Abstract
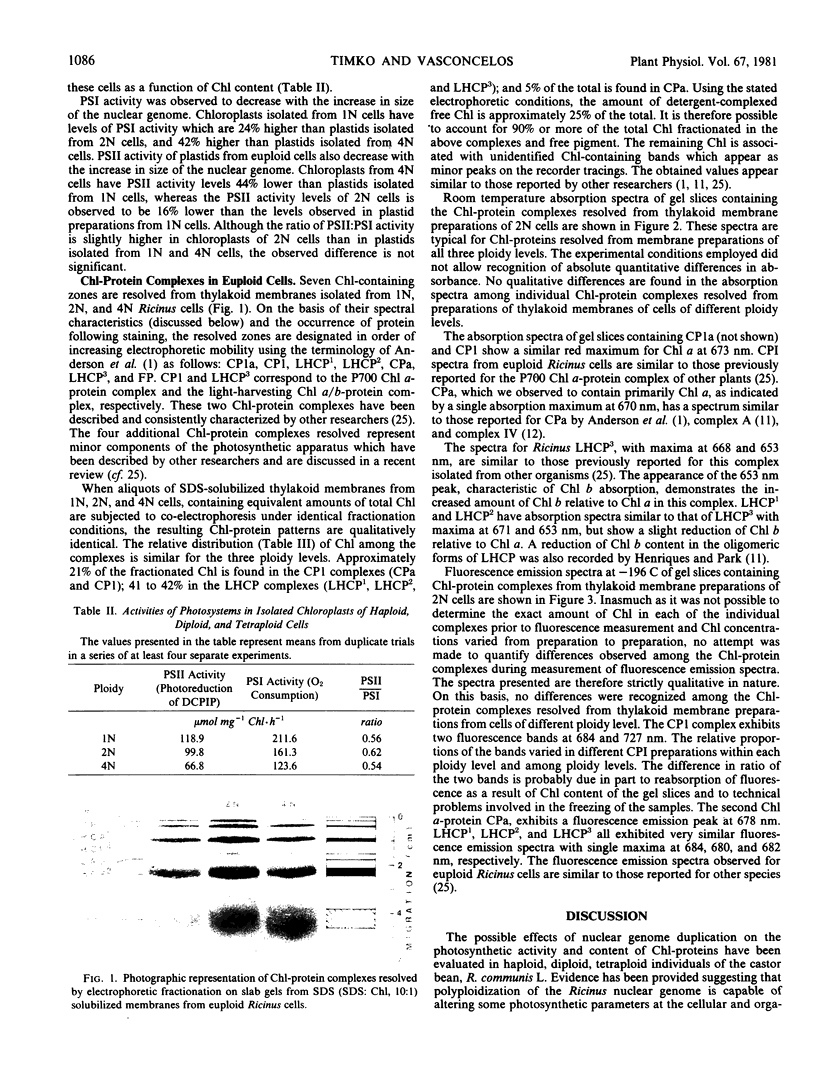
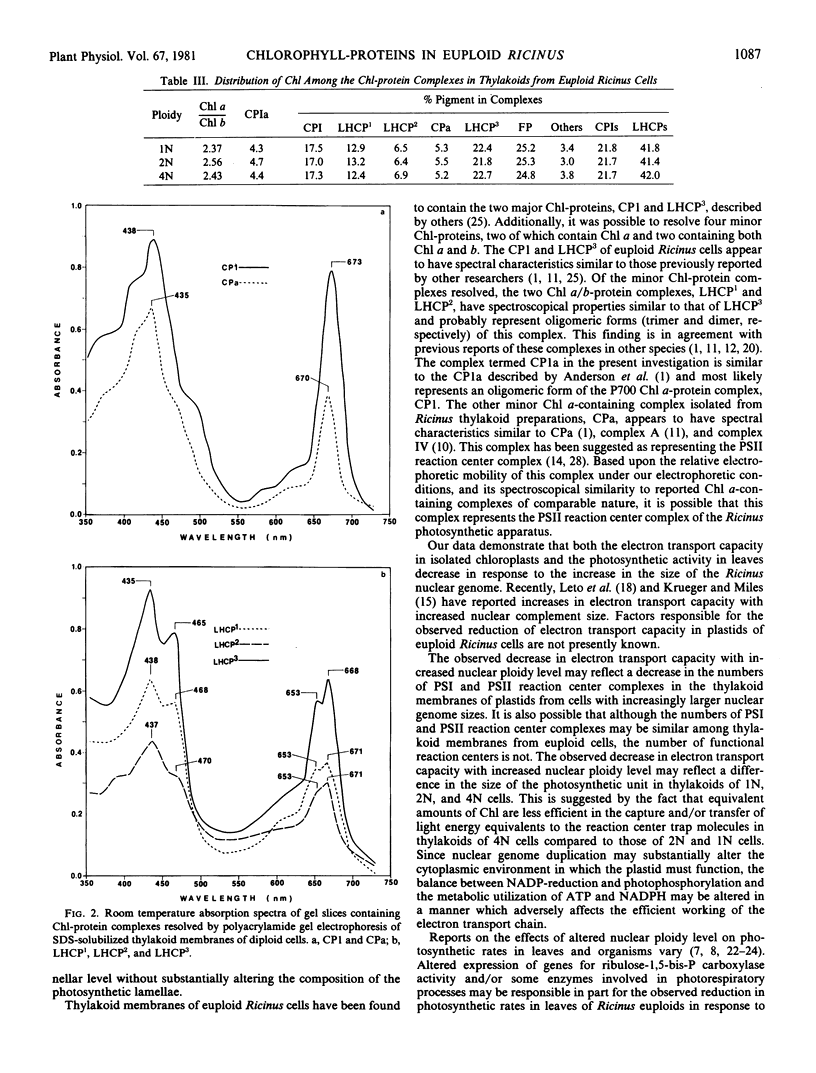
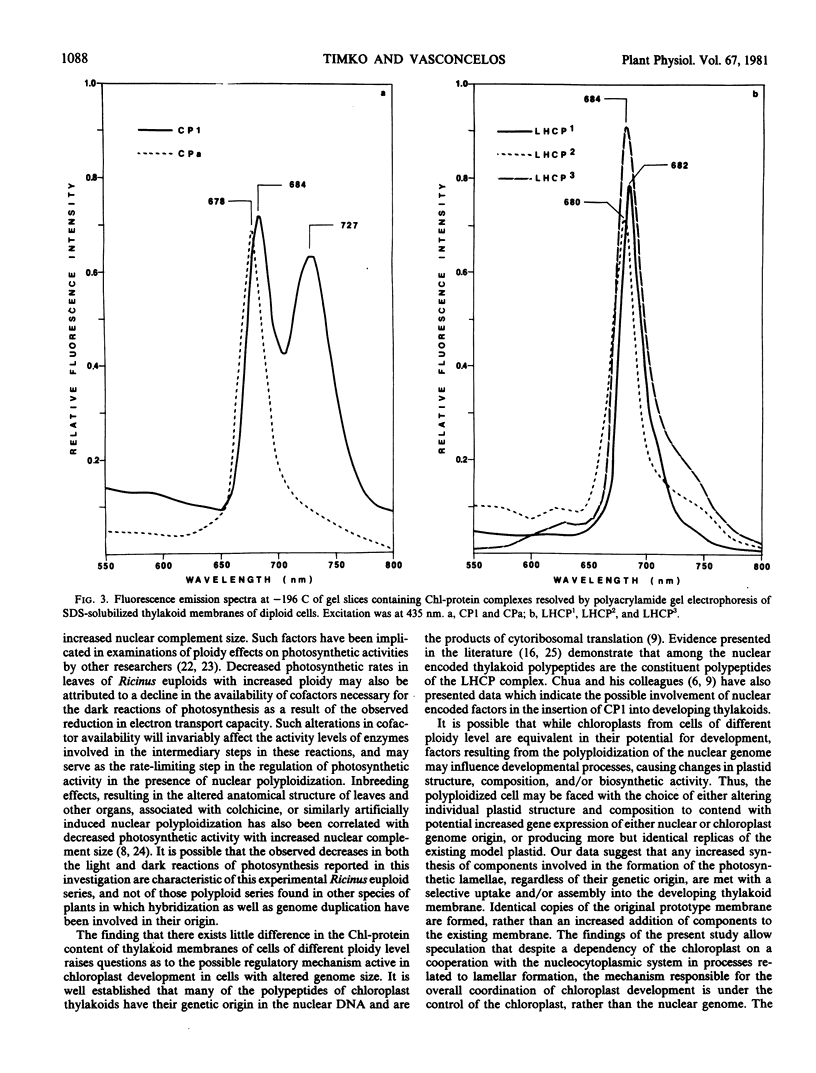
The effects of nuclear genome duplication on the chlorophyll-protein content and photochemical activity of chloroplasts, and photosynthetic rates in leaf tissue, have been evaluated in haploid, diploid, and tetraploid individuals of the castor bean, Ricinus communis L. Analysis of this euploid series revealed that both photosystem II (2,6-dichlorophenolindophenol reduction) and photosystem I oxygen uptake (N,N,N′,N′-tetramethyl-p-phenylenediamine to methyl viologen) decrease in plastids isolated from cells with increasingly larger nuclear complement sizes. Photosynthetic O2-evolution and 14CO2-fixation rates in leaf tissue from haploid, diploid, and tetraploid individuals were also found to decrease with the increase in size of the nuclear genome. Six chlorophyll-protein complexes, in addition to a zone of detergent complexed free pigment, were resolved from sodium dodecyl sulfate-solubilized thylakoid membranes from cells of all three ploidy levels. In addition to the P700-chlorophyll a-protein complex and the light-harvesting chlorophyll a/b-protein complex, four minor complexes were revealed, two containing only chlorophyll a and two containing both chlorophyll a and b. The relative distribution of chlorophyll among the resolved chlorophyll-protein complexes and free pigment was found to be similar for all three ploidy levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaggio A. E., Stetler D. A. Polyploidy and gene dosage effects on chloroplasts of fern gametophytes. Exp Cell Res. 1971 Aug;67(2):287–294. doi: 10.1016/0014-4827(71)90411-3. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Spectral characterization of five chlorophyll-protein complexes. Plant Physiol. 1978 Dec;62(6):856–860. doi: 10.1104/pp.62.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M., Vernon L. P. Polypeptide Composition of Photosynthetic Membranes from Chlamydomonas reinhardi and Anabaena variabilis. Plant Physiol. 1974 May;53(5):777–778. doi: 10.1104/pp.53.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miles C. D., Markwell J. P., Thornber J. P. Effect of nuclear mutation in maize on photosynthetic activity and content of chlorophyll-protein complexes. Plant Physiol. 1979 Nov;64(5):690–694. doi: 10.1104/pp.64.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N. C., Smillie R. M., Henningsen K. W., Von Wettstein D. Composition and Function of Thylakoid Membranes from Grana-rich and Grana-deficient Chloroplast Mutants of Barley. Plant Physiol. 1979 Jan;63(1):174–182. doi: 10.1104/pp.63.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Nelson C. J., Asay K. H. Ribulose bisphosphate carboxylase: altered genetic expression in tall fescue. Plant Physiol. 1977 Jan;59(1):38–41. doi: 10.1104/pp.59.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Photosynthetic and Photorespiratory Carbon Metabolism in Mesophyll Protoplasts and Chloroplasts Isolated from Isogenic Diploid and Tetraploid Cultivars of Ryegrass (Lolium perenne L.). Plant Physiol. 1980 Mar;65(3):489–494. doi: 10.1104/pp.65.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Vasconcelos A. C., Fairbrothers D. E. Euploidy in Ricinus. I. Euploidy and gene dosage effects on cellular proteins. Biochem Genet. 1980 Feb;18(1-2):171–183. doi: 10.1007/BF00504367. [DOI] [PubMed] [Google Scholar]
- Wessels J. S., Borchert M. T. Polypeptide profiles of chlorophyll . protein complexes and thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):78–93. doi: 10.1016/0005-2728(78)90163-9. [DOI] [PubMed] [Google Scholar]