Abstract
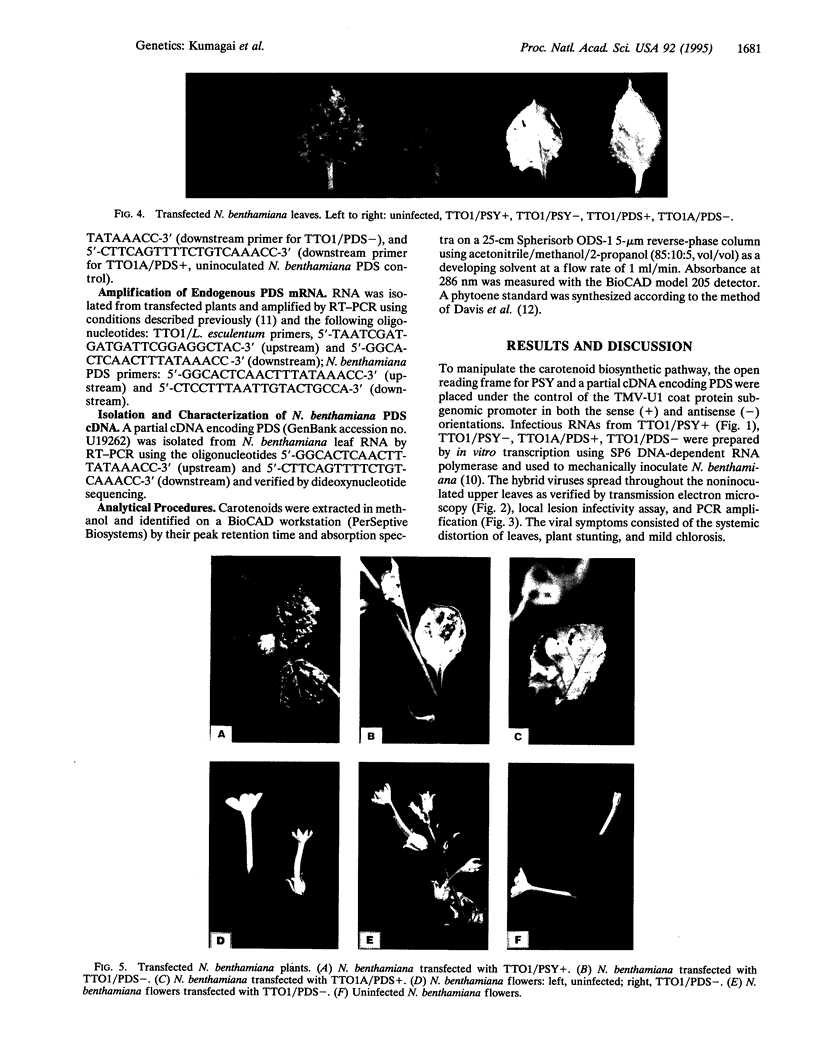
The carotenoid biosynthetic pathway in higher plants was manipulated by using an RNA viral vector. A cDNA encoding phytoene synthase and a partial cDNA encoding phytoene desaturase (PDS) were placed under the transcriptional control of a tobamovirus subgenomic promoter. One to two weeks after inoculation, systemically infected Nicotiana benthamiana plants were analyzed for phytoene. Leaves from transfected plants expressing phytoene synthase developed a bright orange phenotype and accumulated high levels of phytoene. Cytoplasmic inhibition of plant gene expression by viral RNA was demonstrated with an antisense RNA transcript to a partial PDS cDNA derived from tomato. The leaves of the plants transfected with the antisense PDS sequence developed a white phenotype and also accumulated high levels of phytoene. A partial cDNA to the corresponding N. benthamiana PDS gene was isolated and found to share significant homology with the tomato antisense PDS transcript. This work demonstrates that an episomal RNA viral vector can be used to deliberately manipulate a major, eukaryotic biosynthetic pathway. In addition, our results indicate that an antisense transcript generated in the cytoplasm of a plant cell can turn off endogenous gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R., Herz E., Dodds S., Ruther M. Access to hospital care for California and Michigan Medicaid recipients. Health Care Financ Rev. 1991 Summer;12(4):99–104. [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O., Lehto K. M. Regulation of tobamovirus gene expression. Adv Virus Res. 1990;38:307–342. doi: 10.1016/S0065-3527(08)60865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogbo O., Laferriére A., D'Harlingue A., Camara B. Carotenoid biosynthesis: Isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7054–7058. doi: 10.1073/pnas.85.19.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J., Kearney C. M., Hilf M. E., Dawson W. O. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7204–7208. doi: 10.1073/pnas.88.16.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray R. G., Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol. 1993 Jul;22(4):589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Bartley G. E., Scolnik P. A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993 Apr;5(4):379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J., Picton S., Shabbeer J., Schuch W., Grierson D. Molecular biology of fruit ripening and its manipulation with antisense genes. Plant Mol Biol. 1992 May;19(1):69–87. doi: 10.1007/BF00015607. [DOI] [PubMed] [Google Scholar]
- Kumagai M. H., Turpen T. H., Weinzettl N., della-Cioppa G., Turpen A. M., Donson J., Hilf M. E., Grantham G. L., Dawson W. O., Chow T. P. Rapid, high-level expression of biologically active alpha-trichosanthin in transfected plants by an RNA viral vector. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):427–430. doi: 10.1073/pnas.90.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Aoyagi M., Yamanashi Y., Saito H., Ikawa S., Meshi T., Okada Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J Biochem. 1984 Dec;96(6):1915–1923. doi: 10.1093/oxfordjournals.jbchem.a135026. [DOI] [PubMed] [Google Scholar]
- Pecker I., Chamovitz D., Linden H., Sandmann G., Hirschberg J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mur L. A., Beld M., Mol J. N., Stuitje A. R. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990 Apr;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]