Abstract
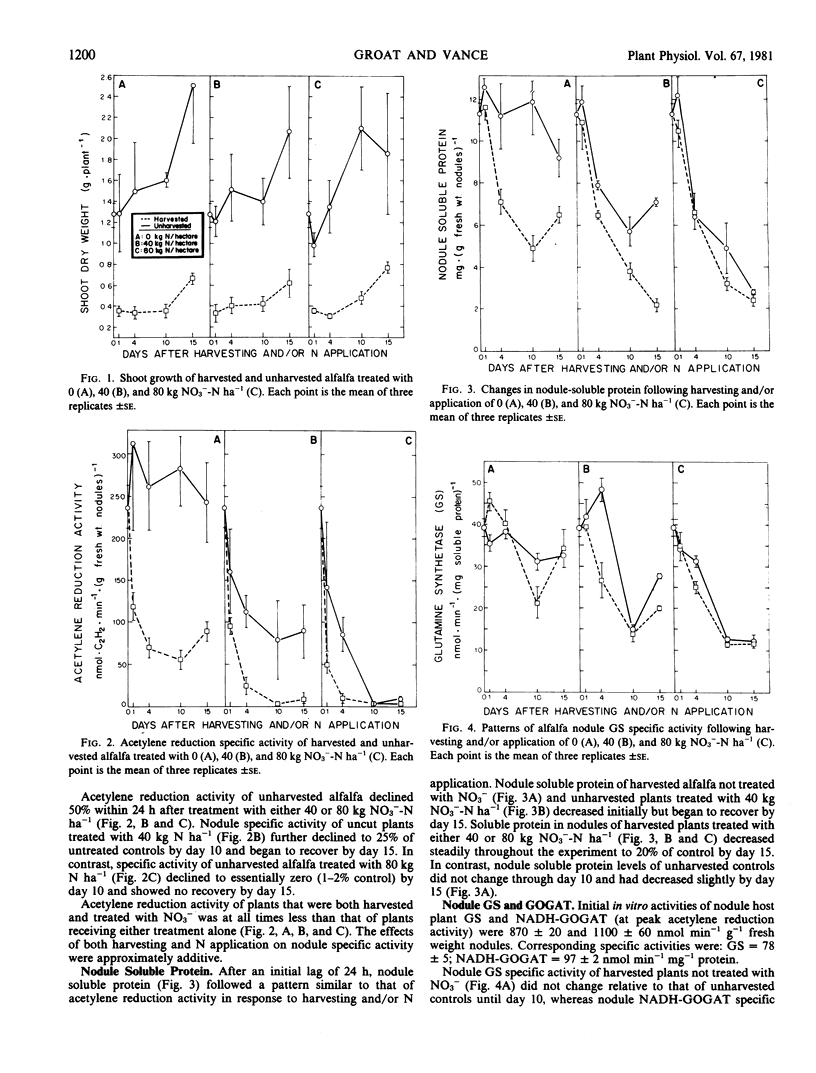
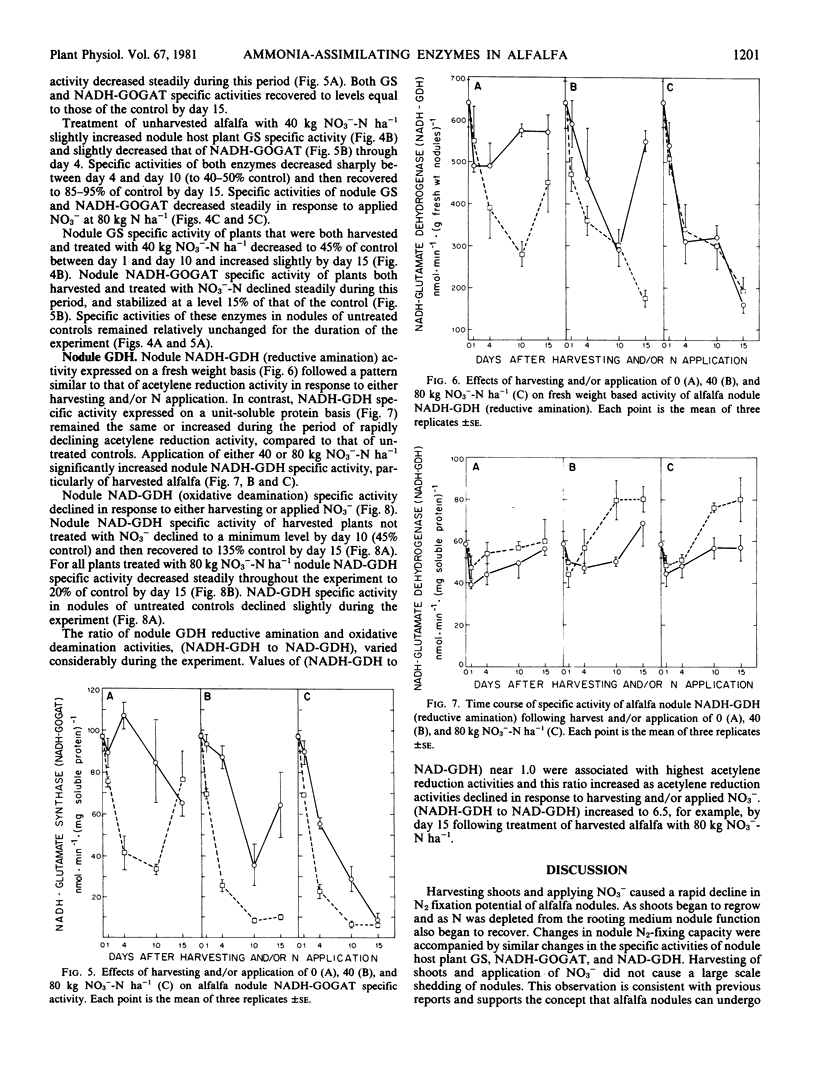
Nitrogenase-dependent acetylene reduction activity of glasshouse-grown alfalfa (Medicago sativa L.) decreased rapidly in response both to harvesting (80% shoot removal) and applied NO3− at 40 and 80 kilograms N per hectare. Acetylene reduction activity of harvested plants grown on 0 kilogram N per hectare began to recover by day 15 as shoot regrowth became significant. In contrast, acetylene reduction activity of all plants treated with 80 kilograms NO3−-N per hectare and harvested plants treated with 40 kilograms NO3−-N per hectare remained low for the duration of the experiment. Acetylene reduction of unharvested alfalfa treated with 40 kilograms N per hectare declined to an intermediate level and appeared to recover slightly by day 15. Changes in N2-fixing capacity were accompanied by similar changes in levels of nodule soluble protein.
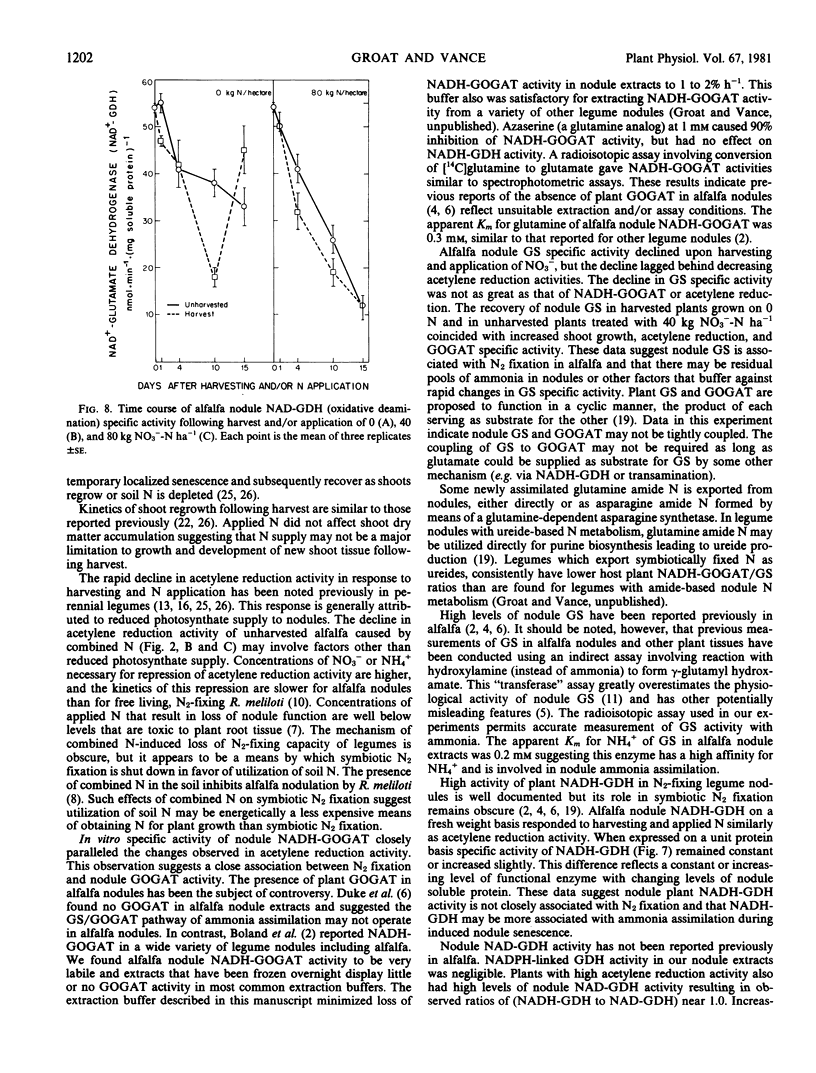
After an initial lag of 24 hours, specific activities of alfalfa nodule glutamine synthetase, NADH-glutamate synthase, and NAD-glutamate dehydrogenase (oxidative amination) decreased similar to but less rapidly than acetylene reduction activity. Increased specific activities of these nodule enzymes occurred as acetylene reduction activity increased and shoot growth resumed. The observed rates of glutamine synthetase and glutamate synthase were sufficient to assimilate ammonia produced via symbiotic N2 fixation. Nodule NADH-dependent glutamate dehydrogenase (reductive amination) specific activity was not associated with changes in acetylene reduction activity.
The data indicate that host plant glutamine synthetase and NADH-glutamate synthase function to assimilate symbiotically fixed N and that NADH-dependent glutamate dehydrogenase may function in ammonia assimilation during senescence in alfalfa nodules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Kamberger W. Regulation of symbiotic nitrogen fixation in root nodules of alfalfa (Medicago sativa) infected with Rhizobium meliloti. Arch Microbiol. 1977 Oct 24;115(1):103–108. doi: 10.1007/BF00427852. [DOI] [PubMed] [Google Scholar]
- McParland R. H., Guevara J. G., Becker R. R., Evans H. J. The purification and properties of the glutamine synthetase from the cytosol of Soya-bean root nodules. Biochem J. 1976 Mar 1;153(3):597–606. doi: 10.1042/bj1530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Schilling N., Shaffer P. W. Initial Organic Products of Fixation of [N]Dinitrogen by Root Nodules of Soybean (Glycine max). Plant Physiol. 1978 Jun;61(6):980–983. doi: 10.1104/pp.61.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Patterson R. P., Raper C. D., Gross H. D. Growth and Specific Nodule Activity of Soybean during Application and Recovery of a Leaf Moisture Stress. Plant Physiol. 1979 Oct;64(4):551–556. doi: 10.1104/pp.64.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Farnden K. J. Induction of glutamate synthase during nodule development in lupin. FEBS Lett. 1975 Jul 15;55(1):33–37. doi: 10.1016/0014-5793(75)80950-1. [DOI] [PubMed] [Google Scholar]


