Abstract
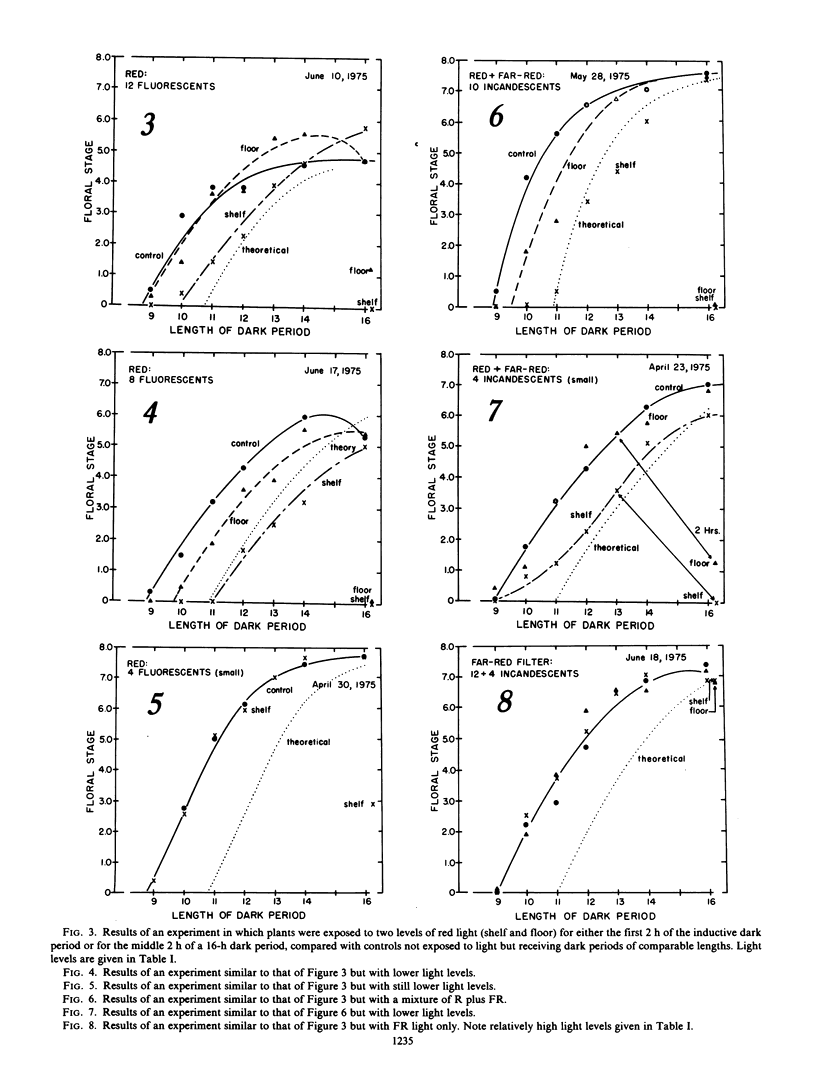
Six experiments studied the effects of low levels of red and far-red light upon the initiation of measurement of the dark period in the photoperiodic induction of flowering in Xanthium strumarium L. (cocklebur), a short-day plant, and compared effects with those of comparable light treatments applied for 2 hours during the middle of a 16-hour inductive dark period. Red light, or red plus far-red, at levels that inhibit flowering when applied during the middle of the inductive dark period, either had no effect on the initiation of dark measurement (i.e., were perceived as darkness), or they delayed the initiation of dark measurement by various times up to the full interval of exposure (2 hours). Far-red light alone had virtually no effect either at the beginning or in the middle of the dark period. These results confirm that time measurement in the photoperiodic response of short-day Xanthium plants is not simply the time required for metabolic dark conversion of phytochrome. Results also suggest that the pigment system (phytochrome?) and/or responses to it may be significantly different as they function during twilight (initiation of dark measurement), and as they function during a light break several hours later. Possible mechanisms by which cocklebur plants detect the change from light to darkness are discussed.
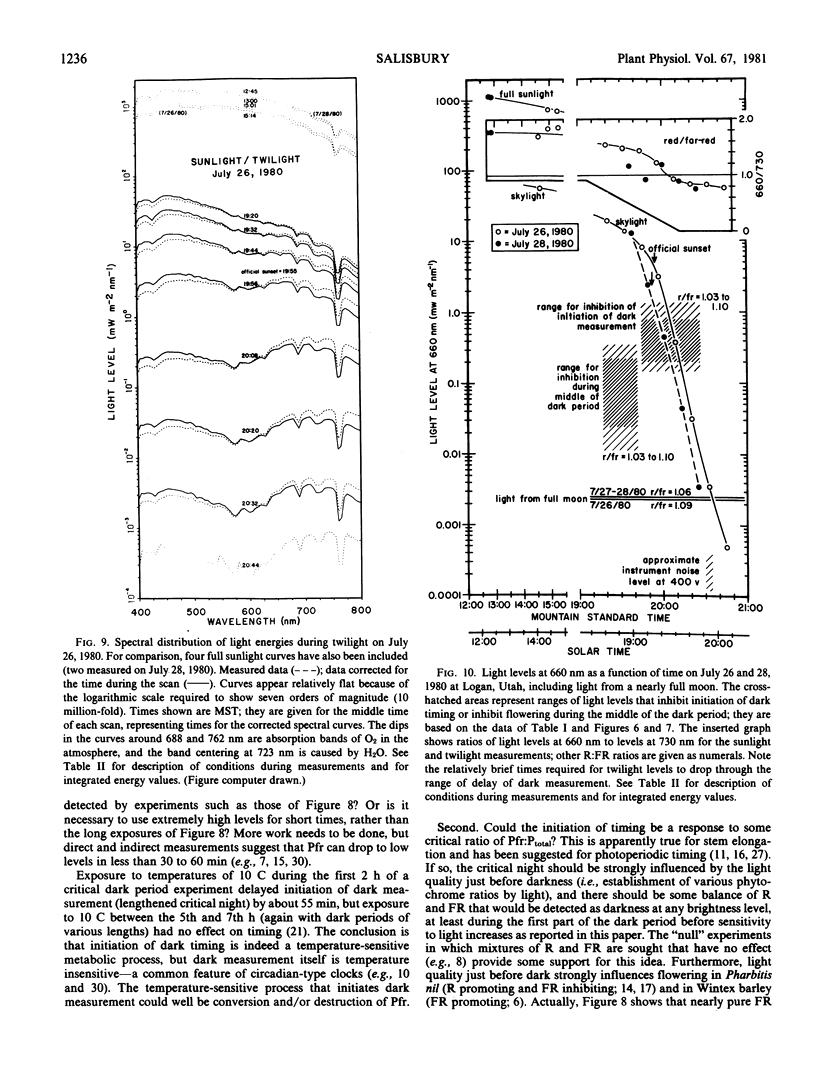
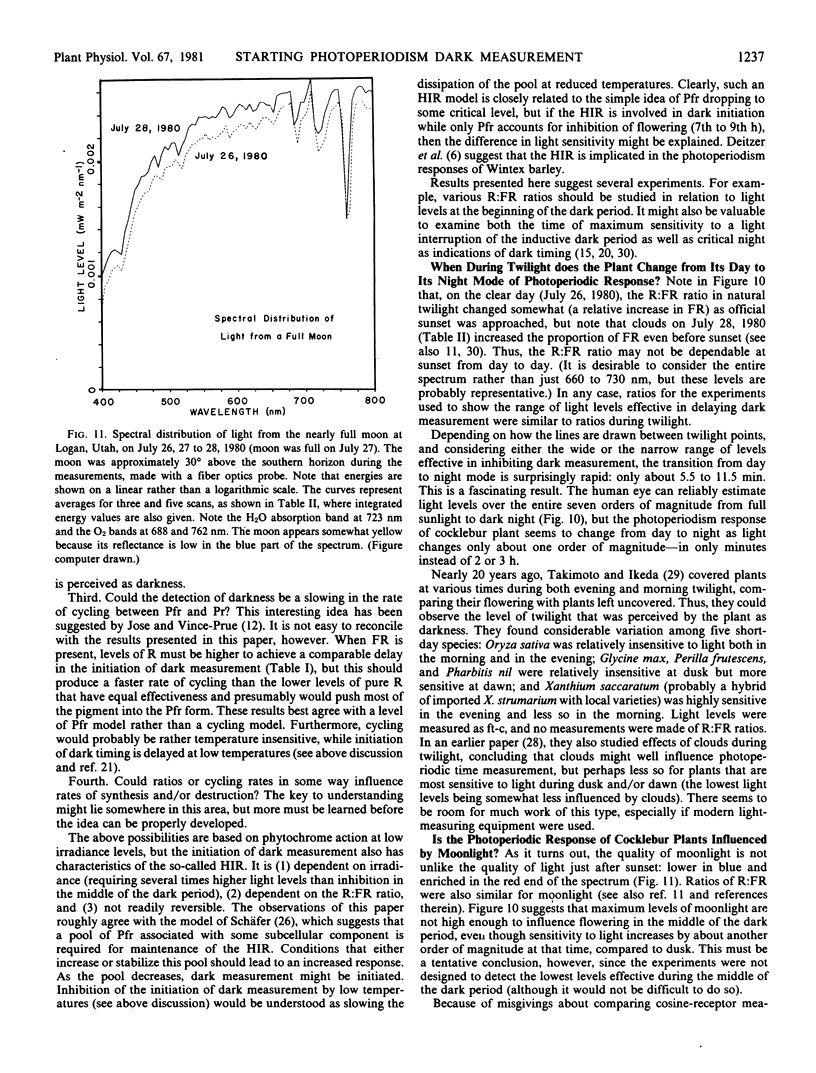
Comparing experimental results with spectral light measurements during twilight and with measurements of light from the full moon led to two conclusions: First, light levels pass from values perceived by the plant as full light to values perceived as complete darkness in only about 5.5 to 11.5 minutes, although twilight as perceived by the human eye lasts well over 30 minutes. Second, cocklebur plants probably do not respond to light from the full moon, even when most sensitive, 7 to 9 hours after the beginning of darkness.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borthwick H. A., Hendricks S. B., Parker M. W. The Reaction Controlling Floral Initiation. Proc Natl Acad Sci U S A. 1952 Nov;38(11):929–934. doi: 10.1073/pnas.38.11.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünning E., Moser I. Interference of moonlight with the photoperiodic measurement of time by plants, and their adaptive reaction. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1018–1022. doi: 10.1073/pnas.62.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitzer G. F., Hayes R., Jabben M. Kinetics and time dependence of the effect of far red light on the photoperiodic induction of flowering in wintex barley. Plant Physiol. 1979 Dec;64(6):1015–1021. doi: 10.1104/pp.64.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. J. Photoreversibility of Flower Initiation. Plant Physiol. 1956 Jul;31(4):279–284. doi: 10.1104/pp.31.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss H. D., Salisbury F. B. Aspects of clock resetting in flowering of xanthium. Plant Physiol. 1967 Nov;42(11):1562–1568. doi: 10.1104/pp.42.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury F. B., Bonner J. The Reactions of the Photoinductive Dark Period. Plant Physiol. 1956 Mar;31(2):141–147. doi: 10.1104/pp.31.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury F. B. The Dual Role of Auxin in Flowering. Plant Physiol. 1955 Jul;30(4):327–334. doi: 10.1104/pp.30.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


