transfection 10–100 fold and sustained considerable expression at lower doses where unmodified PEI transfection was negligible [55].
Conjugation of whole proteins to polymers and lipids has also been shown to increase specificity and transfection efficiency. For example, polymer–antibody conjugates could potentially be used to enhance gene delivery to virtually any cell type. Li et al. were able to successfully target pulmonary endothelial cells with an anti-PECAM antibody–PEI conjugate that was 10 times more efficient than PEI [56]. Anti-ErbB2 antibody conjugates were also shown to selectively target breast cancer tissue [57]. Other options for protein-polymer/lipid conjugation include transferrin, which is taken up at a higher rate in tumor tissue than typical somatic cells [58]. Conjugation of transferrin to PEI (i.e. “transferrinfection”) yielded a conjugate that is 10–100× more efficient than PEI alone [55].
2.3. Cellular invasion – Non-specific but highly efficient cell penetrating peptides
In addition to receptor binding, some viruses are able to further enhance transduction with cell penetrating peptides (CPPs), which are also known as protein transduction domains or PTDs. CPPs are defined as short cationic peptides (5–40 residues) that typically contain short repeats of arginine or lysine [59] and are able to translocate across biological membranes independently of receptors (reviewed extensively in [60–62]). CPP functions include cellular invasion, endosomal escape, or translocation of DNA or viral proteins into the nucleus. The first CPP was discovered within the Trans-Activator of Transcription (TAT) from HIV-1 [63], but many other CPPs have since been discovered [64] and synthetic CPPs have also been designed [65]. Aside from HIV-1, CPPs have been found in both mammalian [66] and plant [67] viruses, including Vp22 from herpes virus. Vp22 is thought to quickly translocate into the host cell nucleus and spread to neighboring cells as well [68]. Interestingly, CPPs have also been found in a wide variety of eukaryotes including Drosophila (pAntp [69]), venomous wasps (Transportan [70]), toads (Buforin II [71]), and mice (pVEC [72]). The mechanisms associated with each CPP are both diverse and unclear. For example, some CPPs are ineffective at low temperatures, suggesting that they require endocytosis [73]. Transfection by pAntp may also be enhanced by endosomolytic agents like hemagglutinin (HA2, see next section), further suggesting that some CPPs simply induce endocytosis [74]. On the other hand, some CPPs are fully functional at low temperatures and can even transfect quiescent cells [73].
Even though CPP mechanisms remain unclear, it has clearly been shown that CPPs are powerful transport vehicles. Conjugates of TAT and the massive β-galactosidase protein (465 kDa) are readily taken up in vitro, although at a slower rate than TAT conjugates with smaller cargoes [75]. TAT has even been shown to translocate 90 nm beads into nuclei, which is quite a feat considering nuclear pore diameters vary from 9 to 40 nm [76]. Therefore, it is not surprising that polymer– TAT conjugates enhance transfection 10–70 fold compared to PEI and chitosan in vitro [77,78]. Enhancement of transfection by PEI–PEG–TAT conjugates has also been observed in vivo, although to a lesser degree (3 fold) [79].
Another interesting application of CPPs is the expression of CPP-transgene fusions for intercellular transport. In these systems, CPPs are expressed as fusion tags on the target proteins, allowing the target protein to spread to neighboring cells which were not transfected. For example, Lai et al. used Vp22 to transport EGFP from transfectants to neighboring cells [80], while Suzuki et al. showed an overall 4.3-fold enhancement of Vp22-lacZ activity in myocardial cells [81]. This technique has also been used to increase the distribution of p53 (a tumor suppressor protein) intratumorally in vivo [82,83].
2.4. Endosomal escape – Fusogenic peptides
Endocytosis has three main outcomes – (1) acidic degradation of cargo within mature endosomes (i.e. lysosomes), (2) recycling of endosomal cargo back to the extracellular space, or (3) endosomal disruption and release of cargo [84]. Viruses have developed sophisticated ways to ensure the safe release of their nucleic acids into the cytoplasm. Helenius et al. were the first to discover that decreases in endosomal pH actually induced endosomal disruption by the influenza virus [85]. Further investigation revealed that the capsid protein hemagglutinin (HA) undergoes a conformational change around pH 5.0, exposing an amphipathic α-helical HA2 domain [86,87]. The amphipathic nature of the HA2 domain allows it to fuse with the endosomal membrane and destabilize it, facilitating the release of the endosomal contents [88]. Many other viruses have similar “fusogenic” peptides (reviewed in [88]), including the gp41 domain of the HIV gp160 protein [89].
Like CPPs, fusogenic peptides have also been used to enhance non-viral gene delivery. The HA2 domain has been used to enhance gene delivery with both cationic polymers and lipids, including poly-lysine [90], Transfectam® [91], and lipofectamine [92]. A gp41-PEI conjugate also enhanced transfections >10 fold in HeLa cells [93] and a peptide from the herpesvirus glycoprotein H enhanced transfection 5–10 fold in a variety of cell lines (MCF-7, AD293, and HepG2) [94]. Synthetic fusogenic peptides like GALA (WEAALAEALAEALAEHLAEALAEALEALAA) have also been shown to enhance liposomal gene delivery up to 100-fold [95,96]. It is important to mention that cationic polymers with protonable amines (e.g. PEI [97] and PAMAM [98]) also have an inherent mechanism of endosomal disruption, in which the polymer binds excess protons in the endosome, thereby protecting the DNA from degradation. This “proton sponge” effect also attracts negatively charged chlorine ions into the endosome, causing an increase in osmotic pressure that eventually bursts the endosome. No viruses have been observed to utilize this mechanism of endosomal escape, but it has been reported that addition of 10 histidines (which also bind protons) to the TAT sequence enhanced its transfection efficiency up to 7000 × compared to TAT alone [99].
2.5. Nuclear import – Enhancers and nuclear localization signals
The final (and probably most critical) physical barrier for gene delivery is the nuclear membrane. Many gene delivery techniques rely on the breakdown of the nuclear membrane during mitosis to transport plasmids into the nucleus, but this strategy is ineffective for quiescent cells [100]. Alternatively, plasmids may be actively transported into the nucleus through nuclear pore complexes (NPCs). Although nuclear pores are quite small during interphase (∼9 nm), they can actively transport proteins as large as 25–50 MDa, which is much larger than plasmid DNA (MW = 2–10 MDa) [101]. Transport through the NPC is tightly regulated by importin α, which binds to proteins containing a nuclear localization signal (NLS) that contains several basic amino acids [102].
Since plasmids are too large to passively diffuse through the NPC [103,104], most viral genomes contain DNA sequences that are bound by viral or host proteins with NLS tags (e.g. the large T-antigen of SV40 – PKKKRV and TAT – GRKKRRQRRRAP), allowing the viral DNA to “hitch a ride” through the NPC with the NLS-tagged protein [105–107]. These DNA sequences, also known as enhancers, are commonly associated with promoter regions and other protein binding sites [108]. For example, the native SV40 enhancer contains both the SV40 origin of replication and early/late promoters [109]. Following microinjection, plasmids with a minimal SV40 enhancer (72 bp long [104]) rapidly accumulate in the nucleus and express levels of luciferase that are 100-fold higher than plasmids without enhancers (pBr322) [104]. The SV40 enhancer was also shown to enhance transgene expression 20-fold in mouse muscle cells [110]. Therefore, it is no surprise that this simple genetic element is included in most commercial expression plasmids.
Direct conjugation of viral proteins containing NLS tags to polymers has also been shown to significantly enhance non-viral gene delivery. For example, a peptide consisting of four repeats of the SV40 NLS (4×PKKKRKV) was shown to bind DNA and transfect cells 100–1000 times better than poly-lysine and twice as well as PEI. Gene expression was also observed in as little as 2 hours after transfection, while PEI transfections took at least 8–24 h [111]. The SV40 NLS has also been improved by adding a phosphorylation site (SSDDE) to the C-terminus [112,113]. Finally, conjugation of the hexon protein from adenovirus (hex) to PEI also enhanced transfection 8×, even though there were no significant differences in polyplex uptake between PEI and the hex–PEI conjugate [114].
2.6. Transgene maintenance – Integration and episomes
Once viral DNA enters the nucleus, it may integrate into the host cell genome or remain an independent nuclear plasmid or “episome”. Viruses have developed many different intriguing ways to integrate into specific areas of the genome [115–118], but they pose significant risks of host gene modification and oncogenesis, making them unfavorable for non-viral gene delivery. However, a new technique which uses a zinc finger recombinase to target specific genomic locations may be a much safer way to integrate transgenes into the genome [119].
Episomal replication is a relatively safer way to maintain transgenes in the nucleus, but it is also much more complex. Viral episomes must contain origins of replication (ori) [120] and scaffold/matrix attachment regions (S/MARs) [121] to ensure replication of the episome and segregation of episomes into daughter cells following mitosis. Some episomal viruses, such as the Epstein–Barr Virus (EBV), also require another viral protein (EBNA-1) to initiate episomal replication [122]. Plasmids containing the EBV origin (oriP) and an expression cassette for EBNA-1 have been shown to maintain steady levels of luciferase expression in mouse heart and lung tissue up to 11 weeks after initial transfection [123]. Likewise, papovavirus episomes have also been shown to persist in human cells for up to 2 months [124,125].
2.7. Transgene expression – Promoters and enhancers
Even after plasmids enter the nucleus, transgene expression can still be challenging. Viruses have developed several different genetic elements to enhance transgene expression, including promoters and various enhancers. Viral promoters from Simian Virus 40 (SV40) [126], Cytomegalovirus (CMV) [127] and Rous Sarcoma Virus (RSV) [128] are commonly used in both viral and non-viral gene therapy, since they provide a high level of expression in a variety of cell lines. However, several studies have shown that specific cell types favor one promoter over another [129]. For example, CMV and RSV expression are roughly equivalent in A5 cells, but 100 times more effective than SV40 [130]. Despite the efficiency of these viral promoters, it is important to note that they are prone to silencing by interferons and tumor necrosis factors as part of the innate immune system in some cell types [131–133]. Consequently, some gene therapy plasmids now include human promoters, such as the ubiquitously active elongation factor 1 α (EF1α) promoter, which gives similar expression levels as CMV or RSV, but for longer periods [130].
Viruses have also been found to enhance transgene expression by incorporating specialized RNA sequences into their open reading frames which increase mRNA stability (polyadenylation tails) and nuclear export (post-transcriptional regulatory elements PREs). Addition of polyA tails to mRNA transcripts is a well known way to protect transcripts from exonuclease digestion, thereby prolonging the life of the transcript and potentially increasing transgene expression. Indeed, addition of the SV40 polyA tail to a CMV-driven luciferase vector significantly enhanced expression 17–50 fold in a variety of cell lines (HeLa, HepG2, and ECV304) in vitro and up to 125 fold in vivo [134]. Much like the SV40 enhancer mentioned previously, the SV40 polyA tail is also included in many commercial expression plasmids.
Another interesting enhancer of transgene expression is the Woodchuck hepatitis Post-translational Regulatory Element (WPRE). When the WPRE is added to the 5′ end of mRNA transcripts, expression is enhanced as much as 700-fold in mouse liver [135]. It has been suggested that the WPRE enhances expression by assuming a tertiary structure which is bound by the host protein CRM-1 and assists in nuclear export. Alternatively, it has also been shown that the structure of the WPRE may prevent read-thru and excessive transcription.
3. Parasitic HGT: A. tumefaciens
A. tumefaciens is a unique bacterial parasite that invades a wide variety of plants to induce tumor formation (i.e. crown gall disease). Other species in the Agrobacterium genus also cause similar infections in plants, including Agrobacterium rhizogenes (hairy root disease) [136], Agrobacterium rubi (cane gall) [137] and Agrobacterium vitis (crown gall in grape) [138], but A. tumefaciens is the most well-known and studied for its ability to transfer genes into plant cells. The transduced bacterial genes initiate uncontrolled cell division, production of substrates and enzymes beneficial to the bacterium, and formation of a tumor (“crown gall”) in which the bacterium feeds [139]. Interestingly, only strains of A. tumefaciens which possess a tumor-inducing (Ti) plasmid are virulent [140]. The Ti plasmid consists of segments of transfer DNA (T-DNA) that are ultimately incorporated into the plant host genome for tumor formation and synthesis of opines, which are utilized by A. tumefaciens as a carbon/nitrogen source. The Ti plasmid also contains a virulence (Vir) region, which encodes several genes (VirA-H [141]) that are necessary for the transformation process itself.
While crown gall disease is a common problem in the agricultural industry, Agrobacterium-mediated gene transfer has become the leading method of transgenic plant production [141]. A. tumefaciens plant infection may occur in vitro, in which case the plant cells or tissues are cultivated before plant regeneration, or in planta, during which a portion of the whole plant is targeted. Many different crop species (including corn [142,143], wheat [144], soybeans [145], rice [146], and many others) have been stably transformed with A. tumefaciens by replacing the existing genes in the T-DNA region of the Ti plasmid with transgenes. Examples of successful Agrobacterium transformation include the transfer of beneficial genes for drought [147,148] or herbicide resistance [149,150]. Many other excellent reviews describing the mechanisms of A. tumefaciens gene delivery in plants are available [141,151–155]. This section will highlight the existing and potential applications of this phenomenon to non-viral gene delivery, including the roles of bacterial and host proteins in the transformation process.
3.1. Mechanism of Agrobacterium-mediated T-DNA delivery
The A. tumefaciens transformation process begins in the soil, where signaling molecules (sugars, phenols, and/or acetosyringone [156,157]) released by wounded plants activate the VirA receptor (Fig. 2). VirA then phosphorylates the transcription factor VirG, which expresses several more virulence (Vir) genes on the Ti plasmid [158]. Several chromosomal proteins are also expressed (chvA, chvB, and exoC) which are involved in bacterial/plant cell attachment [159]. Meanwhile, the endo-nucleases VirD1 and VirD2 cut the T-DNA region out of the Ti plasmid to yield single stranded T-DNA (i.e. the T-strand). The VirD2 protein also stabilizes the T-strand by covalently binding to its 5′ end [151]. Following plant cell adhesion, 11 VirB proteins (VirB1-11) and VirD4 form a type IV secretion system (T4SS) that transports the VirD2/T-strand complex into the plant cell. In addition to the VirD2/T-strand complex, the VirD5, VirE2, VirE3, and VirF proteins are also shuttled into the plant cell [160]. These additional proteins are involved in downstream processes including T-DNA protection from cellular nucleases [161] and nuclear import [162].
Fig. 2.
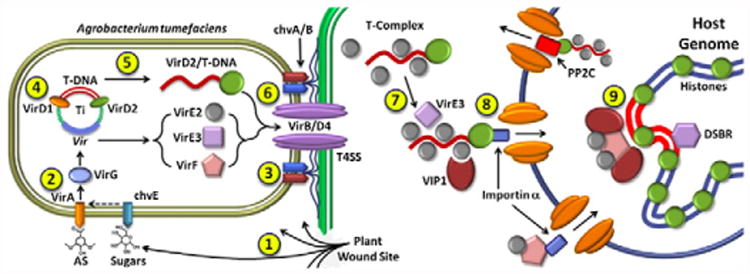
(1) A plant wound releases signal molecules which initiate the infection process by activating VirA and chvE. (2) VirA phosphorylates VirG, resulting in transcriptional activation of other Vir genes. (3) Chromosomal proteins (chv A/B) initiate plant cell attachment. (4) VirD1 and VirD2 prepare single stranded T-DNA through endonuclease activity. (5) VirD2 covalently binds T-DNA. (6) VirD2/T-DNA and other Vir proteins are transferred to the plant via the VirB/VirD4 T4SS complex. (7) VirD2/T-DNA is coated with VirE2, VirE3, and VIP1 to form the mature T-complex. (8) Importin α facilitates nuclear uptake of the T-complex and VirF. (9) The T-complex binds to the nucleosome and integrates into the genome. Alternatively, PP2C may bind to the T-complex for nuclear export.
3.2. Transformation events within the host cell
Even after the T-strand and Vir proteins are transported into the host cell, the formidable challenges of nuclear membrane transport and genomic integration still remain. Both bacterial and host proteins play key roles in these processes and understanding their interactions is an important step in potentially applying Agrobacterium-mediated gene transfer techniques to enhance gene delivery to mammalian hosts. A key bacterial protein in nuclear transport is VirE2, which coats the VirD2-T strand to form a “T-complex”. Both VirD2 and VirE2 have NLS tags which enhance nuclear transport in plant cells, but it has also been suggested that VirE2 changes the conformation of the T-strand to ease transport through the nuclear pore [163]. It is interesting to note that VirE2 alone is unable to facilitate nuclear transport of T-DNA in non-plant cells, suggesting that the NLS sequence of VirE2 is host-specific. However, point mutations in the VirE2 NLS or addition of VirD2 enables T-strand nuclear transport in Xenopus oocytes [164], Drosophila embryos [164], and HeLa cells [165]. Additionally, VirD2 has been shown to strongly interact with plant TATA box-binding protein (TBP) in Arabidopsis cells in vivo, suggesting a potential bacterially-encoded role in chromosomal targeting and/or T-DNA integration [166].
Another bacterial protein involved in transformation within the host cell is VirE3, which has been shown to interact with the host transcription factor pBrp to activate genes necessary for tumor formation in tobacco, sunflowers, and tomatoes [167]. VirE3 also has 2 NLS sequences and interacts with karyopherin α and VirE2 to enhance T-complex nuclear transport in plant cells [162]. In a similar fashion, the plant protein VIP1 (VirE2-interacting Protein 1) has been shown to interact with virD2/ssDNA complexes to facilitate nuclear import in mammalian and yeast cells [168]. Indeed, deliberate overexpression of VIP1 in plants strongly increases transformation by A. tumefaciens [169]. However, since VIP1 is not abundantly expressed in all plants, A. tumefaciens must still express VirE3 to complement VIP1 activity. In plants lacking VIP1 expression, VirE3 can actually rescue VirE2 nuclear import and T-DNA expression [162]. In contrast to VIP1 and VirE3, the plant protein phosphatase 2C (PP2C) encoded by DIG3 interacts with VirD2 and actually reduces its nuclear uptake [170], suggesting a potential mechanism by which a plant defends itself against infection.
Unfortunately, the mechanism of A. tumefaciens T-strand genomic integration is still poorly understood. However, Lacroix et al. showed that VIP1 can bind to core histones, allowing the T-complex to associate with plant nucleosomes (a precursor to genomic integration). Lacroix also showed that the bacterial protein VirF binds the T-complex after it associates nucleosome [171]. It has recently been shown that VirF contains an F-box motif which targets both VirE2 and VIP1 for protease degradation, thereby releasing the T-strand for subsequent genomic integration [172]. No bacterial proteins which integrate the T-strand into the genome have been identified, implying that T-strand integration may rely on host proteins. There is now strong evidence suggesting that the T-strand may randomly integrate into the genome at double stranded breaks (DSBs) with the help of host cell DNA repair enzymes involved in the homologous recombination (HR) and non-homologous end joining (NHEJ) pathways (reviewed in detail here [173]). It has been demonstrated in yeast that the plant proteins Yku70, Rad50, Mre11, Xrs2, Lig4 and Sir4, involved in the NHEJ pathway are involved in host genome T-DNA integration [174]. Proteins involved in HR that are required for T-DNA integration into yeast genome include Rad51 and Rad52 [174].
Histones and chromatin packaging may also influence T-strand integration. Indeed, overexpression of certain histones significantly enhances transient transgene expression [160]. It has also been shown that interactions between VIP1 and histone H2A are necessary for tumor formation, suggesting that H2A and other histones may play a role in T-strand integration [175]. Additionally, a high throughput screen of plant gene expression levels of tobacco BY-2 cells during A. tumefaciens infection revealed an increase during the late stage of infection in the expression of core histones (including H2A) around which genomic DNA is wound [176]. A virus-induced gene silencing approach in Nicotina benthamiana also implicated the importance of core histones in genomic integration during A. tumefaciens infection, as H3 silencing did not reduce transient transformation but did reduce T-DNA integration [177]. Other plant genes identified with variable expression levels during the latter stage of A. tumefaciens infection included those involved in cell cycle progression and growth [176]. Identifying these plant genes directly involved in A. tumefaciens infection and their interactions with bacterially-encoded genes is vital in obtaining a thorough understanding of the transformation process, which can lead to better design of plants recalcitrant to Agrobacterium infection (i.e. protection from crown gall tumor formation in crops) and provide insight into methods by which human gene therapy can be improved.
3.3. Examples of human cell transfection with A. tumefaciens
In addition to plants, A. tumefaciens is able to infect and transform a wide range of other eukaryotic hosts. In 1995, the first example of A. tumefaciens infection of a non-plant host was demonstrated with the budding yeast Saccharomyces cervisiae [178]. In the years since, A. tumefaciens transformation has also been achieved (with mixed success) in many other eukaryotic hosts. Cases of Agrobacterium infection and proliferation have been observed in human patients, suggesting that Agrobacterium may have some clinical potential as a gene delivery vector [179,180]. However, tail vein injections of A. tumefaciens in mice yielded no transgene expression, even though the bacteria remained viable [181].
On the other hand, some limited success with Agrobacterium mediated transformation of animal cells has been achieved in vitro. HeLa, HEK393, and PC12 cells were transformed by A. tumefaciens with a low efficiency (10–20 cells per million) [182]. Interestingly, mutations to the chromosomal chv proteins prevented attachment of A. tumefaciens to HeLa cells and independent mutations in the VirA, VirB, VirD, VirE, and VirG loci all resulted in complete loss of transformation [182]. Therefore, while the same proteins may be responsible Agrobacterium-mediated transformation of plant and animal cells, key differences in animal cells limit the efficiency of the process.
While the efficiency of Agrobacterium mediated transformation may be limited in mammalian hosts, it is interesting to note that some human pathogens possess similar machinery for host cell manipulation. For example, Helicobacter pylori [183] and Legionella pneumonia [184] both use a T4SS to inject effector proteins into human cells that enhance infection. It has also recently been shown that the mammalian parasite Bartonella henselae can transfer plasmid DNA into human endothelial cells in vitro. While a T4SS and a VirD2 analog (Mob) have been identified in B. henselae, no NLS containing proteins have been observed, suggesting that nuclear transport of B. henselae DNA relies on the breakdown of the nuclear envelope during mitosis [185]. These findings point to similarities in transfer and processing of T-DNA in B. henselae and A. tumefaciens, but their mechanisms between of nuclear import may be quite different. Elucidating the complete set of proteins involved in B. henselae transformation of human cells may prove valuable in developing new human gene therapy techniques.
4. Accidental HGT: T.cruzi
T. cruzi is an intracellular parasite which causes Chagas disease, a potentially fatal illness that causes heart disease in millions of people in Latin America [186–188]. Early treatment with benzidazole or nifurtimox can eradicate the parasite in 60–90% of patients [189], but some patients still suffer an autoimmune response that affects the heart after the parasite has been eliminated [190,191]. Teixeira et al. reported that this immune response is triggered by a HGT event between T. cruzi and human cells which induces the expression of cell surface antigens [192–194]. In addition, T.cruzi may also infect germ line cells and vertically transfer its genetic material to the offspring of the original patient [193,195].
T. cruzi has some unique characteristics (intracellular replication and special genetic elements) which enhance HGT. The intracellular stage of the T. cruzi life cycle (see Fig. 3) [196] significantly increases the exposure of the host nucleus to T. cruzi DNA. In fact, HGT appears to be relatively common between endosymbionts and their hosts. The most common examples are mitochondria and chloroplasts, which have successfully transferred many of their genes to the nuclei of various eukaryotes [197]. Buchnera aphidicola, a bacterial symbiont of aphids, has also transferred several of its own essential genes to the nuclei of specialized “bacteriocyte” cells in aphids [198]. In this curious case of obligate mutualism, B. aphidicola cannot replicate outside of bacteriocyte cells, while the aphid host relies on B. aphidicola to produce essential nutrients [199,200]. Aphids and other insects have also obtained large numbers of genes from the endosymbiotic bacteria Wolbachia pipientis. Like T. cruzi, this endosymbiont also infects reproductive cells, thereby allowing both horizontal and vertical transfer of its genetic material [201].
Fig. 3.
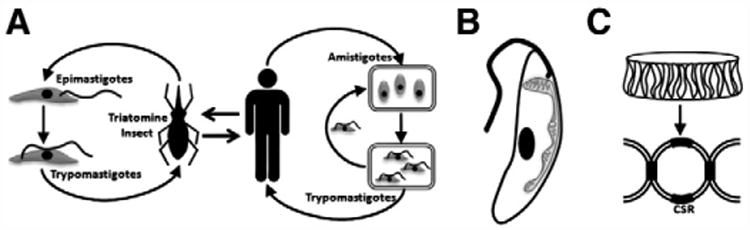
(A) Life cycle of T. cruzi. The parasite is transmitted by the triatomine insect via ingestion of the epimastigote form from infected human hosts. Epimastigotes mature to trypomastigotes in the insect gut and are excreted after the triatomine bites a human host. The trypomastigotes invade host cells and convert to a non-flagellar amastigote form for replication. The amastigotes then mature into trypomastigotes that infect other host cells or are ingested by the triatomine insect. (B) Anatomy of a T. cruzi trypomastigote. Aside from the nuclear DNA, T. cruzi cells also have a single mitochondrion which has a densely packed disk of plasmid DNA (kinetoplast) at the base of its flagellum. (C) The kinetoplast consists of large maxicircles and smaller minicircles that are concatenated at conserved sequence regions (CSRs).
While endosymbiosis definitely increases the probability of HGT, the preceding examples only occurred very gradually over millions of years. In contrast, multiple HGT events have been observed in up to 30% of patients with active T. cruzi infections [14]. This relatively high HGT frequency may be due to a unique feature of T. cruzi – minicircle DNA. Aside from its nuclear DNA, T. cruzi also has a dense network of concatenated plasmids within its mitochondrion called a kinetoplast (see Fig. 3). Like other mitochondria, the DNA in the kinetoplast contains essential genes for mitochondrial function (cytochrome oxidase, NADH dehydrogenase, etc.) on large plasmids called “maxicircles” (20–40 kb) [202–204]. However, the kinetoplast also contains approximately 15,000 small “minicircle” plasmids (∼1.5 kb) which contain genes for guide RNAs that edit maxicircle transcripts prior to translation [205–208]. Interestingly, the diameter of these minicircles (30 nm [209]) is close to the maximum diameter of the nuclear pore complex (9–40 nm [210,211]), potentially easing nuclear transport of T. cruzi minicircles into the nucleus for HGT [212].
It is important to mention that there are other parasites with kinetoplasts (order Kinetoplastida) that have minicircles, yet they have not been observed to cause HGT. The crucial difference between T. cruzi and other kinetoplastids lies in its minicircle DNA sequence. All minicircles contain variable regions with species-specific “guide RNA” genes and conserved sequence regions (CSRs) consisting of three conserved sequence blocks (CSBs) that help form the dense kinetoplast network by providing sites for concatenation [213–215]. CSR sequences from many different species are highly similar (see [215] for a detailed comparison), but each species differs in the number of CSBs per minicircle. For example, the minicircles of Leishmania tarentolae only have 1 CSR [213], while T. cruzi minicircles have four CSRs that are evenly distributed within each minicircle [216]. This relatively high number of CSRs in T. cruzi may significantly increase the probability of HGT [217], since the minicircle CSB sequences bear some similarity to the Long Interspersed Nuclear Element-1 (LINE-1) retrotransposon, which is ubiquitous in the human genome [218]. Indeed, genomic analysis of patients with Chagas disease reveals that T. cruzi minicircle sequences are frequently inserted into LINE-1 sequences throughout each chromosome [14,217].
In summary, the high frequency of HGT events associated with T. cruzi is probably due to three unique traits. First of all, its intracellular nature directly exposes the host cell nuclei to large amounts of DNA from T. cruzi. The small size of T. cruzi minicircles eases their entry into nuclear pores, while the sequence of CSBs may allow the minicircle genes to permanently integrate into the host genome. It is still unclear how these HGT events may cause heart disease in patients without active T. cruzi infections and further work will need to be done to determine if any T. cruzi genes are actively expressed after integration into the host genome. Future work should also focus on the effects of minicircle DNA integration into LINE-1 retrotransposons, which are known to disrupt genes [219–221] and have been associated with certain diseases [222,223].
4.1. T. cruzi as a delivery vector
The abilities of T.cruzi to evade the immune system, invade cells, and transfer genetic material to the host genome make it an attractive system for drug or gene delivery, following appropriate engineering. Techniques have been developed to transfect T. cruzi [224,225] and one patent even describes the use of genetically modified T. cruzi as vectors for gene delivery [226]. This technology has not yet been tested in vivo, but there have been intriguing studies on minicircle DNA plasmids and transposons for gene delivery.
4.2. Synthetic minicircle DNA plasmids
Traditionally, cationic polymers or lipids are mixed with bacterial plasmids to prepare polyplexes for non-viral gene delivery [227]. The size of these polyplex can have a significant effect on transfection efficiency [228], since polyplex size can influence polyplex uptake [229] and nuclear transport [212]. No gene delivery studies have been performed with T. cruzi minicircle plasmids to our knowledge, but Kay et al. developed a bacterial system which produces synthetic minicircle plasmids for transfection [230]. In this system, parental plasmids are split into two daughter plasmids after arabinose induction of a ϕ31 integrase gene in a genetically modified Escherichia coli strain. Arabinose also induces expression of an I-SceI restriction endonuclease that degrades the bacterial backbone plasmid. The remaining minicircle plasmid, which contains an expression cassette for the gene of interest, may then be purified using conventional plasmid isolation techniques [230]. Polyplexes made with these synthetic minicircles have been used to transfect a variety of cell lines and found to increase target gene expression upto560-fold higher than polyplexes made with traditional plasmids [231]. Minicircle plasmids have also been shown to maintain stable target gene expression three times longer than conventional plasmids [232].
Aside from their small size, minicircle plasmids have other advantages over traditional plasmids. Minicircle polyplexes are more resistant to the shear stresses which occur during polyplex formation and transfection [233]. Minicircles also lack bacterial DNA elements (e.g. origins of replication and antibiotic resistance genes) which have been previously observed to silence expression of target genes [234]. These bacterial DNA elements may contain unmethylated CpG motifs, which activate an innate immune responses that interfere with gene delivery in vivo and silence transgene expression [235,236]. Finally, since minicircle plasmids lack bulky bacterial elements, they require considerably less cationic polymer or lipid per plasmid for polyplex formation, making minicircle polyplexes much less toxic than other polyplexes [237]. One interesting alternative to minicircle plasmids is plasmids which use other genes for selection instead of antibiotic resistance markers, including genes that confer resistance totoxins or complement bacterial growth. While these plasmids are still much larger than minicircle plasmids, the absence or antibiotic resistance genes prevent the transfer of antibiotic resistance to bacteria living within the patient and some potential sequence-specific host responses to the foreign DNA [238].
4.3. Transposon-mediated gene delivery and expression
Transposons similar to the CSBs in T. cruzi minicircles have also been used to enhance and maintain target gene expression in vitro. The most efficient transposons used to date include the Sleeping Beauty (SB) [239], Tol2 [240], and PiggyBac (PB) transposons [241]. The SB transposon has been successfully used to integrate a nitric oxide synthase gene into rat lung cells to prevent pulmonary hypertension [242]. A clinical trial is also underway which uses T cells that have been permanently transformed with the SB transposon to treat lymphoid malignancies [243]. In addition, the PB transposon has been used to create induced pluripotent stem cells by genetically reprogramming fibroblasts [244–246]. The CSBs of T. cruzi minicircles may be an interesting new type of transposon-like elements, but further work will need to be done to determine how CSBs promote integration into the host genome and detect any side effects associated with such integration.
5. Intercellular nucleic acid (RNA/DNA) transfer between eukaryotic cells
In addition to parasitic or viral gene transfer, several examples of nucleic acid transfer between eukaryotic cells of the same organism have also been reported (thoroughly reviewed in [247]). For example, intercellular transfer of short interfering RNAs (siRNAs) was first observed in the propagation of systemic RNA interference. In plants, siRNA transfer occurs mostly through channels in the cell wall called plasmodesmata that allow the transport of proteins, RNA, and other small molecules between neighboring plant cells [248,249]. Interestingly, extracellular RNA transport may be facilitated by the Phloem Small RNA-binding Protein (PSRP1), which selectively binds small (∼25 nucleotide) single stranded RNAs [250].
Even though mammalian cells lack plasmodesmata, systemic RNAi propagation has also been observed in animals. In C. elegans, siRNA transport has been shown to rely on the transmembrane proteins SID– 1 and SID–2, which bind extracellular siRNAs and facilitate their uptake [251,252]. Another avenue for cell-to-cell RNA transfer is tunneling nanotubes (TNT's), which are fragile extensions of the plasma membrane that physically connect neighboring cells [253,254]. TNT diameters may be as large as 700 nm and they have been implicated in intercellular transport of HIV-1 particles [255], endosomes, and organelles [254,256]. However, they seem to restrict the passage of small molecules, suggesting that nucleic acid transport must be facilitated by endosomes or some type of carrier molecule [247].
Recent evidence has shown that RNAs may also be transported by exosomes or microvesicles. Exosomes are small membrane-bound vesicles (40–100 nm) that are formed inside endosomes during endosomal maturation and recycling, while microvesicles are much larger (50–1000 nm) and are formed by budding of the plasma membrane. Isolated exosomes and microvesicles have been shown to contain up to 1300 different mRNA molecules which are functionally active [257]. For example, vesicles secreted by embryonic stem cells have been shown to deliver mRNAs to hematopoetic progenitor cells and affect their gene expression profiles [258]. Vesicles containing small RNAs have been found in a wide variety of bodily fluids, including breast milk, suggesting a possible mechanism of genetic transfer between parent and offspring [259,260].
While the majority of intercellular nucleic acid transport deals with RNAs, there have been a few observations of intercellular DNA transport as well. For example, transfer of both nuclear and plastid DNA has been observed at graft junctions in plants [261]. Exosomes secreted from cardiomyocytes have also been found to contain DNA and these “cardiosomes” have even been used to transform fibroblasts [262]. Astrocytes and glioblastoma cells have also been shown to secrete exosomes containing mitochondrial DNA [263]. Aside from exosomes, DNA within apoptotic bodies can also transform cells [264]. Finally, a human antimicrobial peptide (LL-37) is capable of binding extracellular DNA, protecting it from serum nucleases, and facilitating transport across both the cellular and nuclear membranes [265].
Of all the examples discussed in this section, exosomes and microvesicles have shown the highest potential for clinical gene therapy [266]. Electroporation can be used to insert siRNA into exosomes isolated from human blood to produce RNAi delivery vehicles that have successfully silenced MAPK-1 expression in monocytes in vitro [267]. Exosomes have also been labeled with membrane proteins (Lamp2b [268] and rabies virus glycoprotein peptide [269]) to target specific regions in the brain (e.g. neurons, microglia, and oligodendrocytes). Human exosomes from liver stem cells were even shown to transform rat hepatocytes and accelerate hepatic regeneration following partial hepadectomy, suggesting that exosomes are able to cross species barriers for RNA delivery [270]. In addition, microvesicles from endothelial progenitor cells have been shown to promote angiogenesis following pancreas transplantation [271] or ischemia-reperfusion injury in the kidney [272]. Likewise, microvesicles secreted from mesenchymal or liver stem cells have been shown to alleviate the effects of acute and chronic kidney injuries [273,274], peripheral arterial disease [275], myocardial ischemia [276], and myocardial infarction [277]. Altogether, these examples clearly demonstrate the significant role of exosomes and microvesicles in intercellular signaling and suggest that they may be promising vehicles for RNA (and potentially DNA) delivery in vivo.
6. Conclusions
Effective gene delivery techniques could potentially cure hundreds of genetic diseases (e.g. cancer, hemophilia, etc.), but current non-viral gene delivery suffers from low efficiency. However, applying lessons from pathogens that employ horizontal gene transfer has dramatically improved non-viral gene delivery. Viral proteins and gene sequences have been used to improve gene delivery to both the cytoplasm and nucleus. A. tumefaciens has revolutionized the field of plant biotechnology and B. henselae may have a similar impact on human gene therapy. HGT by T. cruzi is still poorly understood, but further investigation of this intracellular parasite will hopefully yield new treatments for patients with Chagas Disease and perhaps new methods to enhance gene delivery. All of these examples clearly show how pathogenic traits that originally caused diseases can be used to cure genetic diseases by significantly enhancing non-viral gene delivery techniques. Exosomes and microvesicles are also exciting new delivery vehicles for RNA and DNA, while new viruses or pathogens with unique HGT techniques may also be awaiting discovery. Therefore, elucidating and applying HGT phenomena to the development of novel non-viral gene delivery techniques may eventually yield safe and effective methods to cure many different genetic diseases.
Acknowledgments
We are grateful to the NIH/NIGMS (Grant 1R01GM093229-01A1) for financial support of this study.
Contributor Information
Jacob J. Elmer, Email: jakelmer@gmail.com.
Kaushal Rege, Email: krege@asu.edu.
References
- 1.Ochiai K, Yamanaka T, Kimura K, Sawada O. Inheritance of Drug Resistance (and its Transfer) Between Shigella Strains and Between Shigella and E. Coli Strains (in Japanese) Hihon Iji Shimpor. 1959;1861 [Google Scholar]
- 2.Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki W, Takagi T. Rapid pathway evolution facilitated by horizontal gene transfers across prokaryotic lineages. PLoS Genet. 2009;5:e1000402. doi: 10.1371/journal.pgen.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The MJ. Human insulin: DNA technology's first drug. Am J Hosp Pharm. 1989;46:S9–S11. [PubMed] [Google Scholar]
- 5.Laffly E, Sodoyer R. Monoclonal and recombinant antibodies, 30 years after. Hum Antibodies. 2005;14:33–55. [PubMed] [Google Scholar]
- 6.Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322:1–15. doi: 10.1016/j.gene.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Moran NA, Jarvik T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 2010;328:624–627. doi: 10.1126/science.1187113. [DOI] [PubMed] [Google Scholar]
- 8.Altincicek B, Kovacs JL, Gerardo NM. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol Lett. 2012;8:253–257. doi: 10.1098/rsbl.2011.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acuña R, Padilla BE, Flórez-Ramos CP, Rubio JD, Herrera JC, Benavides P, et al. Adaptive horizontal transfer of a bacterial gene to an invasive insect pest of coffee. Proc Natl Acad Sci U S A. 2012;109:4197–4202. doi: 10.1073/pnas.1121190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rumpho ME, Worful JM, Lee J, Kannan K, Tyler MS, Bhattacharya D, et al. Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc Natl Acad Sci U S A. 2008;105:17867–17871. doi: 10.1073/pnas.0804968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monier A, Pagarete A, de Vargas C, Allen MJ, Read B, Claverie JM, et al. Horizontal gene transfer of an entire metabolic pathway between a eukaryotic alga and its DNA virus. Genome Res. 2009;19:1441–1449. doi: 10.1101/gr.091686.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H. Phage as agents of lateral gene transfer. Curr Opin Microbiol. 2003;6:417–424. doi: 10.1016/s1369-5274(03)00086-9. [DOI] [PubMed] [Google Scholar]
- 13.de la Riva GA, González-Cabrera J, Vázquez-Padrón R, Ayra-Pardo C. Agrobacterium tumefaciens: a natural tool for plant transformation. Electron J Biotechnol. 1998;1:1–16. [Google Scholar]
- 14.Hecht MM, Nitz N, Araujo PF, Sousa AO, Rosa A de C, Gomes DA, et al. Inheritance of DNA transferred from American trypanosomes to human hosts. PLoS One. 2010;5:e9181. doi: 10.1371/journal.pone.0009181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas CE, Ehrhardt A, Kay Ma. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 16.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavazzana-Calvo M. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 18.Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci U S A. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 20.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 21.Thomas CE, Schiedner G, Kochanek S, Castro MG, Löwenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic. Proc Natl Acad Sci U S A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas W. Viral Capsids and Envelopes: Structure and Function. eLS; Chichester, UK: 2010. [Google Scholar]
- 23.Lodish H, Berk A, Zipursky S, Al E, editors. Viruses: Structure, Function, and Uses. 4th. W. H. Freeman; New York: 2000. [Google Scholar]
- 24.von Köckritz-Blickwede M, Chow Oa, Nizet V. Fetal calf serum contaINS heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malonga H, Neault JF, Arakawa H, Tajmir-Riahi Ha. DNA interaction with human serum albumin studied by affinity capillary electrophoresis and FTIR spectroscopy. DNA Cell Biol. 2006;25:63–68. doi: 10.1089/dna.2006.25.63. [DOI] [PubMed] [Google Scholar]
- 26.Geng YAN, Dalhaimer P, Cai S, Tsai R, Minko T, Discher DE. Shape effects of filamentous versus spherical particles in flow and drug delivery. 2009;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gahéry-Ségard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JS, Ramanathan MP, Muthumani K, Choo AY, Jin SH, Yu QC, et al. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg Infect Dis. 2002;8:1379–1384. doi: 10.3201/eid0812.020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas M, Klibanov aM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 31.de Ilarduya CT, Sun Y, Duzgunes N. Nonviral gene delivery review2.pdf. Eur J Pharm Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Richardson S. Potential of low molecular mass chitosan as a DNA delivery system: biocompatibility, body distribution and ability to complex and protect DNA. Int J Pharm. 1999;178:231–243. doi: 10.1016/s0378-5173(98)00378-0. [DOI] [PubMed] [Google Scholar]
- 33.Murphy JE, Uno T, Hamer JD, Cohen FE, Dwarki V, Zuckermann RN. A combinatorial approach to the discovery of efficient cationic peptoid reagents for gene delivery. Proc Natl Acad Sci U S A. 1998;95:1517–1522. doi: 10.1073/pnas.95.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey JD, Vu HN, Pack DW. A top–down approach for construction of hybrid polymer–virus gene delivery vectors. J Control Release. 2010;144:39–45. doi: 10.1016/j.jconrel.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Kasman LM, Barua S, Lu P, Rege K, Voelkel-Johnson C. Polymer-enhanced adenoviral transduction of CAR-negative bladder cancer cells. Mol Pharmacol. 2009;6:1612–1619. doi: 10.1021/mp9000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim Y, Lee E, Yoon YR, Lee MS, Lee M. Filamentous artificial virus from a self-assembled discrete nanoribbon. Angew Chem. 2008;120:4601–4604. doi: 10.1002/anie.200800266. [DOI] [PubMed] [Google Scholar]
- 37.Malay AD, Heddle JG, Tomita S, Iwasaki K, Miyazaki N, Sumitomo K, et al. Gold nanoparticle-induced formation of artificial protein capsids. Nano Lett. 2012;12:2056–2059. doi: 10.1021/nl3002155. [DOI] [PubMed] [Google Scholar]
- 38.Kichler A, Pages J, Druillennec S, Lenoir C, Delain E, Le Cam E, et al. Efficient DNA Transfection Mediated by the C-Terminal Domain of Human Immunodeficiency Virus Type 1 Viral Protein R Efficient DNA Transfection Mediated by the C-Terminal Domain of Human Immunodeficiency Virus Type 1 Viral Protein R. 2000 doi: 10.1128/jvi.74.12.5424-5431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller K, Nahde T, Fahr a, Müller R, Brüsselbach S. Highly efficient transduction of endothelial cells by targeted artificial virus-like particles. Cancer Gene Ther. 2001;8:107–117. doi: 10.1038/sj.cgt.7700280. [DOI] [PubMed] [Google Scholar]
- 40.Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid–polyethyleneimine conjugates for systemic gene delivery. J Control Release. 2009;133:132–138. doi: 10.1016/j.jconrel.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y, Akita H, Harashima H. Multifunctional Envelope-Type Nano Device (MEND) for Organelle Targeting via a Stepwise Membrane Fusion Process. 1st. Elsevier Inc.; 2012. [DOI] [PubMed] [Google Scholar]
- 42.Akita H, Kudo A, Minoura A, Yamaguti M, Khalil Ia, Moriguchi R, et al. Multilayered nanoparticles for penetrating the endosome and nuclear membrane via a step-wise membrane fusion process. Biomaterials. 2009;30:2940–2949. doi: 10.1016/j.biomaterials.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Bergelson JM. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 44.Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. In & grins aJSQ and U & promote adenovirus internalization but not virus attachment. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 46.Ehrhardt C, Marjuki H, Wolff T, Nürnberg B, Planz O, Pleschka S, et al. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol. 2006;8:1336–1348. doi: 10.1111/j.1462-5822.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 47.Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- 48.Kunzelmann K, Beesley aH, King NJ, Karupiah J, Young Ja, Cook DI. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci U S A. 2000;97:10282–10287. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eierhoff T, Hrincius ER, Rescher U, Ludwig S, Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C βII in influenza virus entry via late endosomes. J Virol. 2003;77:460–469. doi: 10.1128/JVI.77.1.460-469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park J, Singha K, Son S, Kim J, Namgung R, Yun CO, et al. A review of RGD-functionalized nonviral gene delivery vectors for cancer therapy. Cancer Gene Ther. 2012;19:741–748. doi: 10.1038/cgt.2012.64. [DOI] [PubMed] [Google Scholar]
- 52.Kunath K, Merdan T, Hegener O, Häberlein H, Kissel T. Integrin targeting using RGD–PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5:588–599. doi: 10.1002/jgm.382. [DOI] [PubMed] [Google Scholar]
- 53.Juliano RL, Ming X, Nakagawa O, Xu R, Yoo H. Integrin targeted delivery of gene therapeutics. Theranostics. 2011;1:211–219. doi: 10.7150/thno/v01p0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho KC, Kim SH, Jeong JH, Park TG. Folate receptor-mediated gene delivery using folate–poly(ethylene glycol)–poly(L-lysine) conjugate. Macromol Biosci. 2005;5:512–519. doi: 10.1002/mabi.200500018. [DOI] [PubMed] [Google Scholar]
- 55.Kircheis R, Blessing T, Brunner S, Wightman L, Wagner E. Tumor targeting with surface-shielded ligand–polycation DNA complexes. J Control Release. 2001;72:165–170. doi: 10.1016/s0168-3659(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Tan Y, Viroonchatapan E, Pitt BR, Huang L, Pitt R. Targeted Gene Delivery to Pulmonary Endothelium by Anti-PECAM Antibody. 2013 doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Stuckert P, Bosch I, Marks JD, Marasco Wa. Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther. 2001;8:555–565. doi: 10.1038/sj.cgt.7700337. [DOI] [PubMed] [Google Scholar]
- 58.Wagner E, Curiel D, Cotten M. Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv Drug Deliv Rev. 1994;14:113–135. [Google Scholar]
- 59.Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 60.Sebbage V. Review cell-penetrating peptides and their therapeutic applications. Biosci Horiz. 2009;2:64–72. [Google Scholar]
- 61.Kerkis A, Hayashi MaF, Yamane T, Kerkis I. Properties of cell penetrating peptides (CPPs) IUBMB Life. 2006;58:7–13. doi: 10.1080/15216540500494508. [DOI] [PubMed] [Google Scholar]
- 62.Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Biology M, Baltimore M. Cellular uptake of the tat protein from human immunodeficiency virus. Dis Markers. 1988;8:33–34. [PubMed] [Google Scholar]
- 64.Handbook of Cell-Penetrating Peptides. Second. CRC Press; 2010. Google eBook. [Google Scholar]
- 65.Oehlke J, Scheller A, Wiesner B, Krause E, Beyermann M, Klaushenz E, et al. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim Biophys Acta Biomembr. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 66.Nakase I, Hirose H, Tanaka G, Tadokoro A, Kobayashi S, Takeuchi T, et al. Cell-surface accumulation of flock house virus-derived peptide leads to efficient internalization via macropinocytosis. Mol Ther. 2009;17:1868–1876. doi: 10.1038/mt.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi X, Droste T, Kao CC. Cell-penetrating peptides derived from viral capsid proteins. Mol Plant Microbe Interact. 2011;24:25–36. doi: 10.1094/MPMI-07-10-0147. [DOI] [PubMed] [Google Scholar]
- 68.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 69.Derossi D, Joliot aH, Chassaing G, Prochiantz a. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 70.Pooga M, Hällbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi S, Takeshima K, Park CB, Kim SC, Matsuzaki K. Interactions of the novel antimicrobial peptide buforin 2 with lipid bilayers: proline as a translocation promoting factor. Biochemistry. 2000;39:8648–8654. doi: 10.1021/bi0004549. [DOI] [PubMed] [Google Scholar]
- 72.Elmquist A, Lindgren M, Bartfai T, Langel U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp Cell Res. 2001;269:237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- 73.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Lundberg P, El-Andaloussi S, Sütlü T, Johansson H, Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21:2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 75.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nitin N, LaConte L, Rhee WJ, Bao G. Tat peptide is capable of importing large nanoparticles across nuclear membrane in digitonin permeabilized cells. Ann Biomed Eng. 2009;37:2018–2027. doi: 10.1007/s10439-009-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suk JS, Suh J, Choy K, Lai SK, Fu J, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27:5143–5150. doi: 10.1016/j.biomaterials.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahmat D, Khan MI, Shahnaz G, Sakloetsakun D, Perera G, Bernkop-Schnürch A. Synergistic effects of conjugating cell penetrating peptides and thiomers on non-viral transfection efficiency. Biomaterials. 2012;33:2321–2326. doi: 10.1016/j.biomaterials.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 79.Kleemann E, Neu M, Jekel N, Fink L, Schmehl T, Gessler T, et al. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG–PEI. J Control Release. 2005;109:299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 80.Lai Z, Han I, Zirzow G, Brady RO, Reiser J. Intercellular delivery of a herpes simplex virus VP22 fusion protein from cells infected with lentiviral vectors. Proc Natl Acad Sci U S A. 2000;97:11297–11302. doi: 10.1073/pnas.97.21.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suzuki K, Murtuza B, Brand NJ, Varela-Carver A, Fukushima S, Yacoub MH. Enhanced effect of myocardial gene transfection by VP22-mediated intercellular protein transport. J Mol Cell Cardiol. 2004;36:603–606. doi: 10.1016/j.yjmcc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Phelan A, Elliott G, O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 83.Wills KN, Atencio IA, Avanzini JB, Neuteboom S, Phelan A, Philopena J, et al. Intratumoral spread and increased efficacy of a p53-VP22 fusion protein expressed by a recombinant adenovirus. J Virol. 2001;75:8733–8741. doi: 10.1128/JVI.75.18.8733-8741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 85.Helenius a, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lear JD, DeGrado WF. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987;262:6500–6505. [PubMed] [Google Scholar]
- 87.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994;269:12918–12924. [PubMed] [Google Scholar]
- 88.Plank C, Zauner W, Wagner E. Application of membrane-active peptides for drug and gene delivery across cellular membranes. Adv Drug Deliv Rev. 1998;34:21–35. doi: 10.1016/s0169-409x(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 89.Freed EO, Myers DJ, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990;87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2N-terminal fusogenic peptides augment gene transfer by transferrin– polylysine–DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A. 1992;89:7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kichler A, Mechtler K, Behr JP, Wagner E. Influence of membrane-active peptides on lipospermine/DNA complex mediated gene transfer. Bioconjug Chem. 1997;8:213–221. doi: 10.1021/bc970009z. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Collins L, Fabre JW. A powerful cooperative interaction between a fusogenic peptide and lipofectamine for the enhancement of receptor-targeted, non-viral gene delivery via integrin receptors. J Gene Med. 2001;3:560–568. doi: 10.1002/jgm.224. [DOI] [PubMed] [Google Scholar]
- 93.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem. 2008;19:920–927. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 94.Kim J. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J Gene Med. 2008;10:646–654. doi: 10.1002/jgm.1184. [DOI] [PubMed] [Google Scholar]
- 95.Li W, Nicol F, Szoka FC. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 96.Sasaki K, Kogure K, Chaki S, Nakamura Y, Moriguchi R, Hamada H, et al. An artificial virus-like nano carrier system: enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Anal Bioanal Chem. 2008;391:2717–2727. doi: 10.1007/s00216-008-2012-1. [DOI] [PubMed] [Google Scholar]
- 97.Boussif O, Lezoualc'h F, Zanta Ma, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou J, Wu J, Hafdi N, Behr JP, Erbacher P, Peng L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem Commun (Camb) 2006:2362–2364. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]
- 99.Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29:2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 100.Wagstaff KM, Jans Da. Nucleocytoplasmic transport of DNA: enhancing non-viral gene transfer. Biochem J. 2007;406:185–202. doi: 10.1042/BJ20070505. [DOI] [PubMed] [Google Scholar]
- 101.Lechardeur D, Verkman aS, Lukacs GL. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv Drug Deliv Rev. 2005;57:755–767. doi: 10.1016/j.addr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- 104.Dean Da, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Exp Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Efthymiadis a, Briggs LJ, Jans Da. The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties. J Biol Chem. 1998;273:1623–1628. doi: 10.1074/jbc.273.3.1623. [DOI] [PubMed] [Google Scholar]
- 106.Cros JF, Palese P. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 2003;95:3–12. doi: 10.1016/s0168-1702(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 107.Chook Y, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–715. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 108.Jones NC, Rigby PW, Ziff EB. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988;2:267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- 109.Dean Da. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 110.Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F, et al. Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Ther. 2001;8:494–497. doi: 10.1038/sj.gt.3301419. [DOI] [PubMed] [Google Scholar]
- 111.Ritter W, Plank C, Lausier J, Rudolph C, Zink D, Reinhardt D, et al. A novel transfecting peptide comprising a tetrameric nuclear localization sequence. J Mol Med. 2003;81:708–717. doi: 10.1007/s00109-003-0483-2. [DOI] [PubMed] [Google Scholar]
- 112.Chan CK, Jans DA. Enhancement of polylysine-mediated transferrinfection by nuclear localization sequences: polylysine does not function as a nuclear localization sequence. Hum Gene Ther. 1999;10:1695–1702. doi: 10.1089/10430349950017699. [DOI] [PubMed] [Google Scholar]
- 113.Chan CK, Jans Da. Enhancement of MSH receptor- and GAL4-mediated gene transfer by switching the nuclear import pathway. Gene Ther. 2001;8:166–171. doi: 10.1038/sj.gt.3301366. [DOI] [PubMed] [Google Scholar]
- 114.Carlisle RC, Bettinger T, Ogris M, Hale S, Mautner V, Seymour LW. Adenovirus hexon protein enhances nuclear delivery and increases transgene expression of polyethylenimine/plasmid DNA vectors. Mol Ther. 2001;4:473–483. doi: 10.1006/mthe.2001.0472. [DOI] [PubMed] [Google Scholar]
- 115.Kotin RM, Menninger JC, Ward DC, Berns KI. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 116.Miller DG, Petek LM, Russell DW. Adeno-associated virus vectors integrate at chromosome breakage sites. Nat Genet. 2004;36:767–773. doi: 10.1038/ng1380. [DOI] [PubMed] [Google Scholar]
- 117.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 118.Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, et al. Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3:848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- 119.Gersbach Ca, Gaj T, Gordley RM, Mercer AC, Barbas CF. Targeted plasmid integration into the human genome by an engineered zinc-finger recombinase. Nucleic Acids Res. 2011;39:7868–7878. doi: 10.1093/nar/gkr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirchmaier aL, Sugden B. Rep*: a viral element that can partially replace the origin of plasmid DNA synthesis of Epstein–Barr virus. J Virol. 1998;72:4657–4666. doi: 10.1128/jvi.72.6.4657-4666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jankelevich S, Kolman JL, Bodnar JW, Miller G. A nuclear matrix attachment region organizes the Epstein–Barr viral plasmid in Raji cells into a single DNA domain. EMBO J. 1992;11:1165–1176. doi: 10.1002/j.1460-2075.1992.tb05157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sugden B, Warren N. A promoter of Epstein–Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989;63:2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tu G, Kirchmaier aL, Liggitt D, Liu Y, Liu S, Yu WH, et al. Non-replicating Epstein–Barr virus-based plasmids extend gene expression and can improve gene therapy in vivo. J Biol Chem. 2000;275:30408–30416. doi: 10.1074/jbc.M004782200. [DOI] [PubMed] [Google Scholar]
- 124.Milanesi G, Barbanti-Brodano G, Negrini M, Lee D, Corallini a, Caputo a, et al. BK virus–plasmid expression vector that persists episomally in human cells and shuttles into Escherichia coli. Mol Cell Biol. 1984;4:1551–1560. doi: 10.1128/mcb.4.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miron S, Mark J. Efficient episomal expression vector for human carcinoma cells transitional. 1993;566:557–566. doi: 10.1089/hum.1993.4.5-557. [DOI] [PubMed] [Google Scholar]
- 126.Byrne BJ, Davis MS, Yamaguchi J, Bergsma DJ, Subramanian KN. Definition of the simian virus 40 early promoter region and demonstration of a host range bias in the enhancement effect of the simian virus 40 72-base pair repeat. Proc Natl Acad Sci U S A. 1983;80:721–725. doi: 10.1073/pnas.80.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 128.Yamamoto T, De Crombrugghe B. Identification of a functional promoter in the long terminal repeat of rous sarcoma virus. Cell. 1980;22:787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]
- 129.Zarrin aa, Malkin L, Fong I, Luk FD, Ghose a, Berinstein NL. Comparison of CMV, RSV, SV40 viral and Vlambda1 cellular promoters in B and T lymphoid and non-lymphoid cell lines. Biochim Biophys Acta. 1999;1446:135–139. doi: 10.1016/s0167-4781(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 130.Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol Ther. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Harms J, Splitter GA. Interferon-gamma inhibits transgene expression driven by SV40 and CMV promoters but augments expression driven by the mammalian MHCI promoter. Hum Gene Ther. 1995;6:1291–1297. doi: 10.1089/hum.1995.6.10-1291. [DOI] [PubMed] [Google Scholar]
- 132.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 133.Gribaudo G, Ravaglia S, Caliendo A, Cavallo R, Gariglio M, Martinotti MG, et al. Interferons inhibit onset of murine cytomegalovirus immediate-early gene transcription. Virology. 1993;197:303–311. doi: 10.1006/viro.1993.1591. [DOI] [PubMed] [Google Scholar]
- 134.Xu ZL, Mizuguchi H, Ishii-Watabe a, Uchida E, Mayumi T, Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272:149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
- 135.Xu ZL, Mizuguchi H, Mayumi T, Hayakawa T. Woodchuck hepatitis virus post-transcriptional regulation element enhances transgene expression from adenovirus vectors. Biochim Biophys Acta, Gen Subj. 2003;1621:266–271. doi: 10.1016/s0304-4165(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 136.Smith E. Crown gall of plants. Phytopathology. 1911;1:7–11. [Google Scholar]
- 137.Lippincott J, Lippincott B. Genus Agrobacterium and plant tumorigenesis. Annu Rev Microbiol. 1975;29:377–405. doi: 10.1146/annurev.mi.29.100175.002113. [DOI] [PubMed] [Google Scholar]
- 138.Burr T, Bazzi C, Sule S, Otten L. Crown gall of grape – biology of Agrobacterium vitis and the development of disease control strategies. Plant Dis. 1998;82:1288–1297. doi: 10.1094/PDIS.1998.82.12.1288. [DOI] [PubMed] [Google Scholar]
- 139.White P, Braun A. A cancerous neoplasm of plants – autonomous bacteria-free crown-gall tissue. Cancer Res. 1942;2:597–617. [Google Scholar]
- 140.Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, et al. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974;252:169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- 141.Păcurar DI, Thordal-Christensen H, Păcurar ML, Pamfil D, Botez C, Bellini C. Agrobacterium tumefaciens: from crown gall tumors to genetic transformation. Physiol Mol Plant Pathol. 2011;76:76–81. [Google Scholar]
- 142.Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T. High efficiency transformation of maize (Zea mays L) mediated by Agrobacterium tumefaciens. Nat Biotechnol. 1996;14:745–750. doi: 10.1038/nbt0696-745. [DOI] [PubMed] [Google Scholar]
- 143.Zhao Z, Gu W, Cai T, Tagliani L, Hondred D, Bond D, et al. High throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol Breed. 2002;8:323–333. [Google Scholar]
- 144.Zale JM, Agarwal S, Loar S, Steber CM. Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep. 2009;28:903–913. doi: 10.1007/s00299-009-0696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu HK, Yang C, Wei ZM. Efficient Agrobacterium tumefaciens-mediated transformation of soybeans using an embryonic tip regeneration system. Planta. 2004;219:1042–1049. doi: 10.1007/s00425-004-1310-x. [DOI] [PubMed] [Google Scholar]
- 146.Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 147.Xiao B, Huang Y, Tang N, Xiong L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet. 2007;115:35–46. doi: 10.1007/s00122-007-0538-9. [DOI] [PubMed] [Google Scholar]
- 148.Yeo ET, Kwon HB, Han SE, Lee JT, Ryu JC, Byu MO. Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol Cells. 2000;10:263–268. [PubMed] [Google Scholar]
- 149.Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E. Molecular basis for the herbicide resistance of roundup ready crops. Proc Natl Acad Sci U S A. 2006;103:13010–13015. doi: 10.1073/pnas.0603638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manickavasagam M, Ganapathi A, Anbazhagan VR, Sudhakar B, Selvaraj N, Vasudevan A, et al. Agrobacterium-mediated genetic transformation and development of herbicide-resistant sugarcane (Saccharum species hybrids) using axillary buds. Plant Cell Rep. 2004;23:134–143. doi: 10.1007/s00299-004-0794-y. [DOI] [PubMed] [Google Scholar]
- 151.Gelvin SB, VanAlfen N, Bruening G, L J. Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol. 2010;48:45–68. doi: 10.1146/annurev-phyto-080508-081852. [DOI] [PubMed] [Google Scholar]
- 152.Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67:16–37. doi: 10.1128/MMBR.67.1.16-37.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gelvin SB. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:223–256. doi: 10.1146/annurev.arplant.51.1.223. [DOI] [PubMed] [Google Scholar]
- 154.McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- 155.Pitzschke A, Hirt H. New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 2010;29:1021–1032. doi: 10.1038/emboj.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palmer AG, Gao R, Maresh J, Erbil WK, Lynn DG. Chemical biology of multi-host/pathogen interactions: chemical perception and metabolic complementation. Annu Rev Phytopathol. 2004;42:439–464. doi: 10.1146/annurev.phyto.41.052002.095701. [DOI] [PubMed] [Google Scholar]
- 157.Wise AA, Voinov L, Binns AN. Intersubunit complementation of sugar signal transduction in VirA heterodimers and posttranslational regulation of VirA activity in Agrobacterium tumefaciens. J Bacteriol. 2005;187:213–223. doi: 10.1128/JB.187.1.213-223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin SG, Prusti RK, Roitsch T, Ankenbauer RG, Nester EW. Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: essential role in biological activity of VirG. J Bacteriol. 1990;172:4945–4950. doi: 10.1128/jb.172.9.4945-4950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cangelosi GA, Hung L, Puvanesarajah V, Stacey G, Ozga DA, Leigh JA, et al. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol. 1987;169:2086–2091. doi: 10.1128/jb.169.5.2086-2091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CM, Regensburg-Tuïnk TJ, Hooykaas PJ. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science. 2000;290:979–982. doi: 10.1126/science.290.5493.979. [DOI] [PubMed] [Google Scholar]
- 161.Citovsky V, Wong ML, Zambryski P. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci U S A. 1989;86:1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lacroix B, Vaidya M, Tzfira T, Citovsky V. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 2005;24:428–437. doi: 10.1038/sj.emboj.7600524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ziemienowicz A, Merkle T, Schoumacher F, Hohn B, Rossi L. Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins. Plant Cell. 2001;13:369–383. doi: 10.1105/tpc.13.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guralnick B, Thomsen G, Citovsky V. Transport of DNA into the nuclei of xenopus oocytes by a modified VirE2 protein of Agrobacterium. Plant Cell. 1996;8:363–373. doi: 10.1105/tpc.8.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ziemienowicz A, Görlich D, Lanka E, Hohn B, Rossi L. Import of DNA into mammalian nuclei by proteins originating from a plant pathogenic bacterium. Proc Natl Acad Sci U S A. 1999;96:3729–3733. doi: 10.1073/pnas.96.7.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bakó L, Umeda M, Tiburcio AF, Schell J, Koncz C. The VirD2 pilot protein of Agrobacterium- transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants. Proc Natl Acad Sci U S A. 2003;100:10108–10113. doi: 10.1073/pnas.1733208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.García-Rodríguez FM, Schrammeijer B, Hooykaas PJJ. The Agrobacterium VirE3 effector protein: a potential plant transcriptional activator. Nucleic Acids Res. 2006;34:6496–6504. doi: 10.1093/nar/gkl877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tzfira T, Vaidya M, Citovsky V. VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 2001;20:3596–3607. doi: 10.1093/emboj/20.13.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tzfira T, Vaidya M, Citovsky V. Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis nuclear protein VIP1. Proc Natl Acad Sci U S A. 2002;99:10435–10440. doi: 10.1073/pnas.162304099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tao Y, Rao PK, Bhattacharjee S, Gelvin SB. Expression of plant protein phosphatase 2C interferes with nuclear import of the Agrobacterium T-complex protein VirD2. Proc Natl Acad Sci U S A. 2004;101:5164–5169. doi: 10.1073/pnas.0300084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lacroix B, Loyter A, Citovsky V. Association of the Agrobacterium T-DNA–protein complex with plant nucleosomes. Proc Natl Acad Sci U S A. 2008;105:15429–15434. doi: 10.1073/pnas.0805641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tzfira T, Vaidya M, Citovsky V. Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature. 2004;431:87–92. doi: 10.1038/nature02857. [DOI] [PubMed] [Google Scholar]
- 173.Magori S, Citovsky V. Epigenetic control of Agrobacterium T-DNA integration. Biochim Biophys Acta. 2011;1809:388–394. doi: 10.1016/j.bbagrm.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van Attikum H, Bundock P, Hooykaas PJ. Non-homologous end-joining proteins are required for Agrobacterium T-DNA integration. EMBO J. 2001;20:6550–6558. doi: 10.1093/emboj/20.22.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li J, Krichevsky A, Vaidya M, Tzfira T, Citovsky V. Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc Natl Acad Sci U S A. 2005;102:5733–5738. doi: 10.1073/pnas.0404118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Veena H Jiang, Doerge RW, Gelvin SB. Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J. 2003;35:219–236. doi: 10.1046/j.1365-313x.2003.01796.x. [DOI] [PubMed] [Google Scholar]
- 177.Anand A, Vaghchhipawala Z, Ryu CM, Kang L, Wang K, del-Pozo O, et al. Identification and characterization of plant genes involved in Agrobacterium-mediated plant transformation by virus-induced gene silencing. Mol Plant Microbe Interact. 2007;20:41–52. doi: 10.1094/MPMI-20-0041. [DOI] [PubMed] [Google Scholar]
- 178.Bundock P, den Dulk-Ras A, Beijersbergen A, Hooykaas PJ. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J. 1995;14:3206–3214. doi: 10.1002/j.1460-2075.1995.tb07323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peiris K, Gee TM, Boswell TC. Bloodstream infection related to heparin infusion caused by Agrobacterium tumefaciens. J Hosp Infect. 2006;62:250–251. doi: 10.1016/j.jhin.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 180.Giammanco GM, Pignato S, Santangelo C, Grimont PAD, Grimont F, Giammanco G. Molecular typing of Agrobacterium species isolates from catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2004;25:885–887. doi: 10.1086/502315. [DOI] [PubMed] [Google Scholar]
- 181.Petrunia IV, Frolova OY, Komarova TV, Kiselev SL, Citovsky V, Dorokhov YL. Agrobacterium tumefaciens-induced bacteraemia does not lead to reporter gene expression in mouse organs. PLoS One. 2008;3:e2352. doi: 10.1371/journal.pone.0002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V. Genetic transformation of HeLa cells by Agrobacterium. Proc Natl Acad Sci U S A. 2001;98:1871–1876. doi: 10.1073/pnas.041327598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 184.Hubber A, Roy CR. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol. 2010;26:261–283. doi: 10.1146/annurev-cellbio-100109-104034. [DOI] [PubMed] [Google Scholar]
- 185.Schröder G, Schuelein R, Quebatte M, Dehio C. Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. Proc Natl Acad Sci U S A. 2011;108:14643–14648. doi: 10.1073/pnas.1019074108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Control of Chagas Disease: Second Report of a WHO Expert Committee. World Health Organization; 2002. pp. 1–109. [PubMed] [Google Scholar]
- 187.Teixeira AR. Clinic presentation of Chagas disease. In: Vinaud MC, Castro AM, editors. Emerging Chagas Disease. Bentham Science Publishers; 2009. pp. 104–109. [Google Scholar]
- 188.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 189.Urbina JA. New insights in Chagas disease treatment. Drugs Future. 2010;35:409. [Google Scholar]
- 190.Lauria-Pires L, Braga MS, Vexenat AC, Nitz N, Simões-Barbosa A, Tinoco DL, et al. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. Am J Trop Med Hyg. 2000;63:111–118. doi: 10.4269/ajtmh.2000.63.111. [DOI] [PubMed] [Google Scholar]
- 191.Teixeira ARL, Nascimento RJ, Sturm NR. Evolution and pathology in Chagas disease–a review. Mem Inst Oswaldo Cruz. 2006;101:463–491. doi: 10.1590/s0074-02762006000500001. [DOI] [PubMed] [Google Scholar]
- 192.Teixeira AR, Lacava Z, Santana JM, Luna H. Insertion of Trypanosoma cruzi DNA in the genome of mammal host cell through infection. Rev Soc Bras Med Trop. 1991;24:55–58. doi: 10.1590/s0037-86821991000100010. [DOI] [PubMed] [Google Scholar]
- 193.Teixeira AR, Argañaraz ER, Freitas LH, Lacava ZG, Santana JM, Luna H. Possible integration of Trypanosoma cruzi kDNA minicircles into the host cell genome by infection. Mutat Res. 1994;305:197–209. doi: 10.1016/0027-5107(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 194.Simões-Barbosa a, Barros aM, Nitz N, Argañaraz ER, Teixeira aR. Integration of Trypanosoma cruzi kDNA minicircle sequence in the host genome may be associated with autoimmune serum factors in Chagas disease patients. Mem Inst Oswaldo Cruz. 1999;94(Suppl. 1):249–252. doi: 10.1590/s0074-02761999000700041. [DOI] [PubMed] [Google Scholar]
- 195.Nitz N, Gomes C, de Cássia Rosa A, D'Souza-Ault MR, Moreno F, Lauria-Pires L, et al. Heritable integration of kDNA minicircle sequences from Trypanosoma cruzi into the avian genome: insights into human Chagas disease. Cell. 2004;118:175–186. doi: 10.1016/j.cell.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 196.Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–481. doi: 10.1016/s0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 197.Thorsness PE, Weber ER. Escape and migration of nucleic acids between chloroplasts, mitochondria, and the nucleus. Int Rev Cytol. 1996;165:207–234. doi: 10.1016/s0074-7696(08)62223-8. [DOI] [PubMed] [Google Scholar]
- 198.Nikoh N, Nakabachi A. Aphids acquired symbiotic genes via lateral gene transfer. BMC Biol. 2009;7:12. doi: 10.1186/1741-7007-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Douglas AE. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol. 1998;43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 200.Sasaki T, Ishikawa H. Production of essential amino acids from glutamate by mycetocyte symbionts of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 1995;41:6. [Google Scholar]
- 201.Dunning Hotopp JC, Clark ME, Oliveira DCSG, Foster JM, Fischer P, Muñoz Torres MC, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- 202.Lukes J, Guilbride DL, Votýpka J, Zíková A, Benne R, Englund PT. Kinetoplast DNA network: evolution of an improbable structure. Eukaryot Cell. 2002;1:495–502. doi: 10.1128/EC.1.4.495-502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stuart K, Feagin JE. Mitochondrial DNA of kinetoplastids. Int Rev Cytol. 1992;141:65–88. doi: 10.1016/s0074-7696(08)62063-x. [DOI] [PubMed] [Google Scholar]
- 204.Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- 205.Thomas S, Martinez LLIT, Westenberger SJ, Sturm NR. A population study of the minicircles in Trypanosoma cruzi: predicting guide RNAs in the absence of empirical RNA editing. BMC Genomics. 2007;8:133. doi: 10.1186/1471-2164-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Simpson L. RNA editing–a novel genetic phenomenon? Science. 1990;250:512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- 207.Hajduk SL, Harris ME, Pollard VW. RNA editing in kinetoplastid mitochondria. FASEB J. 1993;7:54–63. doi: 10.1096/fasebj.7.1.8422975. [DOI] [PubMed] [Google Scholar]
- 208.Rogers K, Gao G. L Simpson, Uridylate-specific 3′ 5′-exoribonucleases involved in uridylate-deletion RNA editing in trypanosomatid mitochondria. J Biol Chem. 2007;282:29073–29080. doi: 10.1074/jbc.M704551200. [DOI] [PubMed] [Google Scholar]
- 209.Rippe K, Mücke N, Langowski J. Superhelix dimensions of a 1868 base pair plasmid determined by scanning force microscopy in air and in aqueous solution. Nucleic Acids Res. 1997;25:1736–1744. doi: 10.1093/nar/25.9.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- 211.Panté N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maucksch C, Bohla A, Hoffmann F, Schleef M, Aneja MK, Elfinger M, et al. Transgene expression of transfected supercoiled plasmid DNA concatemers in mammalian cells. J Gene Med. 2009;11:444–453. doi: 10.1002/jgm.1310. [DOI] [PubMed] [Google Scholar]
- 213.Jasmer DP, Stuart K. Conservation of kinetoplastid minicircle characteristics without nucleotide sequence conservation. Mol Biochem Parasitol. 1986;18:257–269. doi: 10.1016/0166-6851(86)90084-8. [DOI] [PubMed] [Google Scholar]
- 214.Kidane GZ, Hughes D, Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984;27:265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- 215.Ray DS. Conserved sequence blocks in kinetoplast minicircles from diverse species of trypanosomes. Mol Cell Biol. 1989;9:1365–1367. doi: 10.1128/mcb.9.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Degrave W, Fragoso SP, Britto C, van Heuverswyn H, Kidane GZ, Cardoso MA, et al. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988;27:63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 217.Simões-Barbosa A, Argañaraz ER, Barros AM, Rosa A de C, Alves NP, Louvandini P, et al. Hitchhiking Trypanosoma cruzi minicircle DNA affects gene expression in human host cells via LINE-1 retrotransposon. Mem Inst Oswaldo Cruz. 2006;101:833–843. doi: 10.1590/s0074-02762006000800003. [DOI] [PubMed] [Google Scholar]
- 218.Babushok DV, Kazazian HH. Progress in understanding the biology of the human mutagen LINE-1. Hum Mutat. 2007;28:527–539. doi: 10.1002/humu.20486. [DOI] [PubMed] [Google Scholar]
- 219.Januszyk K, Li PWL, Villareal V, Branciforte D, Wu H, Xie Y, et al. Identification and solution structure of a highly conserved C-terminal domain within ORF1p required for retrotransposition of long interspersed nuclear element-1. J Biol Chem. 2007;282:24893–24904. doi: 10.1074/jbc.M702023200. [DOI] [PubMed] [Google Scholar]
- 220.Goodier JL, Zhang L, Vetter MR, Kazazian HH. LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol Cell Biol. 2007;27:6469–6483. doi: 10.1128/MCB.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ogiwara I, Miya M, Ohshima K, Okada N. Retropositional parasitism of SINEs on LINEs: identification of SINEs and LINEs in elasmobranchs. Mol Biol Evol. 1999;16:1238–1250. doi: 10.1093/oxfordjournals.molbev.a026214. [DOI] [PubMed] [Google Scholar]
- 222.Lucchinetti E, Feng J, da Silva R, Tolstonog GV, Schaub MC, Schumann GG, et al. Inhibition of LINE-1 expression in the heart decreases ischemic damage by activation of Akt/PKB signaling. Physiol Genomics. 2006;25:314–324. doi: 10.1152/physiolgenomics.00251.2005. [DOI] [PubMed] [Google Scholar]
- 223.Fassot C, Briet M, Rostagno P, Barbry P, Perret C, Laude D, et al. Accelerated arterial stiffening and gene expression profile of the aorta in patients with coronary artery disease. J Hypertens. 2008;26:747–757. doi: 10.1097/HJH.0b013e3282f4b3d0. [DOI] [PubMed] [Google Scholar]
- 224.Kelly JM, Ward HM, Miles MA, Kendall G. A shuttle vector which facilitates the expression of transfected genes in Trypanosoma cruzi and Leishmania. Nucleic Acids Res. 1992;20:3963–3969. doi: 10.1093/nar/20.15.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Martínez-Calvillo S, López I, Hernández R. pRIBOTEX expression vector: a pTEX derivative for a rapid selection of Trypanosoma cruzi transfectants. Gene. 1997;199:71–76. doi: 10.1016/s0378-1119(97)00348-x. [DOI] [PubMed] [Google Scholar]
- 226.Cosenza LW. Sacromastigophoric Therapeutic Agent Delivery System. 2003 [Google Scholar]
- 227.Davis ME. Non-viral gene delivery systems. Curr Opin Biotechnol. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 228.Wagner E, Cotten M, Foisner R, Birnstiel ML. Transferrin–polycation–DNA complexes : the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991;88:4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. J Liposome Res. 2006;16:237–247. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- 230.Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- 231.Mayrhofer P, Schleef M, Jechlinger W. Use of minicircle plasmids for gene therapy. Methods Mol Biol. 2009;542:87–104. doi: 10.1007/978-1-59745-561-9_4. [DOI] [PubMed] [Google Scholar]
- 232.Wu J, Xiao X, Zhao P, Xue G, Zhu Y, Zhu X, et al. Minicircle-IFNgamma induces antiproliferative and antitumoral effects in human nasopharyngeal carcinoma. Clin Cancer Res. 2006;12:4702–4713. doi: 10.1158/1078-0432.CCR-06-0520. [DOI] [PubMed] [Google Scholar]
- 233.Catanese DJ, Fogg JM, Schrock DE, Gilbert BE, Zechiedrich L. Supercoiled minivector DNA resists shear forces associated with gene therapy delivery. Gene Ther. 2012;19:94–100. doi: 10.1038/gt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 235.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 236.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 237.Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. An araC-controlled bacterial cre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 2001;276:23018–23027. doi: 10.1074/jbc.M010873200. [DOI] [PubMed] [Google Scholar]
- 238.Vandermeulen G, Marie C, Scherman D, Préat V. New generation of plasmid backbones devoid of antibiotic resistance marker for gene therapy trials. Mol Ther. 2011;19:1942–1949. doi: 10.1038/mt.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ivics Z, Hackett PB, Plasterk RH, Izsvák Z. Molecular reconstruction of sleeping beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 240.Wu SCY, Meir YJJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, et al. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci U S A. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fraser MJ, Smith GE, Summers MD. Acquisition of host Cell DNA sequences by baculoviruses: relationship between host dna insertions and fp mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis viruses. J Virol. 1983;47:287–300. doi: 10.1128/jvi.47.2.287-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu L, Liu H, Visner G, Fletcher BS. Sleeping beauty-mediated eNOS gene therapy attenuates monocrotaline-induced pulmonary hypertension in rats. FASEB J. 2006;20:2594–2596. doi: 10.1096/fj.06-6254fje. [DOI] [PubMed] [Google Scholar]
- 243.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the sleeping beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells bythe piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lucas WJ, Lee JY. Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol. 2004;5:712–726. doi: 10.1038/nrm1470. [DOI] [PubMed] [Google Scholar]
- 249.Lucas WJ, Bouché-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, et al. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 1995;270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- 250.Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee FM, et al. A systemic small RNA signaling system in plants. Plant Cell. 2004;16:1979–2000. doi: 10.1105/tpc.104.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 252.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci U S A. 2007;104:10565–10570. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 254.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 255.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 256.Onfelt B, Nedvetzki S, Benninger RKP, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 257.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 258.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 259.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stegemann S, Bock R. Exchange of genetic material between cells in plant tissue grafts. Science. 2009;324:649–651. doi: 10.1126/science.1170397. [DOI] [PubMed] [Google Scholar]
- 262.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 264.Bergsmedh A, Szeles A, Henriksson M, Bratt A, Folkman MJ, Spetz AL, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A. 2001;98:6407–6411. doi: 10.1073/pnas.101129998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sandgren S, Wittrup A, Cheng F, Jönsson M, Eklund E, Busch S, et al. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J Biol Chem. 2004;279:17951–17956. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 266.Lee Y, El Andaloussi S, Wood MJa. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 267.Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 269.Lentz TL. Rabies virus binding to an acetylcholine receptor alpha-subunit peptide. J Mol Recognit. 1990;3:82–88. doi: 10.1002/jmr.300030205. [DOI] [PubMed] [Google Scholar]
- 270.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21:1305–1320. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- 272.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 273.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 275.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012;25:75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 276.Lai RC, Chen TS, Lim SK, Arslan F, Timmers L, Pasterkamp G, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 277.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]


