Abstract
Despite advances in medicine and biomedical sciences, cancer still remains a major health issue. Complex interactions between tumors and their microenvironment contribute to tumor initiation and progression and also contribute to the development of drug resistant tumor cell populations. The complexity and heterogeneity of tumors and their microenvironment make it challenging to both study and treat cancer. Traditional animal cancer models and in vitro cancer models are limited in their ability to recapitulate human structures and functions, thus hindering the identification of appropriate drug targets and therapeutic strategies. The development and application of microfluidic 3D cancer models has the potential to overcome some of the limitations inherent to traditional models. This review summarizes the progress in microfluidic 3D cancer models, their benefits, and their broad application to basic cancer biology, drug screening, and drug discovery.
Keywords: Microfluidics, 3D in vitro system, Cancer, Tumor microenvironment, Biomimetics, High-throughput screening, Drug testing
1. Introduction
Cancer is a complex disease, developing in a heterogeneous microenvironment that consists of stromal cells, signaling molecules, and various extracellular matrix (ECM) compositions[1,2]. When tumor cells activate the surrounding stroma they create an environment where they can grow and spread. This microenvironmental alteration contributes to the development of resistance to treatment[3,4]. For example, BRAF-mutant melanoma cells activate stromal fibroblasts to overexpress HGF, which results in the increased resistance of melanoma cells to RAF inhibitor treatment[5]. In addition to microenvironmental heterogeneity within a single patient, cancer also differs greatly from patient to patient making treatment challenging[6]. Due to the heterogeneity and complexity of cancer, more in vivo-like approaches that consider multiple parameters of the microenvironment are necessary. Two-dimensional (2D) models are flat surfaces, such as petri dishes, to which cells adhere. 2D systems can be coated with desired proteins such as collagen to study the biochemical response of the cells to those proteins. However, 2D systems are limited in their ability to mimic the complex and inherently 3D conditions present in vivo. 2D systems do not incorporate the structural and mechanical properties that define the in vivo microenvironment. 3D in vitro systems address this issue by embedding cells in an ECM where cells often replicate in vivo structure more faithfully. More recently micro scale organotypic models go a step further to recreate organ structure on a chip.
The use of 3D culture is particularly important in cancer as the interactions between cancer cells and the surrounding microenvironment are known to create a context that promotes tumor progression[7]. More importantly, 3D in vitro cancer models are capable of providing enhanced quantitative information on complex cell-cell and cell-ECM interactions. By using the 3D in vitro cancer models, researchers can more readily tease apart specific interactions, which can be difficult using animal models. For example, 3D conditions activated cancer-related signaling pathways such as H-RasV12-induced IL6-STAT3 and initiated ECM dependent responses that were not seen in 2D conditions[8]. Moreover, recent evidence suggests that highly invasive breast cancer cells show different response to different stiffness of ECM in 3D conditions. That is, the invasive breast cancer cells migrate faster and farther in a stiffer collagen gel (higher concentration)[9–11]. It has also recently been shown that stromal fibroblasts in 3D conditions are more functionally active and produce higher concentrations of signaling molecules, subsequently facilitating the invasive progression of ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) in breast cancer[12]. Several other researchers have observed that tumor cells and various stromal cells respond differently to the mechanical tension and the chemical compositions within the ECM[13,14]. Thus, 3D in vitro cancer models have been slowly gaining the attention of cancer researchers, clinicians, and the pharmaceutical industry over the past two decades[15–18].
3D in vitro systems have demonstrated the potential to overcome limitations of traditional 2D in vitro systems and to reveal new biological insights. However traditional 3D systems (e.g. transwells) offer limited spatial organization and cell-cell interactions. Moreover, the large amount of sample volume required limits the utility of the system as a high throughput screening (HTS) platform. Accordingly, there has been increased interest in novel 3D culture systems that can provide improved biological models and functionality while reducing required volumes and cost. In particular, microscale 3D in vitro models represent a potential alternative to improve both functionality and throughput of traditional 3D systems (Fig. 1). In this review, we summarize progress in microfluidic 3D cancer models, their benefits, and their broad applications to basic cancer biology, drug screening, and drug delivery. We begin by describing simple single channel systems and move on to describe more complex and highly functionalized systems.
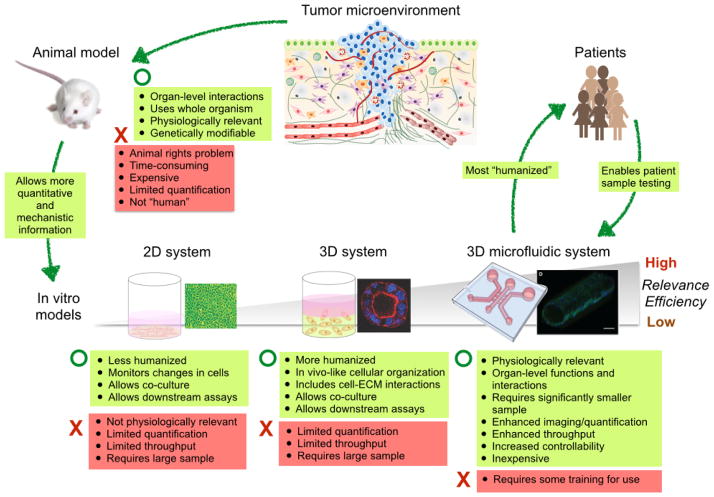
Fig. 1.
Comparison of existing cancer research models. The illustration of tumor microenvironment is adopted from Ref. [100] and the microfluidic channel image is reproduced from Ref. [59] with permission.
2. Simple but informative single channel microscale 3D in vitro cancer models
2-1. Different physical properties of 3D microsystems
Of the many potential advantages of 3D microsystem-based disease models, two are particularly important when comparing 3D microsystems to traditional 3D systems. For certain types of experiments (e.g. use of primary cells), a significant advantage is simply that a smaller sample size is needed to obtain the same results as the conventional system. The other critical advantage is the ability for 3D microscale systems to provide functionality (e.g. spatial/temporal control) that is difficult or impossible to achieve in traditional 3D systems. In 2D culture, the micro scale culture environment offers enhanced sensitivity to cell signaling due to the physical properties of the microchannel environment including the dominance of diffusion over convection. In the conventional systems, fluctuations in temperature, solute concentration, or dissolved gas concentration can lead to surface tension differences at the gas–solution interface. These fluctuations in turn cause rapid convection and mass transfer. For this reason, convective mixing is dominant over diffusion even in the absence of mechanically driven flow in macroscale cell culture systems and secreted molecules are swept away by convection. However, because of the small scale and the spatial constraints, the convective mixing is considerably reduced in static (no flow) culture in microchannels and molecular transport is largely governed by diffusion[19]. The transportation of molecules by diffusion enables the formation of gradients, and the retention of these molecules in close proximity to the cells, increasing response sensitivity. For example, the role of the stroma in Hedgehog signaling-mediated prostate cancer cell growth was recapitulated in vitro for the first time because of the enhanced paracrine signaling, which had only previously been observed in xenograph mouse models[20]. In microscale 3D systems, however, the microchannel environment does not considerably affect cell signaling as cell-ECM effects dominate. The transport of signaling molecules is governed mostly by diffusion through the ECM in both conventional multi-well plates and microchannels. Therefore the results of autocrine and paracrine signaling of monoculture and co-cultures of tumor cells and stromal cells from 3D macro and 3D micro systems are similar[21].
ECM polymerization, however, becomes more controllable in microchannels as compared to conventional culture flasks. Particularly for natural polymers such as collagen I and Matrigel, polymerization is affected by increased heat transfer from ambient environment to the ECM solution due to the increased surface-to-volume ratio of the microchannels[22]. By changing the temperature, pH, and incubation time, different diameters and alignment of collagen fibers are achievable[22,23]. The diameter and alignment of fibers are factors that could influence invasion and migration of cancer cells. Another important aspect of the microscale system is that the system significantly reduces the amount of required ECM gel (200μl vs. 2μl) and number of cells (105 cells vs. 103 cells) for the same analysis on the macroscale. With macroscale technologies, smaller volumes can be achieved by using multi-well plates such as 96 or 192 array well plates. However, in traditional well plates, the individual wells are in a vertical orientation. In other words, the cylindrical wells are tall and thin. This shape hinders efficient imaging of samples because imaging is done from the bottom of the narrow cylinder and has to scan through multiple layers, subsequently causing difficulties for quantitative analysis. Unlike conventional multiwells, microchannels are cylinders stretched out in a linear, horizontal orientation. In this way, when imaging is done from the bottom of the channel, it has fewer layers to scan through.
2-2. Microfluidic 3D screening tools
The reduced volume in microfluidic 3D systems facilitates the system’s capability as an HTS tool to investigate numerous microenvironmental components influencing tumor development and progression. It allows screening of a broader array of microenvironmental parameters and increases our understanding of how the interplay among these parameters regulates cancer development and progression. Microfluidic 3D screening platforms can provide new information not previously obtainable. For example, Montanez-Sauri et al. demonstrated how the effects of different ECM proteins such as collagen I, fibronectin, and laminin can be efficiently measured at a significantly reduced cost by using an array of microfluidic channels[24]. The study revealed that breast cancer epithelial cells become more responsive to the ECM composition when in co-culture with stromal fibroblasts compared to mono-culture. More importantly, the reduced number of cells required for each endpoint (~103 cells/endpoint rather than >105 cells/endpoint) enables high content studies using limited numbers of primary cells obtained from a small biopsy (e.g., typically < 106 mammary fibroblasts per 0.25cm2 breast tissue biopsy). The small scale of the system allows efficient use of patient cells as only 1000~2000 cells are required per endpoint. Using the approach, Su et al. identified considerable inter-individual heterogeneity of paracrine interactions between T47D breast cancer cells and cancer-associated fibroblasts (CAF) or normal mammary fibroblasts (NMF) from breast carcinoma tissue samples and adjacent normal mammary gland tissue from 28 patients[25]. These data demonstrate the promising applications of a microscale 3D in vitro system as a clinical tool to understand the unique characteristics of each patient’s tumor at reduced cost, time, and labor.
2-3. Application of various ECM materials
In addition to naturally-derived ECM polymers, such as collagen, hyaluronan, and fibrin, various other polymers such as alginate, polyethylene glycol (PEG), and poly(lactic-co-glycolic) acid (PLGA), have been applied to microfluidic 3D in vitro cancer models to provide more controlled mechanical and chemical properties and geometry of the ECM. Naturally-derived ECM materials are advantageous because they are biocompatible and provide in vivo-like biochemical and biomechanical properties. However, naturally-derived ECM materials have limitations such as batch-to-batch variation, relatively high cost, and a limited range of properties. In order to overcome the limitations of naturally-derived ECM polymers, the use and development of many synthetic polymers with appropriate cell adhesion peptides has been increasing. Various properties such as architecture, stiffness, porosity, shape, and the concentration of adhesion peptides are readily controllable with synthetic polymers. Recently, stacks of paper have been introduced as a useful ECM substitute; lowering the cost of material while possessing unique features since the paper stacks can be de-stacked later for further analysis of cell behavior within the layers at different chemical or oxygen concentrations[26]. By using the attributes of microfluidics, cell-embedded microparticles and fibers can also be fabricated. With proper controls of structure and adhesion peptides, in-vivo like cell functions are achieved from synthetic materials. For example, Fischbach et al. demonstrated that cancer cells angiogenic activity was significantly increased in 3D alginate ECM with arginylglycylaspartic acid(RGD)-peptide incorporation which is comparable with data from in vivo models[27].
2-4. Generation of 3D multicellular tumor spheroids
3D multicellular tumor spheroids are becoming an important research tool for cancer research as well as drug screening because they mimic aspects of the structures and functions of solid tumors in vivo. Spheroids were traditionally generated by culturing a few thousand cells on an ultra-low adhesion surface or in hanging drops on the underside of culture plate lids, thus only allowing cell-cell adhesion instead of cell adhesion to the surface. In addition, Liu et al. used an ultra-sound trap method to generate aggregates of HepG2 hepatocarcinoma cells, which helped sustain in vivo-like functionalities[28]. It is reported that extended culture of the generated tumor spheroids in vitro shows metabolic and proliferative gradients, similar to tumors in in vivo conditions[29]. However, traditional methods make it difficult to obtain uniform spheroid size, and the spheroids are difficult to handle for follow-up experiments such as drug screening. The multicellular tumor spheroids of different sizes might exhibit different drug resistances due to differences in the penetration of the drug, and thus affect the outcome of the screening.
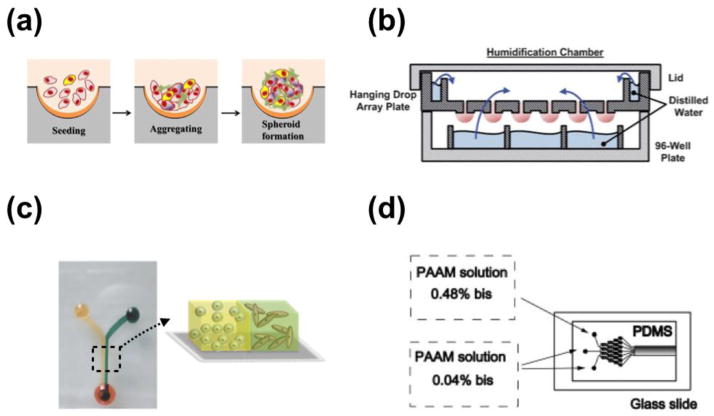
Recently, microscale systems have been developed to offer better control of spheroid size and to simplify operation. Microfabricated well arrays (Fig. 2a) were developed to increase the production of homogeneous tumor spheroids by using the geometrical confinement to trap similar numbers of cells in each well[30–34]. Other research groups have developed micro-chambers or micro-posts in microfluidic channels to trap clusters of cells to control spheroid size and simplify liquid handling procedures[35]. These methods successfully create in vivo-like tumor spheroids with defined size control, however, there are several limitations. For example, the operational processes are not compatible with HTS, the spheroids are composed of one cell type lacking paracrine interactions, and the spheroids are still suspended in a liquid medium lacking interaction with the ECM structure and proteins. To overcome the first two of these issues, Tung et al. developed a 384-format hanging drop array plate (Fig. 2b) that is compatible with existing liquid handlers and plate readers, and capable of incorporating multiple cell types (co-, or tri-cultures) into a single spheroid[36,37]. The study demonstrated that, compared to 2D cultures, 3D spheroids of the epithelial carcinoma cell line A431.H9 were more resistant to a conventional anticancer drug treatment (10 μM of 5-fluorouracil treatment), suggesting the presence of quiescent cells in the spheroids[36]. In order to achieve cell-ECM interaction, cell spheroids have been embedded in 3D ECM by using either microinjection of cells or magnetic force-based cell patterning for high-throughput quantitative cancer invasion screens[38]. In the microinjection platform, cell-polymer suspensions are micro-injected into multiwell plates containing a collagen gel at predetermined x-y-z positions. The pre-defined coordinates of each individual spheroid are compatible with fully automated imaging and image analysis, facilitating the practical implementation of this method as an HTS tool.
Fig. 2.
Representative microfluidic 3D systems. (a) schematic illustration of tumor spheroids generated in microfabricated well. Reproduced from Ref. [34] with permission. (b) Illustration of he hanging drop array plate to culture 3D spheroids. The 384 hanging drop array plate is sandwiched between a 96-well plate filled with distilled water and a standard-sized plate lid. Distilled water from the bottom 96-well plate and the peripheral water reservoir prevent serious evaporation of the small volume hanging drops. Reproduced from Ref. [36] with permission. (c) 3D micro-compartmentalization driven by laminar flow in microchannels. Reproduced from Ref. [40] with permission. Drops of cell containing polymer solutions are loaded onto the inlet ports. Laminar flow leads to two side-by-side 3D compartments. (d) Schematic diagram of the microfluidic gradient device used for photopolymerization of hydrogels. Reproduced from Ref. [101] with permission. the device consists of a pat- terned PDMS mold attached to an activated glass slide. All of the inlets are filled with a solution containing 8 % acrylamide and the photoinitiator. To generate a gradient in the crosslinker concentration, 0.04 % bis was added to two adjacent inlets, and 0.48 % bis to the third inlet.
3. Micro-compartmentalization for enhanced temporal and spatial control
3-1. Gradient of biochemical factors
Microfluidic compartmentalization provides unique functionality and is emerging as a useful tool to examine complex interactions of tumors and various microenvironmental components through improved spatial and temporal control. Micro-compartmentalization has been mostly achieved by creating confined and separate compartments in a single device. This is also achieved by utilizing laminar flow in the microsystem that allows parallel flow in the same compartment of different ECM solutions having matching viscosity (Fig. 2c). Once the ECM solutions are solidified after polymerization, the created compartments are stably maintained[39,40]. There is a wide variety of applications of compartmentalized systems in cancer biology, including studying cell migration and invasion, creating in vivo-like cellular arrangements and many others[39–43]. One useful advantage to 3D compartmentalization is that it can be used to investigate signaling mechanisms involved in chemotaxis[44]. The types of molecular transport such as diffusion are more controllable in microsystems due to the scale of the system and the ability to control the shape, length, and material of channels[45]. Traditionally, cell migration/invasion studies have been conducted using transwells, which is a vertical arrangement of compartments. This vertical arrangement makes it challenging to monitor changes in cells or the ECM during migration, and the number of cells that migrated can only be counted when the experiment is over. However, microfluidic chemotaxis platforms typically have a horizontal arrangement of compartments allowing better monitoring of changes in cells and the ECM during migration. Therefore, researchers using these platforms can conduct more comprehensive analyses on aspects of cell migration such as migration rate, morphology, different sub-populations of cells and the number of cells that have migrated.
3-2. Gradient of biophysical factors
The generation of gradients in 3D microfluidic systems is not limited to the gradient of soluble factors. Gradients of mechanical properties in the ECM such as stiffness and porosity have been achieved in microfluidic systems[46]. It is now well established that cells are capable of sensing mechanical properties of ECMs and respond differently to various mechanical properties[47]. Mechanical interactions between cancer cells and the ECM can accelerate cancer progression. For instance, it has been found that breast carcinogenesis is accompanied by collagen crosslinking, ECM stiffening, and increased focal adhesions[11]. Previous in vitro 3D experiments conducted by Kass et al. demonstrated that stiffening the ECM through an incremental increase in collagen concentration resulted in a more malignant morphology of mammary epithelial cells[48]. Moreover, the rigidity of collagen and collagen fibers perpendicularly aligned to the breast tumor interface promotes the invasion of the tumor cells into the surrounding stroma[9,10]. However, in order to explore any influence caused by mechanical properties of ECMs such as durotaxis, where cell migration is guided by substrate rigidity, it is necessary to generate rigidity gradients in a single well. This approach is not possible in traditional 3D methods which use individual plate wells that can only include a single ECM stiffness at a time. Since studies show that substrate rigidity changes affect malignancy, using a uniform matrix may result in missing this important feature of cancer progression. Microfluidic systems have shown to be promising tools for overcoming this challenge. Zaari et al. have created a stable stiffness gradient of polyacrylamide (PAAM) hydrogel by varying the monomer (acrylamide solution) to crosslinker (0.04% ~ 0.48% bis) ratio using a microfluidic gradient device{Zaari:2004wf}. The microfluidic gradient device used a network of microchannels having three inlets (Fig. 2d), where 0.04% bis was added to two adjacent inlets and 0.48% bis was added to the third inlet to generate a gradient in the crosslinker concentration. As the fluid streams traveled down the network, they were repeatedly split, mixed, and recombined, which eventually establish a chemical gradient across the channel, perpendicular to the flow direction [49,50]. Upon attaining a stable flow in the outlet, the device was exposed to UV for 5 minutes to polymerize the solution. In addition, the desired concentration and stiffness gradients of other materials such as collagen, PEG-DA hydrogel, or Hyaluronic acid-gelatin, are achievable in microfluidic channels by using fluidic shear-driven stretching. Molecules in the center of the channel move faster than a molecule at the channel wall, eventually creating a laterally averaged concentration profile along the channel once polymerization has occurred[46]. Once the gradient of mechanical properties of ECM is successfully created, the systems are used to investigate mechanisms regulating the attachment, growth, invasion/migration of various cells. Using high-resolution imaging technology, such as confocal microscopy or second harmonic generation, physical alterations caused by cell-ECM interactions are monitored[51–54]. Further development and modification of such systems can be employed to investigate mechanical interactions between cells and ECMs and may provide new insights into cancer progression.
3-3. In vivo-like cellular arrangement
Another benefit of micro-compartmentalization in cancer research is the ability to create in vivo-like cellular arrangements in vitro. Tissues are composed of many different cell types that are compartmentalized according to their function within the tissue. Many tissues are highly organized and display clear barriers between regions of the tissue. For example, normal mammary ducts, made up of epithelial cells, are separated from the surrounding stroma by a basement membrane, creating a physical barrier between the two different tissue components. Microtechnology has enabled the recapitulation of compartmentalized in vivo-like environments in vitro by utilizing channel geometry and the physical attributes of microsystems. Zervantonakis et al. created an in vitro 3D microfluidic model of the tumor-vascular interface integrating enhanced in situ monitoring and precise control of microenvironmental components[41]. The study showed that this microfluidic system allowed the regeneration of in vivo like endothelial barrier functions such as permeability. The compartmentalization enabled the incorporation of the third cell type, macrophages, which in turn directed tumor intravasation. Furthermore, 3D microcompartmentalization enables a more precise investigation of distance dependency between tumor and stromal cells. A recent study by Sung et al. demonstrated that breast cancer progression from DCIS to IDC was dependent on the distance between cancer cells and stromal cells. In a single microchannel, cancer cells in closer proximity to stromal fibroblasts showed a more invasive transition while the cancer cells further away from the fibroblasts remained non-invasive[40]. The cancer cells at the interface with fibroblasts were in physical contact with the fibroblasts, which might be an important factor in the progression of cancer subsequent to the initiation by soluble factors. This finding has brought interesting insights into the understanding of the process of cancer progression. That is, the progression from DCIS may be initiated by soluble factors, but physical contact with fibroblasts is maybe required for progression to IDC.
4. Microsystems mimic in vivo complexity and organ level functions
4-1. Tissue-like structures to mimic in vivo-like cellular organization
Continued development and integration of microtechnology with 3D cancer biology has provided new opportunities to generate 3D in vitro cancer models that allow the creation of more complex 3D tissue-level architecture. These models are designed to enable more in depth exploration of functions and interactions compared to traditional methods. Microfabrication and unique physical properties of fluids in microchannels enable fabrication of hollow shaped, or duct-like, structures in vitro, such as mammary duct and blood vessels, showing in vivo like functionalities. By using 3D mouse mammary ducts created by micropatterning of collagen gel (Fig. 2d), Nelson et al. found that the geometry of in vitro mammary ducts dictated the branching position of epithelial cells by distributing different amounts of proteins, such as transformation growth factor-beta (TGF-beta), inhibiting mammary branching morphogenesis[55]. These results demonstrate the importance of tissue geometry during organ morphogenesis and its role in defining the local cellular microenvironment. The system designed by Nelson et al., however, has squared and closed lumens in which continuous flow cannot occur as it would in a natural open and circular lumen. To overcome these issues, other microfluidic systems have been introduced to create duct-like structures by using either microchannel geometry, gel patterning, fluid dynamics, or microfiber generation[56,57]. For example, Bischel et al. created a circular shaped lumen system by using a simple straight microchannel and a viscous fingering method, in which a less viscous solution tunnels through the center of a more viscous solution[58,59]. This system has successfully generated blood vessels in vitro after lining the lumen with endothelial cells. The operation of the viscous fingering method is readily compatible with existing HTS infrastructure as the generation of the entire lumen is achieved via simple pipetting.
4-2. Microfluidic 3D systems for investigating the effect of continuous flow
In duct-like structures such as mammary ducts and blood vessels, continuous luminal flow naturally occurs and is considered an important factor regulating cell-cell and cell-ECM signaling in in vitro settings. For example, in blood vessels, it is likely that fluid shear stress mediates endothelial cell transcription, proliferation, barrier function, and changes in actin cytoskeleton rearrangement[60–64]. Song et al. found that, using a 3D microfluidic vessel model, fluid shear stress exerted by a stream of liquid reduces the number of VEGF-driven sprout vessel formation from endothelial cells which could easily be explored in cancer angiogenesis models[65]. In addition to the luminal flow, interstitial flow in 3D cancer models is also a major factor in stimulating cancer cell migration. Interstitial flow is the main extracellular fluid that exists in the interstitial spaces between tissues to provide the cells with nutrients and a means of waste removal. It has been recently demonstrated that interstitial flow can direct the migration of invasive cancer cells[66], and more importantly, the fluid flow from the blood to the lymphatics can be dramatically increased in cancer and during inflammation[67]. In order to understand the complex mechanisms pertaining to both luminal and interstitial flow using in vitro systems, researchers need more sophisticated tools that will allow the examination of myriad microenvironmental factors such as cell density, cell type, flow velocity, ECM composition/density, and the concentration of signaling molecules. In addition to the microenvironmental components influencing the flow, the local fluid shear stress within microvascular systems is strongly influenced by the intricate architecture of the micro-vasculature networks. Accordingly, several researchers have created 3D microchannel networks replicating some architectural features of microvascular systems[68–72]. Microfluidic 3D in vitro cancer models are arising as very useful tools to meet these needs. These microfluidic 3D systems can incorporate various microenvironmental components into a single system and will allow simultaneous monitoring of flow and cell movement providing a more comprehensive understanding of the effect of continuous flow in tumor microenvironments.
4-3. Organ-on-a-chip to mimic organ-level functions
The continued integration of microfabrication, 3D biology, and microfluidics has led to the development of an organ-on-a-chip [73]. An organ-on-a-chip is a 3D microfluidic cell culture device that mimics some of the functions of a biological organ. Organs in the human body are formed by multiple tissues that serve a common function. To recapitulate organ-level functions in vitro, various organ-specific dynamic functions such as spatiotemporal chemical gradients and mechanical forces such as cyclic strain, compression, and fluid shear stresses need to be integrated. Even though the advances in 3D microfluidic models have made considerable contributions toward mimicking tissue-level structures and functions, most existing models still fail to fully mimic the organ-specific functions in the in vivo microenvironment. This lack of proper in vitro systems consequently results in the use of animal models when investigating organ-level interactions, which are expensive, time consuming, imprecise because animals differ greatly from humans, and are surrounded by ethical issues. The development of humanized 3D in vitro models that feature organ-level functions will greatly impact the capability to understand human pathophysiology. Along these lines, a mechanically active microsystem reconstituting the critical functional alveolar-capillary interface of the human lung has been developed[74]. The system is able to successfully simulate the mechanical contraction and expansion that enables the lung to intake nanoparticulates and stimulate their transport into the underlying microvascular channels. Additionally, many microfluidic 3D systems have been introduced in attempts to recreate structures and functions of various organs such as brain, gut, liver, heart, kidney, and breast[75–81]. Even though the current focus of these organ-on-a-chip systems is to provide low-cost alternatives to animals for drug screening and toxicology applications, the continued development of such systems has a potential to bring a significant impact to cancer research, for example, by providing cytotoxicity information on cancer drugs.
4-4. Body-on-a-chip to recapitulate multi-organ interactions
One practical goal of microfluidic in vitro 3D cancer models is to recreate in vivo-like structures and functions to provide a more extensive understanding of complex interactions in the tumor microenvironment. These models can be employed as drug screening platforms to predict human drug responses. However, most of these models focus on cell or tissue-level interactions and disregard multi-organ interactions which is particularly important when attempting to understand the response of the human body to pharmaceuticals or pharmaceutical interactions[82]. Micro cell culture analogs (μCCA), a.k.a body-on-a-chip, have been recently developed to mimic interactions between organs (Fig. 3). The μCCA combines micro-compartmentalization, the 3D in vitro model, and a circulatory system. The μCCA is composed of a series of interconnected compartments representing different organs such as liver, marrow, and tumor (e.g. colon cancer) to provide a platform for mimicking pharmacokinetic and pharmacodynamic profiles of a drug in humans[83]. The compartments are connected by fluid channels mimicking circulating blood flow. As an initial testing of the system, the cytotoxic effect of Tegafur, an oral prodrug of 5-fluorouracil (5-FU), on each cell line was tested using the μCCA with cell-embedded hydrogel. Interestingly, the μCCA was able to reproduce the metabolism of Tegafur to 5-FU in the liver and consequent death of cells by 5-FU. This result was not obtainable from the same samples in a 96-well plate. Even though the μCCA is still in its early stage of development, the data demonstrate the importance of organ level interactions in predicting drug response. Given the fact that it is impossible to recapitulate the entire human body in vitro, the body-on-a-chip concept that incorporates key organs onto a single screening platform is an important step forward in development of alternatives to animal models.
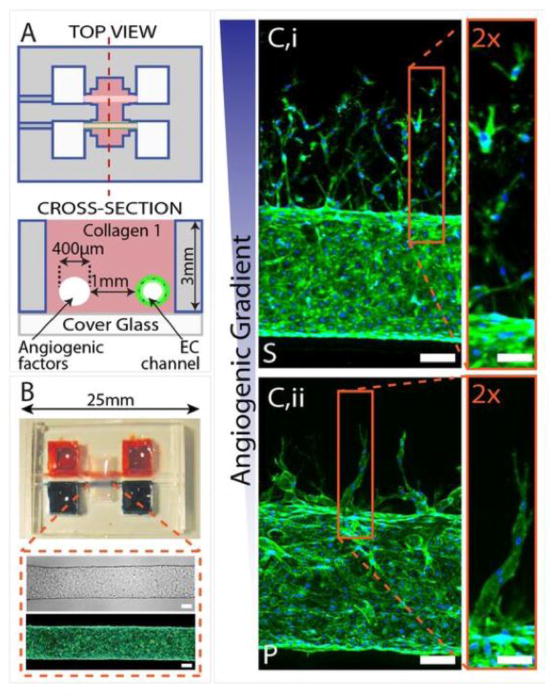
Fig. 3.
Three-dimensional formation of endothelial sprouts and neovessels in a microfluidic device. (A) Device schematic. Parallel cylindrical channels are encased in a 3D collagen matrix within a microfabricated PDMS gasket and connected to fluid reservoirs. One channel is coated with endothelial cells and perfused with medium and the other channel is perfused with medium enriched with angiogenic factors. (B) Photograph of the device. Zoom shows phase (Upper) and fluorescent (Lower) micrographs of an endothelialized channel. F-actin and nuclei are labeled with phalloidin (green) and DAPI (blue), respectively. (C) Representative confocal immunofluorescence images of sprouting and migrating endothelial cells in response to gradients of different proangiogenic factors. Reproduced from Ref. [102] with permission.
5. Practical applications and current challenges
The unique functionalities of 3D microfluidic systems enables many new avenues in cancer research that have been difficult to explore using traditional 3D cancer models. New findings, insights, and applications have been achieved via the emerging integration of 3D microfluidic systems and 3D cancer biology. Importantly, 3D microfluidic systems provide more visual and quantitative evidence of cancer development and progression in more complex microenvironments because the morphology, migration, and proliferation of cells can be monitored and analyzed more efficiently with enhanced throughput. Moreover, in microfluidic 3D systems, many microenvironmental factors such as cell density, the number of cell types, spatial arrangement, and ECM properties become more controllable. Several such systems have already been commercialized (summarized in Table 2). For example, the iuvo™ system is being used by researchers for a variety of 3D assays including viability, migration/invasion, and drug toxicity (http://www.bellbrooklabs.com/products-services/iuvo-microconduit-array-platform/3d-cell-based-assay-service). Likewise, ibidi® provides several μ-slides for 3D applications including chemotaxis and angiogenesis (http://ibidi.com/applications/cell-based-microscopy-assays/3d-cell-microscopy/). Another example is the Perfecta3D® Hanging Drop Plates, which target both basic cancer research and drug screening (http://3dbiomatrix.com/store/perfecta3d-hanging-drop-plates/hdp1384/).
Table 2.
Summary of commercially available microscale 3D in vitro systems.
| Company/Product | Advantages | Applications |
|---|---|---|
| BellBrook Labs/luvo 3D cell-based assays |
|
|
| lbldi/μ-slides |
|
|
| 3D Biomatrix/Perfecta3D Hanging Drop Plates |
|
|
One of the most practical applications of microfluidic 3D cancer systems is in the drug screening and development area. The successful integration of microfluidics, 3D cancer biology, and HTS infrastructure can provide alternatives to animals and humans in the drug development process, specifically, the screening of chemicals cytotoxicity. The current drug development process heavily relies on animal trials and subsequent human clinical trials. A major draw back to using animal models for drug screening is that animal metabolism and cellular response to chemical signals can differ considerably from those in humans[82]. This could partly explain the fact that only 19% of new investigational drug compounds were clinically approved for human use during the study period of 1993–2004[84,85]. If the drug development process were supported by more “humanized” in vitro models[86,87] that could enhance the predictability of new drug compounds in humans, a considerable amount of time, money, and effort could be saved.
Strikingly, oncology has one of the lowest clinical success rates for investigational drugs. The success rate for oncology drugs is more than three times lower than drugs for cardiovascular disease[85,88]. Given that cancer remains the second most common cause of death in the US in 2013, accounting for nearly 1 of every 4 deaths, it is critical to find out why it is so difficult to develop anti-cancer drugs and to treat cancer properly[89]. It has been discussed that part of the reason for the big failure is the lack of proper “humanized” in vitro testing models accommodating the complexity and heterogeneity of human tumor microenvironments within a single patient as well as among different patients. In addition to microenvironmental heterogeneity, tumors themselves are composed of heterogeneous sub-populations of epithelial cells. Current treatment strategies have been developed to specifically target only certain type of cancer cells. Due to the heterogeneity in tumors, it is highly possible that there will be remaining cancer cells that are not affected by or are resistant to certain doses of a drug. In addition, it is inevitable that some normal cells may also be affected by the treatment, which could also cause detrimental side effects. Therefore, clinicians rely on drug dose-response in normal and tumor tissues to provide a therapeutic window. More importantly, this heterogeneity highlights the need for better screening platforms to better predict cellular responses to treatments.
3D in vitro cancer models have the potential to lead to enhanced predictability of new drug compounds. As a result of mounting evidence from multiple studies, it is now more widely accepted that tumor cells grown in 2D vs. 3D exhibit different responses to the same concentration of drug compounds. That is, tumor cells in 3D conditions are more likely to be resistant to drug compounds. This could be due to different levels of oxygenation in tumor clusters and changes in integrin-based signaling in 3D conditions [90,91]. Even though still in the early stages, several pharmaceutical companies are moving toward adapting 3D in vitro cancer models as anti-cancer drug testing tools. The introduction of microfluidic 3D in vitro cancer models expands the utility of previous 3D in vitro drug screening platforms. Microfluidic 3D cancer models that incorporate stromal cells and various ECM conditions as well as tumor cells while closely mimicking in vivo-conditions will advance our ability to rapidly screen the microenvironment. These microfluidic 3D cancer models will also broaden our understanding of cancer cell/stromal cell/ECM interactions impact cancer development and treatment.
One of the current challenges of microfluidic 3D models is the establishment of reliable endpoint readouts that can be automated and be compatible with high-throughput and high-content analyses. Currently, various microfluidic platforms are being developed to be more user-friendly and automation-compatible for use as HTS tools. However, in order to move one-step forward from the research and development stage of microfluidic 3D models to widespread use in clinical labs and pharmaceutical industries, it is important to improve methods for imaging, detecting, and quantifying signals. The most common methods used with 3D in vitro screening include the quantification of the proliferation and metabolic activity of cancer cells. Both methods collect integrated signals from individual channels, but detailed information is lost. For example, cell-ECM interactions and morphological changes of cells cannot be obtained. One benefit of a 3D in vitro system is that cell and ECM changes can be monitored and quantified more accurately. This could provide crucial information regarding cancer development or responses to certain drug treatments. However, the process is still not compatible with HTS. In attempts to overcome this issue, several research groups have developed various quantitative endpoint and analysis tools to be used in conjunction with high-resolution imaging technology such as confocal microscopy, optical coherence tomography (OCT) and second harmonic generation (SHG). OCT is a non-invasive optical signal acquisition and processing method that uses light waves to take high resolution 3D images of tissues[92]. Because OCT can provide cross-sectional images of tissue structure on the micron scale in situ and in real time, this imaging technology has been applied to various in vitro and in vivo applications as well as clinical applications[93]. SHG is a nonlinear optical method where the emitted light has exactly half the wavelength of the two incident photons. Because collagen is one of the strongest harmonophores, SHG has been widely used to image collagen and capture intrinsic characteristics of collagen networks. To be practical, these imaging techniques need to be accompanied by automated image analysis methods in order to analyze the large amount of information that is obtained via this high-content imaging technique. The development of efficient algorithms allowing automated image analysis will alleviate the bottleneck of information when analyzing images after high-resolution imaging.
Several researchers have integrated the high-resolution imaging technologies with 3D in vitro system to enable quantitative high-content screenings. Klein et al. optimized OCT systems for use with in vitro 3D systems to conduct high-content therapeutic screens[94]. Using the optimized OCT system, the cytotoxic effect of photodynamic therapy (PDT) to ovarian cancer cells, OVCAR-5, was continuously monitored during 24 hrs and was evaluated by quantifying cancer cells surface area per unit volume. Sung et al. used the area-based SHG intensity analysis to further define the invasive progression of the DCIS clusters in microfluidic compartmentalized 3D in vitro model[40]. The area-based analysis quantified the percentage of the collagen area affected by DCIS cells, the percentage of which increased as the DCIS clusters became invasive. The ability to compartmentalize by cell type facilitates readouts from one compartment without image overlap between cell types, thereby improving the signal and simplifying image analysis. In order to automate the quantification of collagen analysis, a new software tool such as CurveAlign was developed[95]. CurveAlign employs the curvelet transform that detects both the scale and orientation of the edges. These analyses can be automated and have potential to enable high-content and high-throughput analyses. The combination of high resolution imaging techniques and computational analysis help to make 3D microfluidic in vitro platforms practical and will provide a tool to quantitatively monitor changes in the tumor microenvironments.
6. Future directions: Microfluidic 3D in vitro cancer models will advance alongside advancements in tissue engineering and biomaterials science
As discussed above, the significant advances in microfluidic 3D in vitro cancer models have provided new capabilities to understand cancer and enabled new avenues of cancer research that had been almost impossible previously. The recent incorporation of tissue engineering principles into microfluidic 3D in vitro cancer models has resulted in the evolution of practical in vivo-like in vitro cancer models. The models recapitulate tissue- or organ-level structures and functions while still allowing efficient and expansive investigation of the dynamics in tumor microenvironments. Traditional tissue regeneration strategies aim to provide the aspects of their original microenvironments necessary to reconstruct the structure and functions associated with a desired tissue. Likewise, the design of tumor microenvironments in vitro, a.k.a. tumor engineering, needs to incorporate aspects of in vivo tumor microenvironment. It is becoming appreciated that the mechanisms known to regulate tissue regeneration and wound healing could be similar to the mechanisms that regulate tumor proliferation and dissemination{Ghajar:2010bf}. Accordingly, tumor engineers who design microfluidic 3D in vitro cancer models need to continue to incorporate parallel advances in tissue engineering. This synergy will benefit both microfluidic tumor engineering and tissue engineering. Similar to microfluidic 3D cancer models and despite the considerable advances in tissue engineering, the tissue-engineered organs have seen limited clinical use due to several challenges. As microscale technologies allow improved control over cellular microenvironments in a high-throughput manner, microfluidic 3D in vitro models can provide a greater understanding of biological mechanisms and better guide the design of more physiologically compatible systems[96]. For both microfluidic tumor engineering and tissue engineering, the contribution from biomaterials science is indispensable. Particularly, in order to construct physiologically relevant 3D tissue in vitro, it is critical to employ suitable materials with the desired degradation rates, products, and suitable mechanical properties for the desired tissue. Smartly designed biomaterials can also be used as a sink or source of signaling molecules such as growth factors within 3D in vitro models[97,98]. Moreover, the ECM can be designed to be cleaved only by specific proteases, which will help better understand relevant signaling mechanisms in cell-ECM interactions[99]. Likewise, the attributes of microfluidic 3D systems have expanded the utility of biomaterials by modulating chemical and mechanical properties of biomaterials, which is difficult to achieve by biomaterials alone. The future of microfluidic 3D in vitro cancer models needs to be accompanied by the progressive development of other disciplines such as tissue engineering and biomaterials science in order to maximize the utility and functionality of the models. Multidisciplinary approaches should also facilitate the translation of the technologies into clinical research.
7. Conclusions
While there are still many remaining questions that need to be answered, it is true that leading questions in cancer biology are becoming more tractable via capabilities made possible by new microfluidic 3D in vitro cancer models. Because of the heterogeneity and complexity of tumor microenvironments, approaches that consider multiple parameters of the microenvironment are necessary. That is, disrupting multiple key interactions between tumors and the microenvironment may offer more effective therapeutic strategies. Innovative technologies that enable efficient high-content analyses while quantitatively manipulating key microenvironmental parameters are needed to shed light on the complex regulation of cancer initiation and progression. One important question is whether we need a complex in vitro system. If complexity is desired, how complex does it have to be to obtain reliable data to yield promising target molecules or provide better predictability of drug performance? The answer may depend on the goal of the experiments or screenings. Most microfluidic 3D in vitro cancer systems could be used in the earliest stages of drug development, typically to identify drug candidates by providing more mechanistic information on cancer development and progression. Such systems may not require organ-level functions or multi-organ interactions. In such cases, it may be more important to focus on cellular level interaction in a more in vivo like environment. However, in order to explore drug toxicity, it may be important to incorporate organ level functions and multi-organ interactions. The continued development and integration of microtechnology, 3D cancer biology, drug screening, and clinical testing will bring significant contributions toward a deeper understanding of the complexity and heterogeneity of cancers and how to successfully treat the cancers.
Fig. 4.
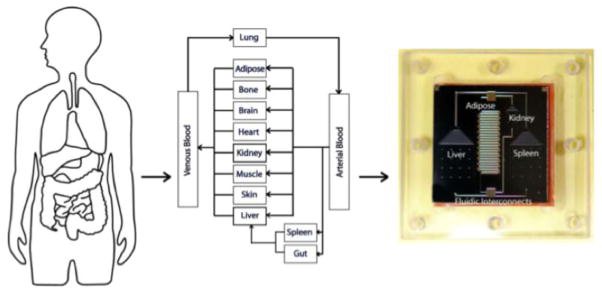
Concept of μCCA development. The human body can be simulated as a series of interconnected compartments. Each organ is represented by a compartment and treated as a chemical reactor, absorber, or holding tank (depending on its function in the body). Reproduced from Ref. [82] with permission.
Table 1.
The major differences of microscale 2D and 3D systems
| microscale 2D system | microscale 3D system | |
|---|---|---|
| Differences between corresponding macro systems |
|
|
| Dominant molecule transport mechanism | diffusion-dominant | |
| Use of ECM proteins |
|
|
| HTS accessibility | compatible | |
| Imaging and analysis | compatible with various imaging technologies and analysis algorithms that are currently available | |
Acknowledgments
This study was supported by NIH grants (K25-CA104162, R01CA107012, 1R33CA137673-01, and R01EB010039BRG), and National Science Foundation award (EFRI-1136903). David J. Beebe has an ownership interest in Bellbrook Labs LLC, which has licensed technology reported in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 4.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo DL. Tumor heterogeneity and personalized medicine. N Engl J Med. 2012;366:956–957. doi: 10.1056/NEJMe1200656. [DOI] [PubMed] [Google Scholar]
- 7.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthuswamy SK. 3D culture reveals a signaling network. Breast Cancer Res. 2011;13:103. doi: 10.1186/bcr2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provenzano PP, Inman D, Eliceiri K, Knittel J, Yan L, Rueden C, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ. Understanding the Impact of 2D and 3D Fibroblast Cultures on In Vitro Breast Cancer Models. PLoS ONE. 2013;8:e76373. doi: 10.1371/journal.pone.0076373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/S0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 14.Arora PD, Narani N, McCulloch CAG. The compliance of collagen gels regulates transforming growth factor-β induction of α-smooth muscle actin in fibroblasts. The American Journal of Pathology. 1999;154:871. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada K, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 17.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi J-P, Knuuttila M, et al. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS ONE. 2010;5:e10431. doi: 10.1371/journal.pone.0010431.g008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Meyvantsson I, Shkel I, Beebe D. Diffusion dependent cell behavior in microenvironments. Lab Chip. 2005;5:1089–1095. doi: 10.1039/b504403k. [DOI] [PubMed] [Google Scholar]
- 20.Domenech M, Bjerregaard R, Bushman W, Beebe DJ. Hedgehog signaling in myofibroblasts directly promotes prostate tumor cell growth. Integrative Biology. 2012 doi: 10.1039/c1ib00104c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer M, Su G, Beebe DJ, Friedl A. 3D microchannel co-culture: method and biological validation. Integrative Biology. 2010;2:371–378. doi: 10.1039/c0ib00001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung KE, Su G, Pehlke C, Trier SM, Eliceiri KW, Keely PJ, et al. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials. 2009;30:4833–4841. doi: 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P, Lin R, Moon J, Lee LP. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed Microdevices. 2006;8:35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- 24.Montanez-Sauri SI, Sung KE, Berthier E, Beebe DJ. Enabling Screening in 3D Microenvironments: Probing Matrix and Stromal Effects on the Morphology and Proliferation of T47D Breast Carcinoma Cells. Integrative Biology. 2013;5:631–640. doi: 10.1039/c3ib20225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su G, Sung KE, Beebe DJ, Friedl A. Functional screen of paracrine signals in breast carcinoma fibroblasts. PLoS ONE. 2012;7:e46685. doi: 10.1371/journal.pone.0046685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derda R, Laromaine A, Mammoto A, Tang SKY, Mammoto T, INGBER DE, et al. Paper-supported 3D cell culture for tissue-based bioassays. Proceedings of the National Academy of Sciences. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Kuznetsova LA, Edwards GO, Xu J, Ma M, Purcell WM, et al. Functional three-dimensional HepG2 aggregate cultures generated from an ultrasound trap: comparison with HepG2 spheroids. J Cell Biochem. 2007;102:1180–1189. doi: 10.1002/jcb.21345. [DOI] [PubMed] [Google Scholar]
- 29.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. 2004;9:273–285. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- 30.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296–4303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- 31.Torisawa YS, Takagi A, Nashimoto Y, Yasukawa T, Shiku H, Matsue T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials. 2007;28:559–566. doi: 10.1016/j.biomaterials.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 33.Markovitz-Bishitz Y, Tauber Y, Afrimzon E, Zurgil N, Sobolev M, Shafran Y, et al. A polymer microstructure array for the formation, culturing, and high throughput drug screening of breast cancer spheroids. Biomaterials. 2010;31:8436–8444. doi: 10.1016/j.biomaterials.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Yeon SE, No DY, Lee SH, Nam SW, Oh IH, Lee J, et al. Application of concave microwells to pancreatic tumor spheroids enabling anticancer drug evaluation in a clinically relevant drug resistance model. PLoS ONE. 2013;8:e73345. doi: 10.1371/journal.pone.0073345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu LY, Di Carlo D, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008;10:197–202. doi: 10.1007/s10544-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 36.Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao AY, Tung YC, Qu X, Patel LR, Pienta KJ, Takayama S. 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol Bioeng. 2012;109:1293–1304. doi: 10.1002/bit.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truong HH, de Sonneville J, Ghotra VPS, Xiong J, Price L, Hogendoorn PCW, et al. Automated microinjection of cell-polymer suspensions in 3D ECM scaffolds for high-throughput quantitative cancer invasion screens. Biomaterials. 2012;33:181–188. doi: 10.1016/j.biomaterials.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 39.Wong A, Perez-Castillejos R, Love J, Whitesides G. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials. 2008;29:1853–1861. doi: 10.1016/j.biomaterials.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, et al. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integrative Biology. 2011;3:439–450. doi: 10.1039/c0ib00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaji H, Camci-Unal G, Langer R, Khademhosseini A. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbagen.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim HY, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9:1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amadi OC, Steinhauser ML, Nishi Y, Chung S, Kamm RD, Mcmahon AP, et al. A low resistance microfluidic system for the creation of stable concentration gradients in a defined 3D microenvironment. Biomed Microdevices. 2010;12:1027–1041. doi: 10.1007/s10544-010-9457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthier E, Surfus J, Verbsky J, Huttenlocher A, Beebe D. An arrayed high-content chemotaxis assay for patient diagnosis. Integr Biol (Camb) 2010;2:630–638. doi: 10.1039/c0ib00030b. [DOI] [PubMed] [Google Scholar]
- 46.Du Y, Hancock MJ, He J, Villa-Uribe JL, Wang B, Cropek DM, et al. Convection-driven generation of long-range material gradients. Biomaterials. 2010;31:2686–2694. doi: 10.1016/j.biomaterials.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo C, Wang H, Dembo M, Wang Y. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: Influence of extracellular matrix composition and organization during development and tumorigenesis. The International Journal of Biochemistry & Cell Biology. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeon NL, Dertinger SK, Chiu DT, Choi IS, Stroock AD, Whitesides GM. Generation of solution and surface gradients using microfluidic systems. Langmuir. 2000;16:8311–8316. [Google Scholar]
- 50.Kim S, Kim HJ, Jeon NL. Biological applications of microfluidic gradient devices. Integr Biol (Camb) 2010;2:584–603. doi: 10.1039/c0ib00055h. [DOI] [PubMed] [Google Scholar]
- 51.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayan C, Levitt JM, Miller E, Kaplan D, Georgakoudi I. Fully automated, quantitative, noninvasive assessment of collagen fiber content and organization in thick collagen gels. J Appl Phys. 2009;105:102042. doi: 10.1063/1.3116626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legant WR, Chen CS, Vogel V. Force-induced fibronectin assembly and matrix remodeling in a 3D microtissue model of tissue morphogenesis. Integrative Biology. 2012;4:1164–1174. doi: 10.1039/c2ib20059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DHT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bischel LL, Lee SH, Beebe DJ. A Practical Method for Patterning Lumens through ECM Hydrogels via Viscous Finger Patterning. J Lab Autom. 2012;17:96–103. doi: 10.1177/2211068211426694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bischel LL, Young EWK, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Cardeña G, Comander J, Anderson KR, Blackman BR, Gimbrone MA. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price GM, Wong KHK, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials. 2010;31:6182–6189. doi: 10.1016/j.biomaterials.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito K, Sakamoto N, Ohashi T, Sato M. Effects of frequency of pulsatile flow on morphology and integrin expression of vascular endothelial cells. Technol Health Care. 2007;15:91–101. [PubMed] [Google Scholar]
- 64.Blackman BR, García-Cardeña G, Gimbrone MA. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 65.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proceedings of the National Academy of Sciences. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shieh AC, Rozansky HA, Hinz B, Swartz MA. Tumor Cell Invasion Is Promoted by Interstitial Flow-Induced Matrix Priming by Stromal Fibroblasts. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 67.Haessler U, Teo JCM, Foretay D, Renaud P, Swartz MA. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integrative Biology. 2012;4:401–409. doi: 10.1039/c1ib00128k. [DOI] [PubMed] [Google Scholar]
- 68.Bellan LM, Singh SP, Henderson PW, Porri TJ, Craighead HG, Spector JA. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;5:1354–1357. doi: 10.1039/B819905A. [DOI] [Google Scholar]
- 69.Huang JH, Kim J, Agrawal N, Sudarsan AP, Maxim JE, Jayaraman A, et al. Rapid Fabrication of Bio-inspired 3D Microfluidic Vascular Networks. Advanced Materials. 2009;21:3567–3571. doi: 10.1002/adma.200900584. [DOI] [Google Scholar]
- 70.Carugo D, Capretto L, Willis S, Lewis AL, Grey D, Hill M, et al. A microfluidic device for the characterisation of embolisation with polyvinyl alcohol beads through biomimetic bifurcations. Biomed Microdevices. 2012;14:153–163. doi: 10.1007/s10544-011-9593-8. [DOI] [PubMed] [Google Scholar]
- 71.Borenstein JT, Tupper MM, Mack PJ, Weinberg EJ, Khalil AS, Hsiao J, et al. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed Microdevices. 2009;12:71–79. doi: 10.1007/s10544-009-9361-1. [DOI] [PubMed] [Google Scholar]
- 72.Prabhakarpandian B, Pant K, Scott RC, Pattillo CB, Patillo CB, Irimia D, et al. Synthetic microvascular networks for quantitative analysis of particle adhesion. Biomed Microdevices. 2008;10:585–595. doi: 10.1007/s10544-008-9170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huh D, Torisawa Y-S, Hamilton GA, Kim HJ, INGBER DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 74.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, INGBER DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim HJ, Huh D, Hamilton G, INGBER DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 76.Sung JH, Yu J, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 77.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip. 2011;11:4165. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grafton MMG, Wang L, Vidi PA, Leary J, Lelièvre SA. Breast on-a-chip: mimicry of the channeling system of the breast for development of theranostics. Integrative Biology. 2011;3:451–459. doi: 10.1039/c0ib00132e. [DOI] [PubMed] [Google Scholar]
- 79.Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 80.Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y, Williams JC, Johnson SM. Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues. Lab Chip. 2012;12:2103. doi: 10.1039/c2lc21142d. [DOI] [PubMed] [Google Scholar]
- 82.Shuler ML, Esch MB. Body-on-a chip: Using microfluidic systems to predict human responses to drugs. Pure and Applied Chemistry. 2010;82:1635–1645. doi: 10.1351/PAC-CON-09-10-44. [DOI] [Google Scholar]
- 83.Sung JH, Shuler ML. A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip. 2009;9:1385–1394. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- 84.DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in Risks Associated With New Drug Development: Success Rates for Investigational Drugs. Clin Pharmacol Ther. 2010;87:272–277. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- 85.Kola I, Landis J. Opinion: Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 86.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a Humanized In Vitro Blood? Brain Barrier Model to Screen for Brain Penetration of Antiepileptic Drugs, Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 87.Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010;10:241–253. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 88.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nature Reviews Drug Discovery. 2006;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 89.Cancer Facts & Figures 2013. American Cancer Society; 2013. pp. 1–64. [Google Scholar]
- 90.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 91.Loessner D, Rizzi SC, Stok KS, Fuehrmann T, Hollier B, Magdolen V, et al. A bioengineered 3D ovarian cancer model for the assessment of peptidase mediated enhancement of spheroid growth and intraperitoneal spread. Biomaterials. 2013;34:7389–7400. doi: 10.1016/j.biomaterials.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature Biotechnology. 2003;21:1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 93.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2:9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein OJ, Jung YK, Evans CL. Longitudinal, quantitative monitoring of therapeutic response in 3D in vitro tumor models with OCT for high-content therapeutic screening. Methods. 2013 doi: 10.1016/j.ymeth.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pehlke CA, Doot J, Sung KE, Riching KM, Nowak R, Provenzano PP, et al. Quantification of Collagen Architecture using the Curvelet Transform. n.d. Submitted. [Google Scholar]
- 96.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proceedings of the National Academy of Sciences. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature Biotechnology. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 98.Cornelia Wiegand U-CH. A superabsorbent polymer-containing wound dressing efficiently sequesters MMPs and inhibits collagenase activity in vitro. Journal of Materials Science Materials in Medicine. 2013;24:2473. doi: 10.1007/s10856-013-4990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwartz MP, Rogers RE, Singh SP, Lee JY, Loveland SG, Koepsel JT, et al. A Quantitative Comparison of Human HT-1080 Fibrosarcoma Cells and Primary Human Dermal Fibroblasts Identifies a 3D Migration Mechanism with Properties Unique to the Transformed Phenotype. PLoS ONE. 2013;8:e81689. doi: 10.1371/journal.pone.0081689.s030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2008;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaari N, Rajagopalan P, Kim SK, Engler AJ, Wong JY. Photopolymerization in microfluidic gradient generators: microscale control of substrate compliance to manipulate cell response. Adv Mater Weinheim. 2004;16:2133–2137. [Google Scholar]
- 102.Nguyen DHT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proceedings of the National Academy of Sciences. 2013;110:6712–6717. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]