Abstract
The success of immunotherapeutic approaches targeting Glioblastomamultiforme demand a robust anti-glioma T cell cytotoxic and memory response. Recent evidence suggests that Rapamycin regulates T cell differentiation. Herein, we tested whether administration of Rapamycin could enhance the efficacy of immunotherapy utilizing Fms-like tyrosine kinase 3 ligand (Ad-Flt3L) and Thymidine kinase/Ganciclovir (Ad-TK/GCV). Using the refractory rat RG2 glioma model, we demonstrate that administration of Rapamycin with Ad-Flt3L + Ad-TK/GCV immunotherapy enhanced the cytotoxic activity of anti-tumor CD8+ T cells. Rats treated with Rapamycin + Ad-Flt3L + Ad-TK/GCV exhibited massive reduction in the tumor volume and extended survival. Rapamycin administration also prolonged the survival of Ad-Flt3L + Ad-TK/GCV treated GL26 tumor bearing mice, associated with an increase in the frequency of tumor-specific and IFN-γ+ CD8+ T cells. More importantly, Rapamycin administration even for a short interval elicited a potent long-lasting central memory CD8+ T cell response. The enhanced memory response translated to an increased frequency of tumor-specific CD8+ T cells within the tumor and IFN-γ release, providing the mice with long-term survival advantage in response to tumor rechallenge. Our data therefore points to Rapamycin as an attractive adjuvant to be used in combination with immunotherapy in a Phase I clinical trial for GBM.
Keywords: Glioblastoma, immunotherapy, HSV1-TK, Flt3L, memory T cells
Introduction
Malignant cancers of the central nervous system, i.e., glioblastoma multiforme grade IV (GBM), are characterized by a high degree of immune suppression, mediated by high levels of TGF-β and/or IL-10 which leads to an increase in T regulatory (Treg) cells, lack of lymphatic drainage, presence of the blood brain barrier and a paucity of antigen presenting cells such as dendritic cells (DCs) within the brain parenchyma (1–3). Taking into account these features, we developed a gene therapy mediated immune-stimulatory approach for GBM which relies on the expression of thymidine kinase (TK) that phosphorylates and activates the pro-drug ganciclovir (GCV) inducing the death of actively proliferating tumor cells with the concomitant release of the endogenous TLR ligand, High-mobility group B1 protein (HMGB1); and Fms-like tyrosine kinase 3 ligand (Flt3L) that increases the recruitment of dendritic cells (DCs) to the tumor microenvironment (4–7). HMGB1 induces the maturation of DCs with the upregulation of costimulatory ligands and MHC II molecules and release of inflammatory cytokines such as IL-12 and IFN-γ polarizing them towards an activated phenotype (8). The activated DCs subsequently prime anti-tumor T cell responses in the draining lymph nodes (dLNs). Cytotoxic CD8+ T cells are well recognized to mediate effective anti-tumor immunity (9). In addition, TH1 skewed CD4+ T cells also play an important role in enhancing the CD8+ T cell mediated anti-tumor immunity. DC vaccination strategies tested in glioma patients in Phase I clinical trials demonstrated significant cytotoxic T cell and memory T cell infiltration in areas of intracranial tumor (10, 11). Efforts have also been made to enhance the potency and selectivity of anti-glioma immune responses by adoptive immune therapy utilizing tumor-infiltrating lymphocytes (12). A 50% reduction in tumor size was observed in 2 out of 5 patients who were administered cytotoxic T lymphocytes generated by treatment with IL-2 and autologous glioma cells (13). Clinical trials have also tested the potency of cytotoxic cells generated by stimulation with phytohaemagglutinin, anti-CD3 antibodies and IL-2 or through the stimulation of peripheral blood lymphocytes with IFN-γ, IL-2, anti-CD3 antibodies and IL-1α (14, 15). Our own data has shown that modulating the T cell infiltration into the tumor microenvironment and enhancing T cell effector functions through the over expression of IFN-γ or IL-2 or inhibition of NF-κB signaling extended the median survival of RG2 tumor bearing rats as compared to Ad-Flt3L + Ad-TK/GCV treated group (16). Over expression of IFN-γ resulted in upregulation of MHC I expression on tumor cells potentially increasing tumor antigen presentation to T cells. Addition of Ad-IL-2 to the Ad-Flt3L + Ad-TK/GCV immunotherapy enhanced the ratio of cytotoxic T lymphocytes/Tregs resulting in augmented anti-tumor CD8+ T cell responses. Similar effects were obtained by the inhibition of NF-κB signaling that resulted in reduced Foxp3 expression in CD4+ T cells and enhanced IFN-γ release by CD8+ T cells (17).
An anti-tumor immune response that elicits tumor regression and long term survival requires the recruitment and expansion of powerful anti-tumor cytotoxic T lymphocytes. In addition, generation of anti-tumor memory T cell response is required for averting tumor relapse and it has been suggested that memory CD8+ T cells may determine the efficacy of anti-tumor therapies (18, 19). An agent that has recently been shown to regulate the magnitude and quality of T cell responses is Rapamycin (20). Rapamycin is a prototypic mTOR inhibitor that has been widely used clinically to prevent renal allograft rejection because of its immunosuppressive properties (21). Rapamycin’s ability to regulate Foxp3 expression, induce Treg differentiation and CD4+ T cell anergy is at least partially responsible for its tolerogenic effects (20) (22–24). Rapamycin also inhibits type I interferon production by pDCs (25). In contrast, several studies have highlighted the role of Rapamycin as an immune stimulatory agent. Rapamycin has been shown to enhance DC maturation and antigen presentation and promote the efficacy of autologous DC vaccination (26). Rapamycin through its effect on the mTOR signaling cascade has also been shown to promote cytotoxic CD8+ T cell responses and induce immunological memory in a T cell intrinsic manner (27) (19) (28). Also, memory T cells generated in the presence of Rapamycin display improved protective capacity against infections. Indeed, Rapamycin has been shown to potentiate the efficacy of vaccines targeting bacteria and viruses in mouse models (27, 29). This prompted the question whether Rapamycin and other mTOR inhibitors could be used to augment the efficacy of vaccines against tumors and in fact Temsirolimus, another mTOR inhibitor has been shown to enhance the anti-tumor effects of heat shock protein cancer vaccines (30).
Here we tested the hypothesis that administration of Rapamycin along with immune-mediated gene therapy would augment the cytotoxic and memory T cell response against GBM. Using two GBM models in rats (RG2) and mice (GL26) we show that Rapamycin enhances the cytotoxic activity of CD8+ T cells. More importantly it also promotes the development of CD8+ memory T cell response. Rapamycin administration for a short duration results in eliciting a vigorous long-lasting anti-glioma memory response. Consequently tumor bearing animals treated with immunotherapy in the presence of Rapamycin show better survival and respond more strongly to a second tumor challenge.
Materials and Methods
Syngeneic glioma models
All animal experiments were performed in accordance with protocols approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan. RG2 cells (20,000 in 3 µl DMEM) were stereotactically implanted in the right striatum of syngeneic Fisher rats (220–250 g; Harlan Laboratories Inc.) at the coordinates of 1.0 mm anterior and 3.2 mm lateral from the bregma and 6.0 mm ventral from the dura as previously described (16, 31). GL26 cells expressing ovalbumin were prepared as before (32). 60,000 GL26-OVA cells in 1µl DMEM were stereotactically implanted in the right striatum of syngeneic C57BL/6 mice (5–7 wk.; Harlan Laboratories Inc.) at the coordinates of 0.5 mm anterior and 2.1 mm lateral from the bregma and 3.2 mm ventral from the dura. Five days after tumor implantation, Fisher rats received an intra-tumoral injection of 3 µl of Ad vectors stereotactically using the same burr hole in four locations at 7.0 mm, 6.0 mm, 5.0 mm and 4.0 mm ventral from the dura for RG2 glioma model. C57BL/6 mice received 1.5µl of Ad vectors in three locations at 3.8 mm, 3.5 mm and 3.2 mm ventral from the dura at 14 days after tumor implantation. Animals were treated with 3 × 108 pfuof Ad-Flt3L or 1 × 108 pfuof Ad-TK or both Ad-Flt3L and Ad-TK. Animals treated with Ad-TK also received 25 mg/kg GCV twice daily for 7 days (mice) or 10 days (rats) starting the day after vector injection.
Rapamycin administration
1.5 mg/kg Rapamycin (LC Laboratories) was administered intraperitonially (i.p.) to the animals daily.
Quantification of DC maturation status
Antibodies for flow cytometry were obtained from BD Biosciences unless indicated otherwise. List of antibody clones used are indicated in supplementary table 1.1 × 106 Flt3L derived DCs were incubated for 48 hrs in media only or in the presence of 50 ng/mL of LPS (Invitrogen) or 10 ng/ml Rapamycin + 50 ng/ml LPS. 48 hrs later DC maturation markers CD80, CD86, MHCII, CD40 and CD25 were analyzed by flow cytometry. BMDCs were stained in FACS buffer (2% FBS in PBS) with anti-rat antibodies CD80, CD86, RT1B (MHC type II), CD40 (eBiosciences) and CD25 for 30 min at 4 °C. Data was acquired using a FACScan flow cytometer and analyzed with Summit software v4.3. 50,000 live cells were recorded for each sample. Release of IL-10, IFN-γ and IL-12 from BMDC was determined in culture supernatant by ELISA (IL-10 and IFN-γ, R&D system; IL-12, Invitrogen). Absorbance was read on Spectramax Plus (Molecular Device) plate reader.
Antigen-specific effector and memory T cell functions
Memory T cells in RG2 tumor bearing rats were analyzed at day 33 post tumor implantation. Single cell suspensions from spleen and dLNs were prepared as before followed by staining with CD3, CD8, CD45RC and CD62L antibodies (7). Memory responses were visualized by staining for CD45RC+ CD62Lhigh cells among the CD3+ CD8+ T cells in the spleen. At least 50,000 live cells (determined by FSC/SSC) were acquired for each spleen and LN sample. For GL26-OVA model, 24 days or 89 days after tumor implantation or 5 days after the second tumor challenge at 90 days (day 95), brains, spleens and dLNs were harvested from mice. Tumor infiltrating immune cells were isolated as before (7) and as described in supplementary text. Cells were counted and labeled with CD8 and CD3 antibodies. Stained cells were incubated with SIINFEKL-H2Kb-tetramer (Beckman coulter) for 30 min at room temperature after which cells were washed and analyzed by flow cytometry. Intracellular IFN-γ staining was performed using the BD Cytofix/Cytoperm kit (BD Biosciences). Antigen-specific memory responses were visualized by staining for CD44high CD62Lhigh cells among the tetramer+ CD3+ CD8+ T cells in the spleen and dLNs of immunized mice. Data was acquired using a FACScan (Beckton Dickinson) flow cytometer and analyzed using Summit software v4.3. At least 20,000 tetramer+ CD8+ T cells were acquired for each sample.
Statistical Analysis
All statistical analysis was carried out using the GraphPad Prism software (version 5.0a). Kaplan-Meier survival curves were analyzed using the Mantel log-rank test. Co-stimulatory ligand expression and cytokine release from DCs was analyzed by one-way ANOVA followed by Bonferroni’s post-test. Antigen-specific cytotoxic and memory CD8+ T cell responses were analyzed using one-way ANOVA followed by Bonferroni post-test. p values of < 0.05 were considered to be significant.
Results
Rapamycin enhances therapeutic efficacy of Ad-Flt3L+Ad-TK/GCV-mediated gene therapy in the RG2 intracranial glioma model
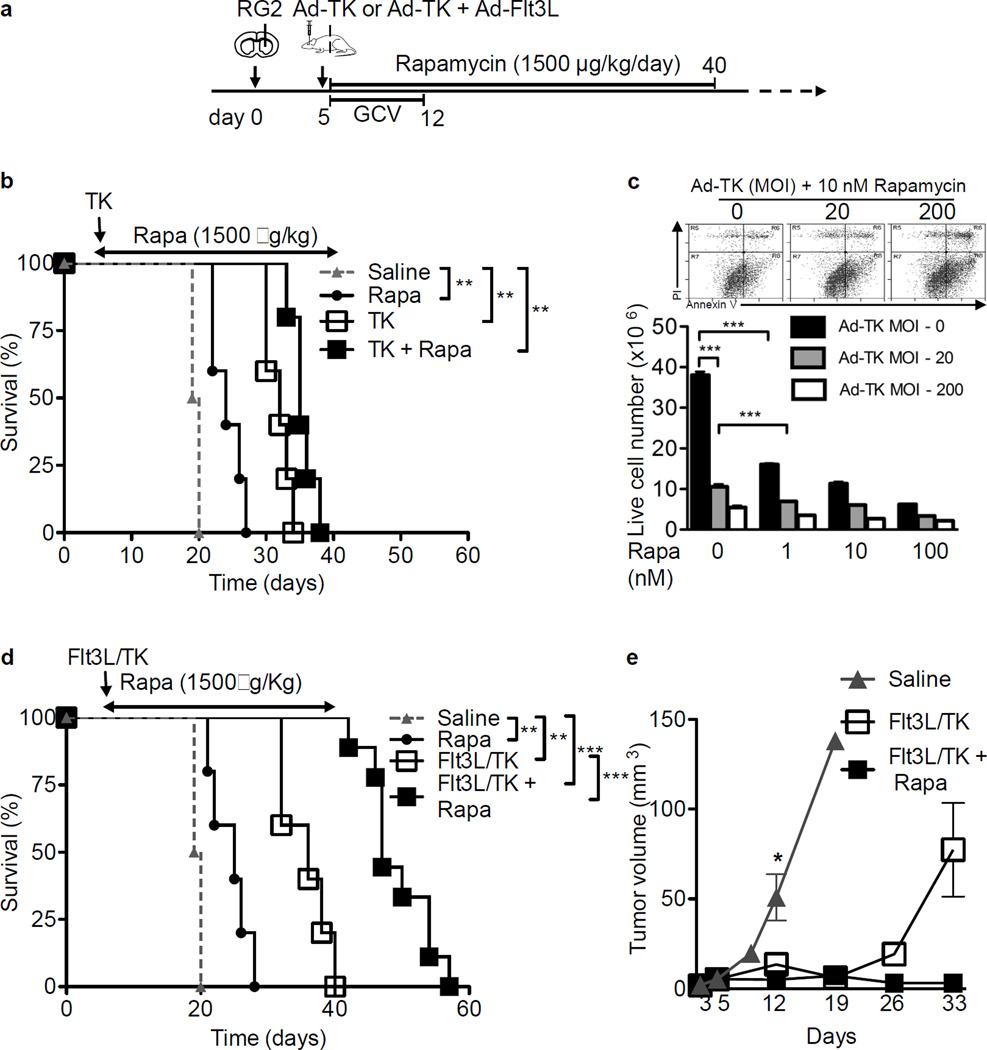
Rapamycin and its analogs have exhibited clinical benefits against tumors such as endometrial and renal cancer either through a direct growth inhibitory effect on cancer cells or through its ability to determine T cell fate (33). To test whether Rapamycin could further enhance the anti-tumor immunity elicited by Ad-TK/GCV or Ad-TK/GCV+Ad-Flt3L gene therapy, rats were implanted with RG2 tumors, and 5 days post tumor implantation, Ad-TK/GCV alone or the combination Ad-Flt3L+Ad-TK/GCV immune-mediated gene therapy was initiated. Rats were also treated with Rapamycin beginning 5 days after tumor implantation until day 40 (Fig. 1A). Administration of Ad-TK/GCV gene therapy to the tumor bearing rats resulted in increase in their median survival period of 19.5 days (saline treated) to 32 days (p < 0.01, Fig. 1B). The median survival time of the animals treated with the Ad-Flt3L+Ad-TK/GCV immunotherapy was also significantly enhanced from 19.5 days (saline treated) to 36 days (p < 0.01, Fig. 1D). In addition, combining Rapamycin administration with Ad-Flt3L+Ad-TK/GCV immunotherapy resulted in an additional increase in the median survival time of tumor bearing rats to 47 days compared to 36 days for the Ad-Flt3L+Ad-TK/GCV immunotherapy alone treated group (p < 0.001, Fig. 1D). In fact, approximately 89% ± 10% of the RG2 tumor bearing rats treated with Rapamycin and immunotherapy survived beyond day 42 by when all tumor bearing rats treated with immunotherapy alone had perished (Fig. 1D). Consistent with the increased survival, rats treated with Ad-Flt3L+Ad-TK/GCV therapy or Rapamycin in combination with Ad-Flt3L+Ad-TK/GCV showed a drastic reduction in the tumor volume at day 12 as compared to the saline treated group (p < 0.01, Fig. 1E). The difference in tumor volume was even more apparent at day 33 when the average tumor volume for Ad-Flt3L+Ad-TK/GCV treated animals was 77.41 ± 26.01 mm3 while rats treated with Rapamycin + Ad-Flt3L+Ad-TK/GCV showed an average tumor volume of 3.1 ± 0.58 mm3. In contrast, Rapamycin administration during Ad-TK/GCV cytotoxic gene therapy failed to further increase the survival of Ad-TK/GCV only treated mice suggesting that Rapamycin potentially modulates the anti-tumor immune surveillance mechanisms mediated by Flt3L immunotherapy (Fig. 1B). Animals treated with Rapamycin alone also showed a significant increase in their survival period (24 days) compared to saline administered rats (19.5 days) indicating a direct effect of Rapamycin on tumor growth (p < 0.01, Figs. 1B and 1D). To examine the effect of Rapamycin on tumor cells, RG2 cells were treated with a combination of Rapamycin (0–100 nM) and Ad-TK (MOI = 0, 20, 200) and 24 hours later, incubated with 25 µM GCV for an additional 48 hrs. Cell viability was assessed by annexin V/PI staining. As positive controls for annexin V and PI staining, cells treated with staurosporine or cells subjected to freeze-thaw cycles were used respectively. Treatment with staurosporine resulted in an increase in annexin V+ cells (apoptosis), multiple cycles of freeze-thawing caused an increase in PI+ cells (necrosis/late apoptosis). AnnexinV/PI double positive cells were increased under both treatments (Supplementary fig. 1). Both Ad-TK/GCV and Rapamycin treatment of RG2 cells lead to a progressive increase in the percentage of apoptotic cells (annexin V positive cells) in a dose dependent manner. In the absence of Ad-TK, Rapamycin treatment of RG2 cells resulted in approximately 57% reduction in cell viability (p < 0.001 vs. 0 nM Rapamycin, Fig. 1C). At the same time, while Ad-TK/GCV treatment significantly reduced RG2 cell viability, Rapamycin treatment resulted in a further decrease in RG2 cell viability (p < 0.001, vs. 0 nM Rapamycin at 20 MOI Ad-TK, Fig. 1C). We also performed western blot analysis on Rapamycin treated RG2 cells. The two most characterized downstream regulators activated by mTOR signaling are the eIF4E binding proteins (4E-BPs) and the ribosomal proteins p70S6 kinase. Tumor cells were first starved of growth factors and then treated with Rapamycin prior to stimulation with 20% FCS. Similar to what has been shown before, while Rapamycin’s inhibition led to the dephosphorylation of p70S6 kinase, it did not target 4E-BP1 phosphorylation status (Supplementary fig. 2) (34).
Figure 1. Rapamycin enhances efficacy of Ad-Flt3L + Ad-TK immunogene therapy for RG2 glioma.
(a) Experimental design to assess the anti-tumor effects of Rapamycin in combination with Ad-TK/GCV (b–c) or Ad-Flt3L+Ad-TK/GCV (d–e). Fisher rats were implanted in the striatum with 20,000 RG2 cells and 5 days later, they were treated with either Ad-TK or Ad-Flt3L + Ad-TK followed by treatment with GCV. Rapamycin were administered i.p. daily between 5 and 40 days after tumor implantation. (b) Kaplan-Meier survival curves of rats (n = 5 animals per group) treated with saline or Rapamycin (Rapa) or Ad-TK/GCV (TK) or Rapamycin + Ad-TK/GCV (TK + Rapa). Survival curves were compared using Mantel-Cox test, ** p < 0.01. (c) RG2 cells were seeded at 5,000 cells per well in 12-well plate and incubated overnight at 37 °C. Cells were treated with a combination of Rapamycin (0, 1, 10 or 100 nM) and Ad-TK (MOI = 0, 20, 200) and 24 hrs later, incubated with 25 µM GCV for an additional 48 hrs. Cells were trypsinized and cell viability was analyzed by flow cytometry using annexin V (FITC) and PI staining. Double negative cells were considered to be viable. Representative flow plots for 10 nM Rapamycin in combination with 0, 20 or 200 MOI of Ad-TK treatment are shown (d) Kaplan-Meier survival curves of rats treated with saline or Rapamycin (Rapa) or Ad-Flt3L + Ad-TK/GCV (Flt3L/TK) or Rapamycin + Ad-Flt3L + Ad-TK/GCV (Flt3L/TK + Rapa). Survival curves were compared using Mantel-Cox test, ** p < 0.01, *** p < 0.005. n = 5–8 animals per group. (e) Brains from RG2 tumor implanted saline or Flt3L/TK or Flt3L/TK + Rapa treated rats were processed for stereology to determine the tumor volume at days 3, 5, 9, 12, 19, 26 and 33 (n = 3–5 animals per time point for each treatment group) after tumor implantation. Data was compared using unpaired 2-tailed Student’s t-test. Mean ± SEM are indicated, * p < 0.05.
Rapamycin affects cell surface markers and cytokine profiles of Flt3L-induced DCs
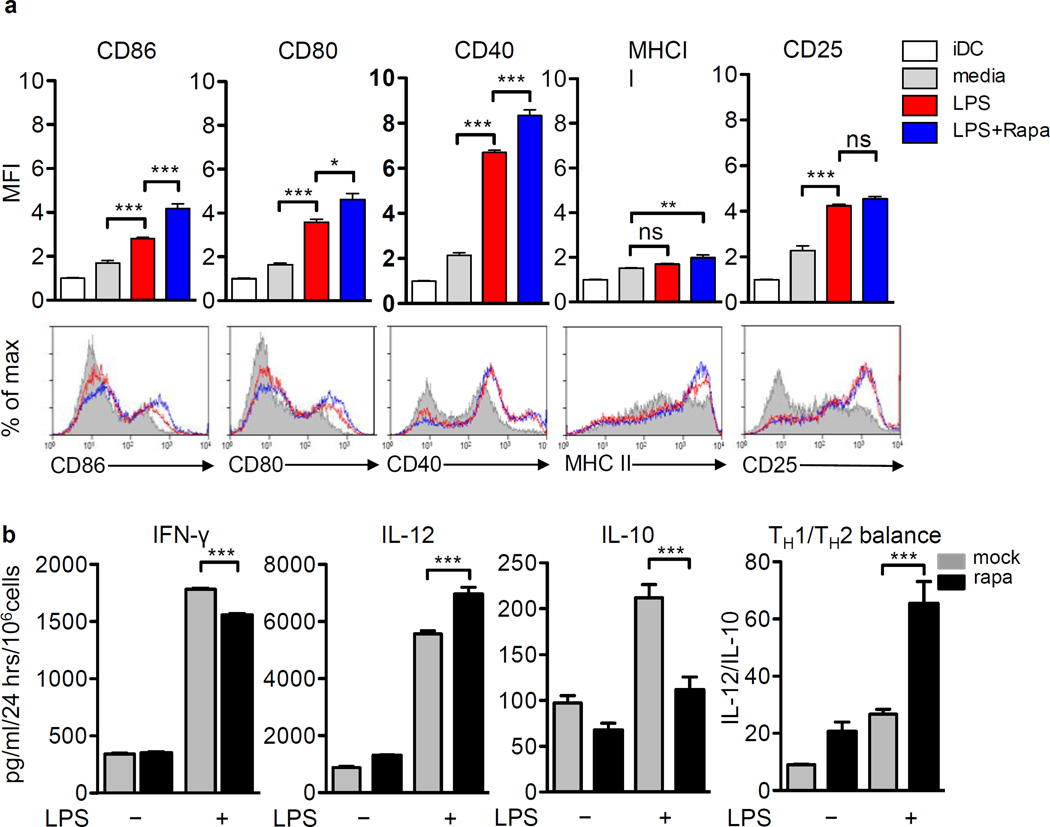
Rapamycin has previously been shown to exert immunosuppressive effects on DCs and bone marrow derived DCs generated in its presence show reduced production of pro-inflammatory cytokines and impaired ability to induce allogeneic T cell responses (35, 36). In contrast, several studies have shown that Rapamycin promotes pro inflammatory cytokine release by DCs and enhances their T cell activation potential (37, 38). The increase in therapeutic efficacy observed after Rapamycin co-administration with Ad-Flt3L + Ad-TK/GCV treatment of RG2 intracranial glioma suggested that Rapamycin could enhance the Flt3L-mediated recruitment and activation of DCs and priming of the anti-GBM T cell response. To examine the effect of Rapamycin on DC activation, expression of co-stimulatory molecules and cytokine release from Rapamycin treated Flt3L derived bone marrow derived DCs was monitored in the presence of LPS. Generation of plasmacytoid DCs (pDCs) and conventional DCs (cDCs) in mouse bone marrow derived DCs was monitored by staining for B220 and CD11c markers on the cells obtained 7 days after Flt3L stimulation (Supplementary fig. 3). However due to the lack of a definitive marker for rat pDCs, FSC/SSC profiles were used to identify total DCs. LPS stimulation upregulated the expression of CD86, CD80 and CD40 on DCs compared to the media treated group (p < 0.001, Fig. 2A). The expression levels of CD86, CD80 and CD40 were slightly enhanced in the presence of Rapamycin by 1.5, 1.3 and 1.2 fold respectively compared to the LPS treated group (p < 0.001 vs. LPS, Fig. 2A). In contrast, Rapamycin stimulation did not enhance LPS induced CD25 and MHCII expression on DCs (Fig. 2A). Since cytokines produced by DCs are crucial for the polarization of CD4+ T cells and the subsequent generation of CD8+ T cell mediated adaptive immunity, we next examined the effect of Rapamycin on the pro-inflammatory (IFN-γ and IL-12) and immune suppressive (IL-10) cytokine release by DCs. While Rapamycin + LPS stimulation resulted in a marginal decrease in IFN-γ secretion (1.1 fold vs. LPS, p < 0.001), Rapamycin + LPS treatment boosted the levels of IL-12 (1.2 fold vs. LPS, p < 0.001) and decreased IL-10 release (2 fold vs. LPS, p < 0.001) by the DCs (Fig. 2B). Overall, presence of Rapamycin during stimulation of DCs with LPS therefore strongly enhanced the TH1/TH2 cytokine ratio (p < 0.001, vs. LPS) indicating a bias towards immune-stimulatory cytokine release. These results prompted us to test whether Rapamycin could also enhance the therapeutic efficacy of anti-glioma DC vaccine. Animals vaccinated with DCs loaded with Ad-TK/GCV-treated tumor cell lysate prior to tumor cell implantation (Supplementary fig. 4A) exhibited increased circulating anti-RG2 antibodies (~ 4 fold increase in relative fluorescence vs. saline, p < 0.001, Supplementary fig. 4B) and increased cytotoxic activity against tumor cells (42.90% ± 1.25% target cell lysis) when compared to the saline vaccinated controls (32.67% ± 0.24% target cell lysis, p < 0.01, Supplementary fig. 4C). However, DC vaccination failed to improve the survival of tumor bearing rats (Supplementary fig. 4D). Interestingly and in agreement with the effect of Rapamycin on DCs observed in vitro, animals immunized with the DC vaccine in the presence of Rapamycin (Supplementary fig. 4E) showed a significant increase in their median survival time (26 days vs. 21 days for saline, p < 0.001, Supplementary fig. 4F).
Figure 2. Rapamycin modulates the expression of costimulatory molecules and cytokine secretion from Flt3L-induced DCs.
(a) Maturation markers on Flt3L-induced rat bone marrow DCs (gated using forward and side scatter profiles) were analyzed by flow cytometry after 7 days culture (iDCs) or after stimulation with media (mock) or 50 ng/ml LPS or 50 ng/ml LPS + 10 ng/ml Rapamycin (LPS + Rapa) for 48 hrs. Overlays from one representative experiment are depicted. Results in (A) are represented as fold change in mean fluorescence intensity (MFI). (b) DCs harvested after 7 days in culture were treated with either 100 ng/ml Rapamycin (Rapa) or saline (mock) in the presence or absence of LPS for 48 hrs. Cytokine secretion was measured by ELISA. TH1/TH2 cytokine balance was estimated by IL-12 vs. IL-10 cytokine ratio. Data in (a) and (b) was analyzed using One-way ANOVA followed by Bonferroni’s post-test and are expressed as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.005, ns not significant.
Rapamycin enhances effector and memory CD8+ T cells’ function induced by Ad-Flt3L+Ad-TK/GCV
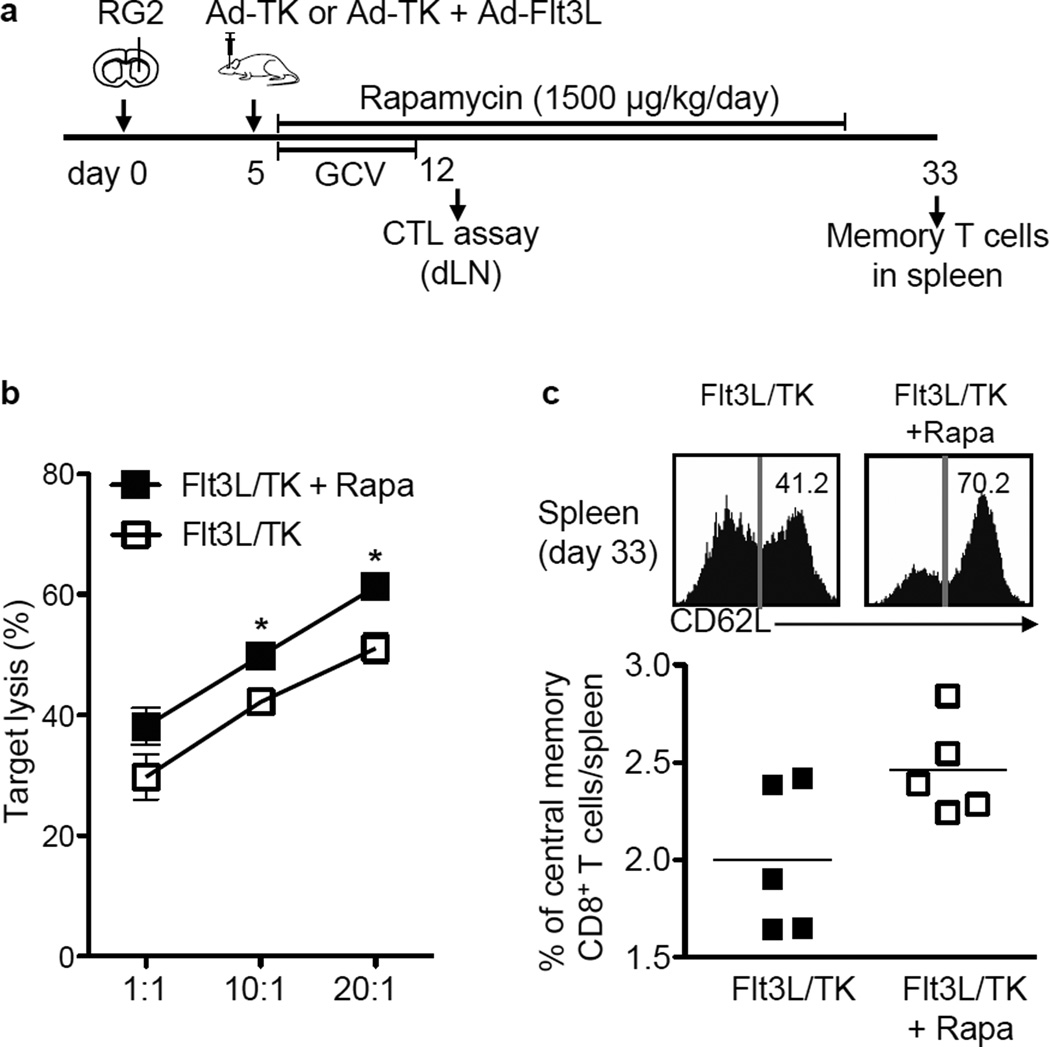
Since Rapamycin promoted an increase in TH1 cytokine release from DCs, which fosters the development of cytotoxic T lymphocyte-mediated immune response against pathogens and tumors, we examined the impact of Rapamycin administration on T cell activation in Rapamycin + Ad-Flt3L+Ad-TK/GCV treated, brain tumor bearing rats. CD3+ T cells from the dLNs of Rapamycin + Ad-Flt3L+Ad-TK/GCV treated tumor bearing animals were harvested on day 12 and tested for their cytolytic activity towards RG2 cells (Fig. 3A). At day 12 the tumor volumes in Ad-Flt3L + Ad-TK/GCV only group are similar to the tumor volumes of rats treated with the combination therapy. Cytotoxicity of T cells isolated from animals treated with gene therapy in the presence of Rapamycin was observed to be significantly higher (p < 0.05, 61.27% ± 1.5% target cell lysis at 20:1 E:T ratio) when compared to the gene therapy alone treated group (51.07% ± 1.02% target cell lysis) indicating that Rapamycin enhanced the cytotoxic activity of tumor infiltrating T cells (Fig. 3B). In addition, Rapamycin elicited an increase in the percentage of central memory CD8+ T cells in the spleen of tumor bearing rats treated with Ad-TK/GCV+Ad-Flt3L immunotherapy compared to the immunotherapy alone group (Fig. 3C).
Figure 3. Rapamycin enhances effector and memory CD8+ T cell function induced by Ad-Flt3L + Ad-TK/GCV.
(a) Fisher rats were implanted in the striatum with 20,000 RG2 cells and 5 days later treated with Ad-Flt3L + Ad-TK followed by GCV administration between 5 and 12 days after tumor implantation. Rapamycin (1500 µg/kg) was administered i.p. daily between 5 and 33 days after tumor implantation. Animals were sacrificed at day 12 or 33 to collect T cells from the draining lymph nodes (dLN) or the spleen respectively. (b) T cells isolated from dLNs of Ad-Flt3L + Ad-TK/GCV (Flt3L/TK) or Ad-Flt3L + Ad-TK/GCV + Rapamycin (Flt3L/TK + Rapa) treated rats were incubated with VivoTag-680 stained RG2 cells for 5 hrs at 37 °C at the indicated ratios. Target lysis was assessed by flow cytometry using Annexin-V and PI.VivoTag-680 positive target cells were gated and the percentage of Annexin V and/or PI positive cells was calculated as dead tumor cells. Specific lysis was determined by comparison with lysis of control target cells that were not co-cultured with lymphocytes. Data was compared using unpaired 2-tailed Student’s t-test. Mean ±SEM are indicated, * p < 0.05. (c) Upper panels show representative histograms and corresponding percentage of memory T cells in the spleen at 33 days after tumor implantation. The column scatter graph shows the percentage of central memory CD8+ T-cell population (CD62Lhigh CD45RC+) among CD8+ T cells (CD8+ CD3+) for each group. Data was compared using unpaired 2-tailed Student’s t-test. Mean ±SEM are indicated.
Rapamycin is required at an early stage to enhance therapeutic efficacy of Ad-Flt3L+Ad-TK/GCV immunotherapy
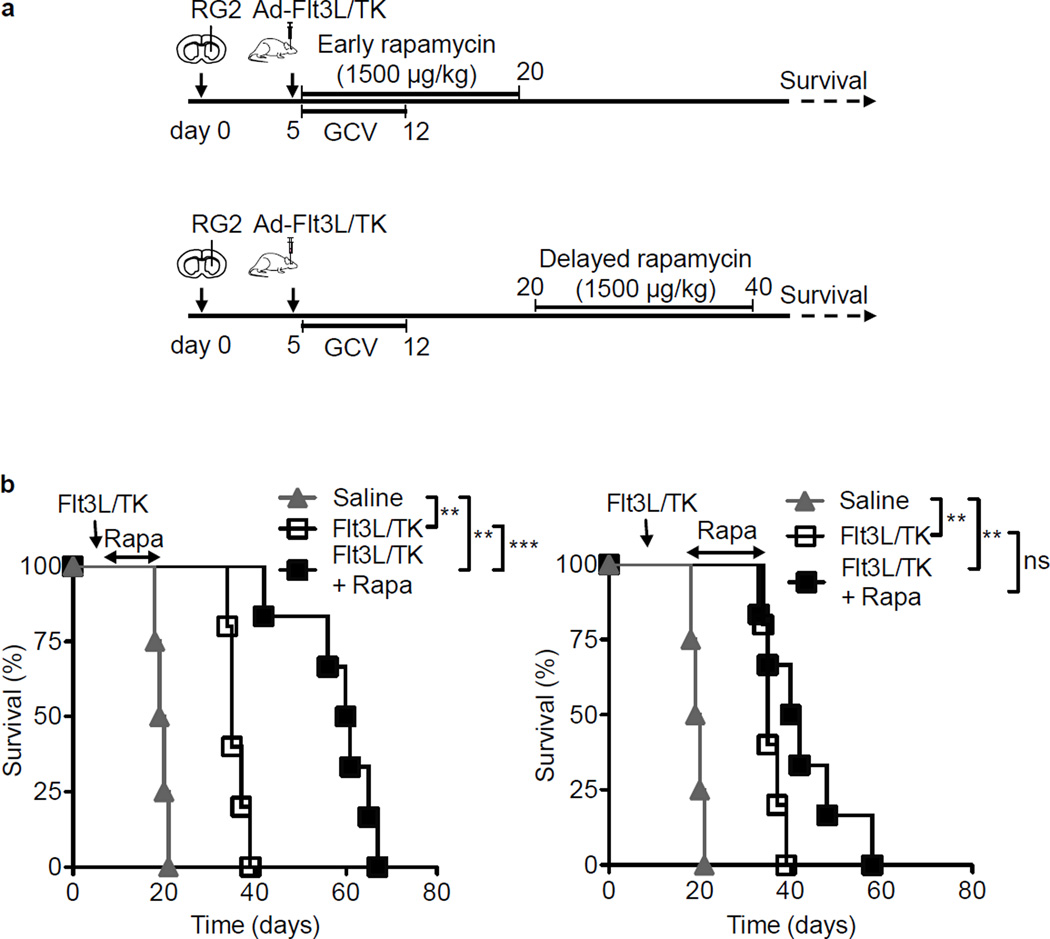
The duration and timing of Rapamycin administration has been shown to influence the development of CD8+ T cell memory response such that a short duration of high dose Rapamycin promoted CD8+ T cell persistence whereas a prolonged high dose regimen abolished memory responses (27) (39). Therefore to facilitate the development of a therapeutic regimen that effectively supports memory CD8+ T cell responses; we next characterized the impact of the timing of Rapamycin administration in our system. We administered Rapamycin to Ad-Flt3L+Ad-TK/GCV treated rats at two different time points post tumor implantation (Fig. 4A). Interestingly, we observed that Rapamycin could enhance the therapeutic efficacy of the Ad-Flt3L+Ad-TK/GCV immunotherapy only when it was administered at the initiation of the treatment (Fig. 4B). Rapamycin administration during the initiation of immunotherapy (between days 5 and 20 post tumor implantation) enhanced the survival of Ad-Flt3L+Ad-TK/GCV treated rats from 35 days to 60 days (p < 0.001, Fig. 4B). In contrast, Rapamycin administration after the initiation of immunotherapy (between days 20 and 40) failed to potentiate the efficacy of the immunogene therapy (p > 0.05 vs. Ad-Flt3L+Ad-TK/GCV, Fig. 4C). Previous data from our lab has shown that the generation of T cell response following immunogene therapy peaks within 7 days post gene therapy administration. Our data therefore suggested that Rapamycin mediates its effects by modulating TK/Flt3L-mediated effector phase of the anti-tumor immune response and was crucially required during the expansion phase of the effector T cells.
Figure 4. Delayed Rapamycin administration does not enhance the efficacy of Ad-Flt3L + Ad-TK/GCV immunotherapy.
(a) Experimental design to assess the time dependent effects of Rapamycin in combination with Ad-Flt3L + Ad-TK/GCV. Fisher rats were implanted in the striatum with 20,000 RG2 cells and 5 days later, they were treated with Ad-Flt3L + Ad-TK followed by treatment with GCV. Rapamycin (1500 µg/kg) was administered i.p. daily either between 5 and 20 days after tumor implantation (b) or between 20 and 40 days post implantation (c). Animals were monitored for survival until moribund. (b) – (c) Kaplan-Meier survival curve of rats treated with Ad-Flt3L + Ad-TK/GCV + Rapamycin (Flt3L/TK + Rapa) for different time points (n = 5–7 animals per group). Survival curves were compared using Mantel-Cox test, ** p < 0.01, *** p < 0.005, ns not significant.
Rapamycin enhances antigen-specific cytotoxic T cell activity induced by Ad-Flt3L + Ad-TK/GCV in a mouse syngeneic intracranial GBM model
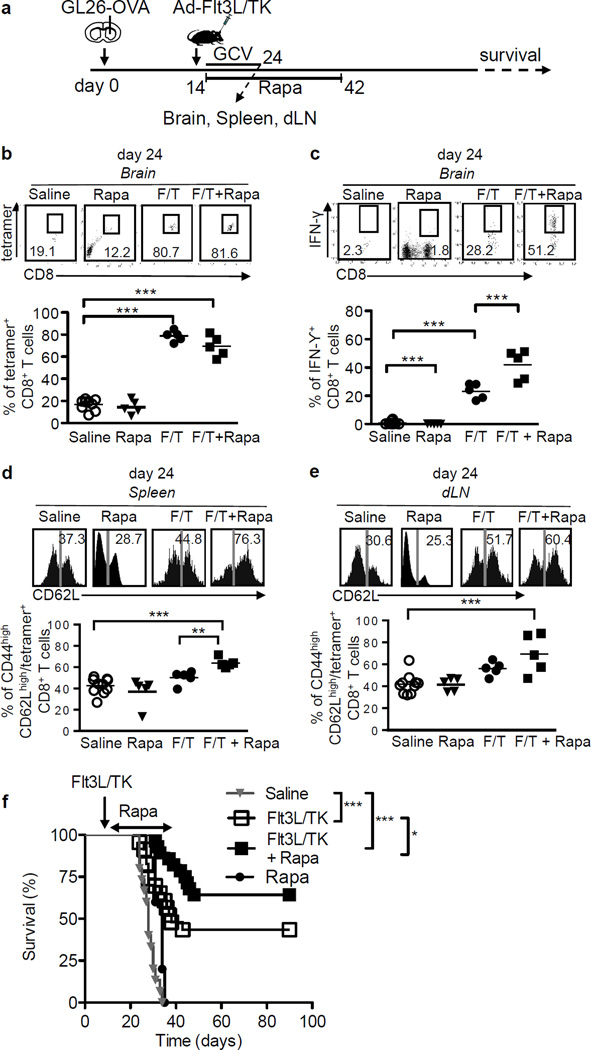
Our data with the RG2 glioma model indicated that Rapamycin enhances the therapeutic efficacy of Ad-Flt3L+Ad-TK/GCV immune-mediated gene therapy by modulating the generation of effector and memory T cell responses. In order to examine the antigen specificity of the cytotoxic and memory T cell response in more detail we made use of theGL26 syngeneic glioma model in mice. GL26-OVA cells expressing the surrogate tumor antigen ovalbumin were implanted in the striatum of wild type C57BL/6 mice and treatment was initiated as indicated (Fig. 5A). The induction of antigen-specific cytotoxic CD8+ T cell responses and immunological memory were visualized using the ovalbumin-derived peptide epitope SIINFEKL-H2Kb tetramer. Flow cytometric analysis of tumor infiltrating lymphocytes labeled with anti-CD8 antibodies and SIINFEKL-H2Kb tetramers revealed more ovalbumin-specific CD8+ T cells among the tumor-infiltrating cells in the Ad-Flt3L+Ad-TK/GCV immunotherapy treated mice (p < 0.001 vs. saline treated group, Fig. 5B). Although combining Rapamycin with the immunogene therapy did not further increase the percentage of tetramer+ CD8+ T cells in the brain, a significant increase in the frequency of CD8+ T producing IFN-γ was observed in these mice (~ 2 fold change vs. immunotherapy alone, p < 0.01) indicating an increase in the activity of CD8+ T cells (Fig. 5C). Consistent with our previous results, Ad-Flt3L+Ad-TK/GCV immunotherapy induced ovalbumin-specific CD8+ T cells in the dLNs (p < 0.05 vs. saline, Fig. 5E). In addition, Rapamycin treatment further enhanced the percentage of ovalbumin-specific central memory CD8+ T cells in the spleen of tumor bearing mice by ~ 1.3 fold (p < 0.05 vs. immunotherapy alone, Fig. 5D). A similar trend was observed for cells from the dLNs (p > 0.05 vs. immunotherapy alone, Fig. 5E). Consistent with the increased cytotoxic T cell activity against tumor, Rapamycin co-administration prolonged the survival of GL26-OVA tumor implanted mice, such that 62% of mice treated with Rapamycin + Ad-Flt3L+Ad-TK/GCV survived beyond day 60 compared to 41% from the immunotherapy treatment alone group (p < 0.05, Fig. 5F). Rapamycin treatment alone failed to enhance the tumor-specific effector or memory T cell responses and consequently provided no survival benefit.
Figure 5. Rapamycin enhances antigen-specific effector T cell function in GL26 induced glioma in mice.
(a) Experimental design to assess the effect of Rapamycin on antigen-specific effector and memory T cell function in GL26 induced glioma. C57BL/6 mice were implanted with 20,000 GL26-OVA in the right striatum of the brain and 14 days later animals received Ad-Flt3L + Ad-TK/GCV treatment in presence (indicated as F/T + Rapa) or absence of (indicated as F/T) Rapamycin administration for 28 days. GL26-OVA tumor bearing mice were also treated with saline or Rapamycin (Rapa) only as controls. (b) – (c) Flow cytometric analysis measuring OVA-specific CD8+ T cells (B) and SIINFEKL-induced IFN-γ production by CD8+ T (c) cells in brain tumor 24 days after tumor implantation. (d) – (e) OVA-specific central memory CD8+ T (CD44+ CD62Lhigh / CD8+ CD3+ tetramer+) cells in spleen (d) and dLN (e) at day 24. Data in (b) – (e) was analyzed using One-way ANOVA, mean ± SEM are indicated, * p < 0.05, ** p < 0.01, *** p < 0.005, ns not significant. (f) Kaplan-Meier survival curves of mice bearing tumors treated with saline or Rapamycin (Rapa) orAd-Flt3L +Ad-TK (Flt3L/TK) or Ad-Flt3L + Ad-TK + Rapamycin (Flt3L/TK + Rapa). n= 5 animals per group. Survival curves were compared using Mantel-Cox test, * p < 0.05, *** p < 0.005.
Rapamycin enhances antigen-specific memory T cell response against glioma
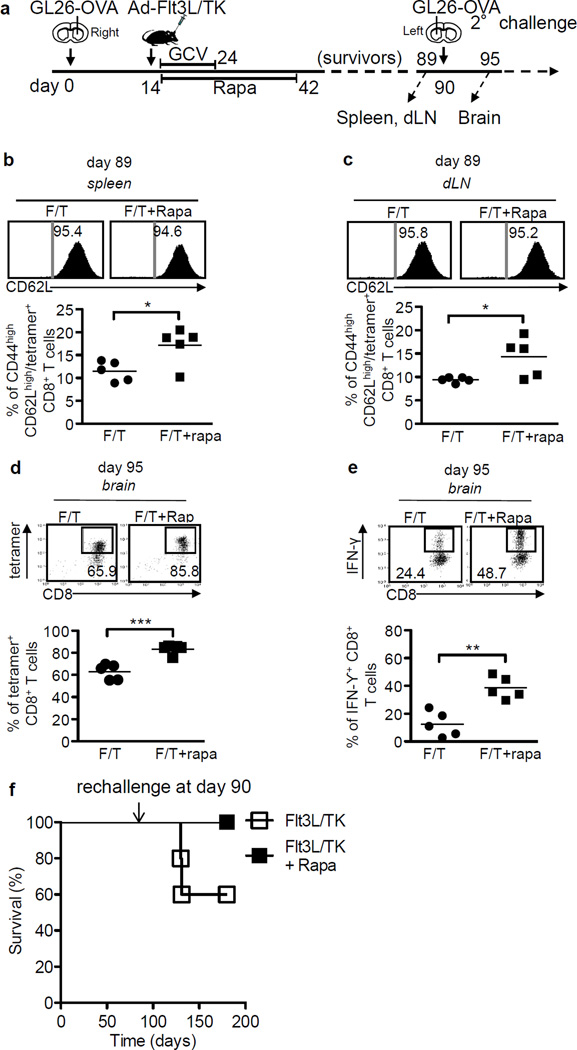
In most cases GBM recurs after treatment (40), thus, development of immunological memory response capable of recognizing brain tumor antigen, leading to the rejection of recurrent GBM becomes crucial for preventing relapse. Analysis of spleens and dLNs 10 days post tumor implantation showed that Rapamycin administration induced higher percentage of antigen-specific memory CD8+ T cells in the spleens of Ad-Flt3L+Ad-TK/GCV treated GL26-OVA tumor bearing mice (~1.3 fold vs. immunotherapy alone, p < 0.05, Fig.5D). To determine the duration and robustness of the T cells’ mediated memory response, mice that survived the first tumor were implanted with a second tumor in the contralateral hemisphere as indicated in the figure (Fig. 6A). One day prior to the second tumor challenge (day 89), spleen and dLNs of these mice were examined for the presence of antigen-specific memory CD8+ T cells. Mice treated with the combination of Rapamycin + Ad-Flt3L+Ad-TK/GCV showed ~1.5 fold higher percentages of antigen-specific CD44high CD62Lhigh tetramer+ CD8+ T cells in the spleen and dLNs (p < 0.05 vs. immunotherapy alone, Figs. 6B and 6C). When rechallenged with tumor, mice also showed increased percentages of tetramer+ CD8+ T cells in the brain (~1.3 fold, p < 0.001 vs. immunotherapy alone, Fig. 6D). Furthermore, tumor-infiltrating CD8+ T cells from Rapamycin + Ad-Flt3L+Ad-TK/GCV treated mice produced IFN-γ at a 3 fold higher frequency than those treated with immune gene therapy alone (p < 0.001, Fig. 6E). Rapamycin administration in combination with Ad-Flt3L+Ad-TK/GCV also conferred long-term survival advantage in response to the second tumor challenge; 100% of the mice in this treatment group survived beyond90 days after the second tumor challenge compared to 60% of the mice from the immune gene therapy alone group (Fig. 6F).
Figure 6. Rapamycin enhances antigen specific memory T cell function in GL26 induced glioma in mice.
(a) Ad-Flt3L + Ad-TK/GCV treated mice from figure 5 that survived to 90 days were given a second tumor challenge in the left striatum. Brains, lymph nodes and spleens were collected at indicated time points. (b) – (c) Percentage of CD44+ CD62Lhigh population among tet+ CD8+ T cells in spleen (b) and dLNs (c) at day 89 are shown. (d) – (E) OVA-specific CD8+ T cells (d) and SIINFEKL-induced IFN-γ production by CD8+ T cells (e) in brain tumors 5 days after the second challenge (at day 95). Data was compared using unpaired 2-tailed Student’s t-test. Mean ± SEM are indicated, * p < 0.05. (f) Survivors were rechallenged at day 90 without further treatment. Survival curves were compared using Mantel-Cox test, *** p < 0.005.
Discussion
Due to the limited success of the current treatment modalities for GBM patients, there is an urgent need to develop novel therapies; particularly those that can inhibit tumor relapse. We have previously shown that immune-mediated gene therapy utilizing adenovirus expressing Flt3L and TK followed by GCV administration exhibits effective antitumor immunity and results in tumor regression in several mouse and rat glioma models (4, 6, 41). An important factor determining the success of these therapeutic approaches is the ability to harness the memory T cell response. Recently there has been considerable interest in the mTOR pathway as a determinant of T cell fate. In particular, several studies have highlighted the use of mTOR inhibitor Rapamycin to modulate CD8+ T cell memory phenotype. Herein we provide evidence that Rapamycin potentiates the therapeutic efficacy of the immune-mediated gene therapy in two syngeneic, intracranial glioma models, RG2 and GL26. We demonstrate that co-administration of Rapamycin with Ad-Flt3L + Ad-TK/GCV enhances the tumor antigen-specific cytotoxic CD8+ T cell responses. Furthermore, Rapamycin strongly favors the generation of a robust memory CD8+ T cell response that in turn boosts the adaptive immune response against a second tumor challenge and confers a survival advantage to the mice.
Although Rapamycin has traditionally been used as an immunosuppressive agent, reports have shown that it can enhance the effect of vaccines targeting bacterial and viral infections (27, 29). We observed that Rapamycin upregulated the LPS-induced expression of co-stimulatory ligands CD80 and CD86 on Flt3L derived DCs. At the same time, Rapamycin stimulation promoted the release of TH1 polarizing cytokines by LPS stimulated DCs indicating that Rapamycin favors the development of TH1 phenotype. Consistent with the effect of Rapamycin on DCs, we observed that administration of Rapamycin at the time of DC vaccination prolonged the survival of RG2 tumor bearing rats. Additionally Rapamycin has been shown to enhance the life span of activated DCs thus increasing the duration of their interaction with T cells. The shortened life span of activated DCs is one of the factors responsible for the modest success of DC vaccination in clinics and could be why DC vaccination alone failed to improve the survival of RG2 tumor bearing rats in the absence of Rapamycin in our system (26). Apart from its effects on DC maturation and kinetics, Rapamycin has been shown to influence CD4+ T cell fate and memory CD8+ T cell development (19, 20, 27, 42). We thus tested whether Rapamycin could enhance the therapeutic efficacy of the Flt3L/TK immunogene therapy, which relies on the recruitment of antigen presenting cells to the tumor environment and priming of T cell responses (5, 7). We observed that combining Rapamycin administration with Ad-Flt3L + Ad-TK/GCV gene therapy led to a remarkable increase in the survival of tumor bearing animals in both RG2 (rat) and GL26 (mice) glioma models. Rapamycin by itself can induce apoptosis and consistent with previous studies it increased the sensitivity of RG2 cells to Ad-TK/GCV and showed a marginal increase in the survival of tumor bearing animals as compared to the saline treated group. However Rapamycin induced tumor cell apoptosis does not appear to be the primary mechanism contributing to tumor regression as no significant difference in survival between Ad-TK/GCV and Ad-TK/GCV +Rapamycin administered animals was observed. Instead our data suggests that Rapamycin mediates its effects by potentiating the TK/Flt3L mediated anti-glioma immune response. The observation that the effect of Rapamycin is abolished if its administration is delayed would support the hypothesis that it is crucially required during the antigen presentation and the subsequent expansion phase of anti-tumor T cells. Accordingly, we observed enhanced cytotoxic T cell responses as indicated by the increased lysis of RG2 tumor cells by activated T cells isolated from the dLNs of tumor bearing animals. GL26-OVA tumor implanted mice also showed elevated percentages of tumor-specific and IFN-γ producing CD8+ T cells. Of note, minimal contraction of antigen-specific CD8+ T cells was observed at day 89 compared to the frequency of antigen-specific CD8+ T cells at day 24 in the Rapamycin treated group. Since no significant differences in the percentage of antigen-specific CD8+ T cells in the presence or absence of Rapamycin was observed at day 24, it would suggest that Rapamycin modulates the function and quality of T cell responses rather than their proliferation. Our data is in accordance with the observations by Araki et al. who showed that Rapamycin administration during the effector to memory transition phase was crucial to the generation of improved memory T cell responses (27). Thus in our experiments, initiating Rapamycin treatment after day 20 failed to impact the survival of tumor bearing animals. Rapamycin treatment also accelerated memory T cell development as indicated by the increased percentages of CD44high CD62Lhigh CD8+ T cells. This would also imply the generation of a robust central memory response, as CD62L expression is high on central memory T cells. Memory T cells with high CD62L expression are also associated with enhanced proliferative capacity (43). Of note, the effects of Rapamycin administration were long lasting as higher numbers of memory T cells were observed at day 89 along with an increased frequency of tumor-specific IFN-γ+ CD8+ T cells at day 95, even though the treatment with Rapamycin was suspended at day 42. Indeed this enhanced immunological memory was associated with prolonged survival when the mice were challenged with a second tumor implantation. Taken together our data demonstrates that Rapamycin enhances the therapeutic efficacy of Ad-Flt3L + Ad-TK/GCV gene therapy in a two-fold manner; (1) by enhancing the functional quality of the anti-glioma cytotoxic CD8+ T cell response and promoting the maintenance of tumor antigen-specific CD8+ T cells, (2) eliciting a potent CD8+ memory phenotype resulting in heightened anti-tumor effector CD8+ T cell response when rechallenged with the tumor. Moreover, we provide evidence that Rapamycin administration for a short duration is sufficient to boost anti-tumor CD8+ memory T cell response that provides long-term survival advantage in the event of tumor rechallenge. Since relapse is a common occurrence in GBM patients, our studies point to Rapamycin administration as a novel therapeutic target to be used in combination with TK/Flt3L immune-gene therapy in a phase I clinical trial for GBM.
Supplementary Material
Acknowledgements
We are grateful to Karin Murasko for her academic leadership and Donald Tomford, Stephen Napolitan, and Molly Dahlgren for superb administrative support and Rosemary Lemons and Marta Dzaman for superb technical assistance.
Grant Support
This work was supported by National Institutes of Health/ National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants 1UO1-NS052465, UO1-NS052465-04S1, 1RO1-NS074387 and 1RO1-NS057711 to M.G. Castro; NIH/NINDS Grants 1RO1-NS054193, 1RO1-NS061107 and 1RO1-NS082311 to P.R. Lowenstein; MICHR Grant NIH/U21TR000433, Cancer Biology Training Grant NIH/NCI T32-CA009676 and the Neurology Training Grant NIH/NINDS T32-NS007222; The Phase One Foundation and the Department of Neurosurgery, University of Michigan School of Medicine.
List of abbreviations
- Ad-Flt3L
Fms-like tyrosine kinase 3 ligand
- Ad-TK/GCV
Thymidine kinase/Ganciclovir
- GBM
Glioblastomamultiforme grade IV
- Treg
T regulatory
- DCs
Dendritic cells
- HMGB1
High-mobility group B1 protein
- dLNs
Draining lymph nodes
- pDCs
Plasmacytoid DCs
- FSC/SSC
Forward scatter/side scatter
- PI
Propidium iodide
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10:133–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 3.Arko L, Katsyv I, Park GE, Luan WP, Park JK. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2010;128:1–36. doi: 10.1016/j.pharmthera.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King GD, Muhammad AK, Curtin JF, Barcia C, Puntel M, Liu C, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 9.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 11.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich PY, Dutoit V, Tran Thang NN, Walker PR. T-cell immunotherapy for malignant glioma: toward a combined approach. Curr Opin Oncol. 2010;22:604–610. doi: 10.1097/CCO.0b013e32833dead8. [DOI] [PubMed] [Google Scholar]
- 13.Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4:329–336. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51–56. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 15.Ingram M, Buckwalter JG, Jacques DB, Freshwater DB, Abts RM, Techy GB, et al. Immunotherapy for recurrent malignant glioma: an interim report on survival. Neurol Res. 1990;12:265–273. doi: 10.1080/01616412.1990.11739955. [DOI] [PubMed] [Google Scholar]
- 16.Mineharu Y, King GD, Muhammad AK, Bannykh S, Kroeger KM, Liu C, et al. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res. 2011;17:4705–4718. doi: 10.1158/1078-0432.CCR-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mineharu Y, Muhammad AK, Yagiz K, Candolfi M, Kroeger KM, Xiong W, et al. Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-kappaB signaling improves the efficacy of immunotherapy in a brain cancer model. Neurotherapeutics. 2012;9:827–843. doi: 10.1007/s13311-012-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, et al. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34:541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 25.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189:2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–652. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc Natl Acad Sci U S A. 2010;107:4716–4721. doi: 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 34.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 36.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 37.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, et al. Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity. J Immunol. 2012;188:3080–3087. doi: 10.4049/jimmunol.1103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, et al. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11:613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.