Abstract
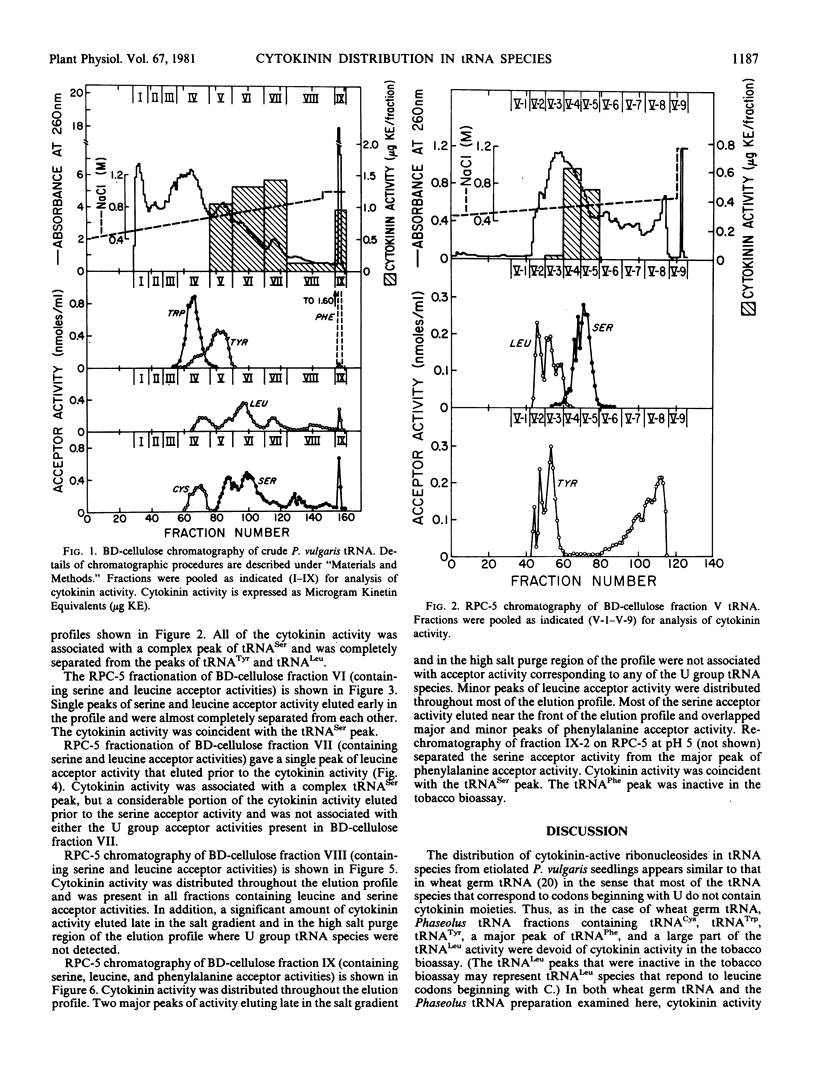
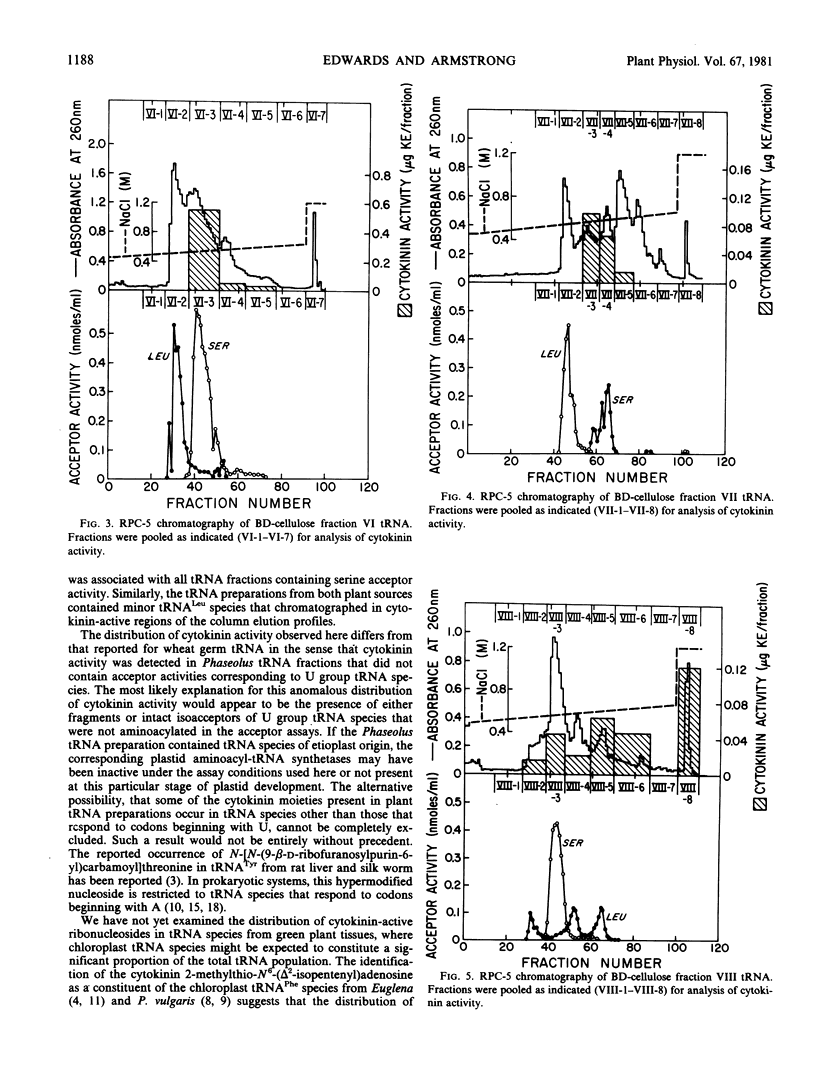
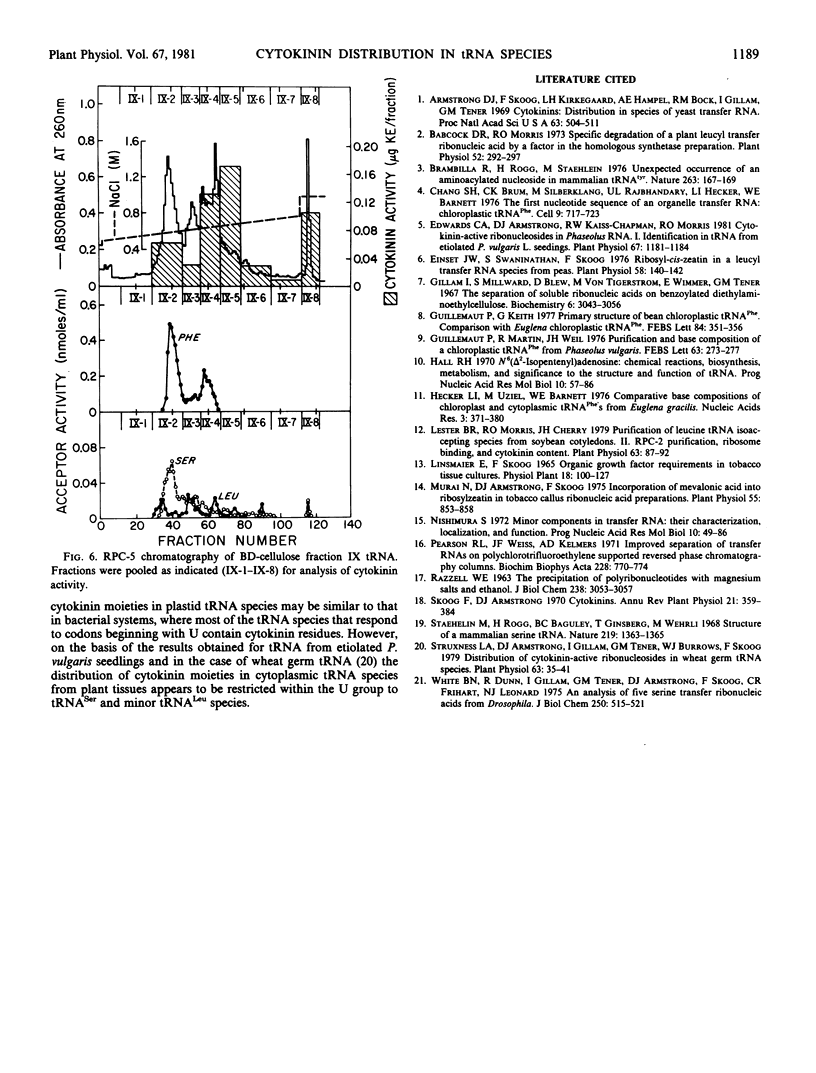
The distribution of cytokinin-active ribonucleosides in tRNA species from etiolated Phaseolus vulgaris L. seedlings has been examined. Phaseolus tRNA was fractionated by benzoylated diethylaminoethyl-cellulose and RPC-5 chromatography, and the distribution of cytokinin activity was compared with the distribution of tRNA species expected to correspond to codons beginning with U. Phaseolus tRNACys, tRNATrp, tRNATyr, a major peak of tRNAPhe, and a large fraction of tRNALeu were devoid of cytokinin activity in the tobacco bioassay. Cytokinin activity was associated with all fractions containing tRNASer species and with minor tRNALeu species. In addition, several anomalous peaks of cytokinin activity that could not be directly attributed to U group tRNA species were detected.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Skoog F., Kirkegaard L. H., Hampel A. E., Bock R. M., Gillam I., Tener G. M. Cytokinins: distribution in species of yeast transfer RNA. Proc Natl Acad Sci U S A. 1969 Jun;63(2):504–511. doi: 10.1073/pnas.63.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D. F., Morris R. O. Specific degradation of a plant leucyl transfer ribonucleic Acid by a factor in the homologous synthetase preparation. Plant Physiol. 1973 Sep;52(3):292–297. doi: 10.1104/pp.52.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R., Rogg H., Staehelin M. Unexpected occurrence of an aminoacylated nucleoside in mammalian tRNATyr. Nature. 1976 Sep 9;263(5573):167–169. doi: 10.1038/263167a0. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Brum C. K., Siberklang M., RajBhandary U. L., Hecker L. I., Barnett W. E. The first nucleotide sequence of an organelle transfer RNA: chloroplastic tRNAphe. Cell. 1976 Dec;9(4 Pt 2):717–723. doi: 10.1016/0092-8674(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Armstrong D. J. Cytokinin-Active Ribonucleosides in Phaseolus RNA: I. IDENTIFICATION IN tRNA FROM ETIOLATED PHASEOLUS VULGARIS L. SEEDLINGS. Plant Physiol. 1981 Jun;67(6):1181–1184. doi: 10.1104/pp.67.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W., Swaminathan S., Skoog F. Ribosyl-cis-zeatin in a Leucyl Transfer RNA Species from Peas. Plant Physiol. 1976 Aug;58(2):140–142. doi: 10.1104/pp.58.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Keith G. Primary structure of bean chloroplastic tRNAPhe. Comparison with Euglena chloroplastic tRNAPhe. FEBS Lett. 1977 Dec 15;84(2):351–356. doi: 10.1016/0014-5793(77)80723-0. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Martin R., Weil J. H. Purification and base composition of a chloroplastic tRANphe from Phaseolus vulgaris. FEBS Lett. 1976 Apr 1;63(2):273–277. doi: 10.1016/0014-5793(76)80110-x. [DOI] [PubMed] [Google Scholar]
- Hall R. H. N6-(delta 2-isopentenyl)adenosine: chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol. 1970;10:57–86. doi: 10.1016/s0079-6603(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Hecker L. I., Uziel M., Barnett W. E. Comparative base compositions of chloroplast and cytoplasmic tRNAPhe's from Euglena gracilis. Nucleic Acids Res. 1976 Feb;3(2):371–380. doi: 10.1093/nar/3.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B. R., Morris R. O., Cherry J. H. Purification of Leucine tRNA Isoaccepting Species from Soybean Cotyledons: II. RPC-2 Purification, Ribosome Binding, and Cytokinin Content. Plant Physiol. 1979 Jan;63(1):87–92. doi: 10.1104/pp.63.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N., Armstrong D. J., Skoog F. Incorporation of mevalonic Acid into ribosylzeatin in tobacco callus ribonucleic Acid preparations. Plant Physiol. 1975 May;55(5):853–858. doi: 10.1104/pp.55.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E. THE PRECIPITATION OF POLYRIBONUCLEOTIDES WITH MAGNESIUM SALTS AND ETHANOL. J Biol Chem. 1963 Sep;238:3053–3057. [PubMed] [Google Scholar]
- Staehelin M., Rogg H., Baguley B. C., Ginsberg T., Wehrli W. Structure of a mammalian serine tRNA. Nature. 1968 Sep 28;219(5161):1363–1365. doi: 10.1038/2191363a0. [DOI] [PubMed] [Google Scholar]
- Struxness L. A., Armstrong D. J. Distribution of Cytokinin-active Ribonucleosides in Wheat Germ tRNA Species. Plant Physiol. 1979 Jan;63(1):35–41. doi: 10.1104/pp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. N., Dunn R., Gillam I., Tener G. M., Armstrong D. J., Skoog F., Frihart C. R., Leonard N. J. An analysis of five serine transfer ribonucleic acids from Drosophila. J Biol Chem. 1975 Jan 25;250(2):515–521. [PubMed] [Google Scholar]


