Abstract
The kinetics of 14C fixation, and inorganic C (Cinorg) accumulation, have been followed in isolated pea mesophyll protoplasts. NaH14CO3 was supplied to the protoplasts in media the pH of which was varied between 7 and 8.
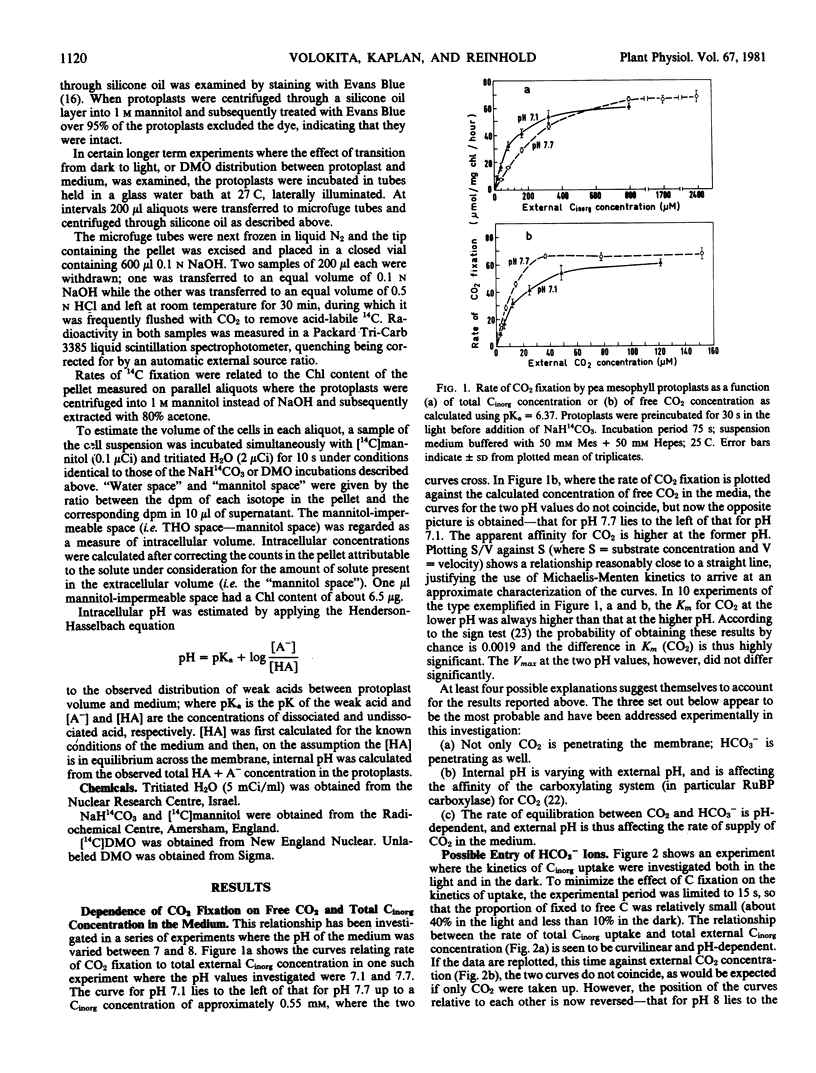
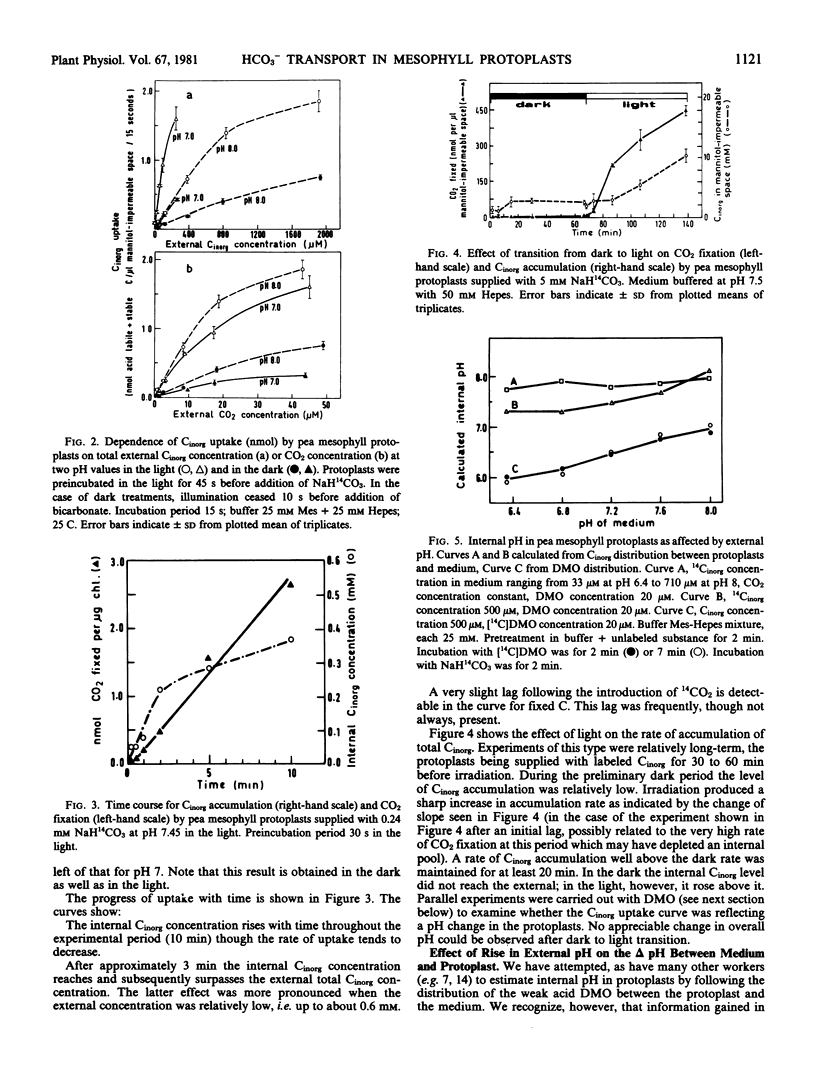
When 14CO2 fixation was plotted against the calculated concentration of free CO2 in the media, the apparent Km for CO2 was observed to rise as external pH increased. The Vmax did not alter significantly. Similarly, when Cinorg uptake, either in the light or in the dark, was plotted against external CO2 concentration the slope of the curves was steeper at higher external pH.
Investigation of the time course of uptake showed that internal Cinorg concentration rose throughout the experimental period, and that in the light it surpassed the external Cinorg concentration after about 3 minutes. Irradiation of protoplasts previously taking up 14Cinorg in the dark brought about a sharp increase in the rate of 14Cinorg accumulation which was sustained for at least 20 minutes.
Estimates of internal pH based on the distribution of labeled 5,5-dimethyloxazoladine-2,4-dione (DMO) between protoplast and medium suggested that internal pH altered relatively little with change in external pH. The values for internal pH as calculated from Cinorg distribution were always higher than those calculated from DMO distribution, i.e. the internal Cinorg concentration was higher than would be predicted on the assumption of passive distribution in accordance with pH.
Addition of carbonic anhydrase to the external solution was without effect either on rate of 14CO2 fixation or Cinorg accumulation.
Various possible interpretations of the results are considered. It is concluded that the most reasonable explanation, consistent with all the data, is that HCO3− ions can cross the protoplast membranes, and that their passage is mediated by a transfer mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Ion Transport in Isolated Protoplasts from Tobacco Suspension Cells: III. Membrane Potential. Plant Physiol. 1979 Dec;64(6):959–962. doi: 10.1104/pp.64.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Rahat M. Energization of the sugar transport mechanism in the plasmalemma of isolated mesophyll protoplasts. Plant Physiol. 1980 Mar;65(3):550–553. doi: 10.1104/pp.65.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P. Uptake of bicarbonate ion in darkness by isolated chloroplast envelope membranes and intact chloroplasts of spinach. Plant Physiol. 1974 Oct;54(4):520–526. doi: 10.1104/pp.54.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Gutknecht J. Solubility of carbon dioxide in lipid bilayer membranes and organic solvents. Biochim Biophys Acta. 1980 Mar 13;596(3):352–358. doi: 10.1016/0005-2736(80)90122-4. [DOI] [PubMed] [Google Scholar]
- Tenhunen J. D., Weber J. A., Yocum C. S., Gates D. M. Solubility of gases and the temperature dependency of whole leaf affinities for carbon dioxide and oxygen: an alternative perspective. Plant Physiol. 1979 May;63(5):916–923. doi: 10.1104/pp.63.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


