Abstract
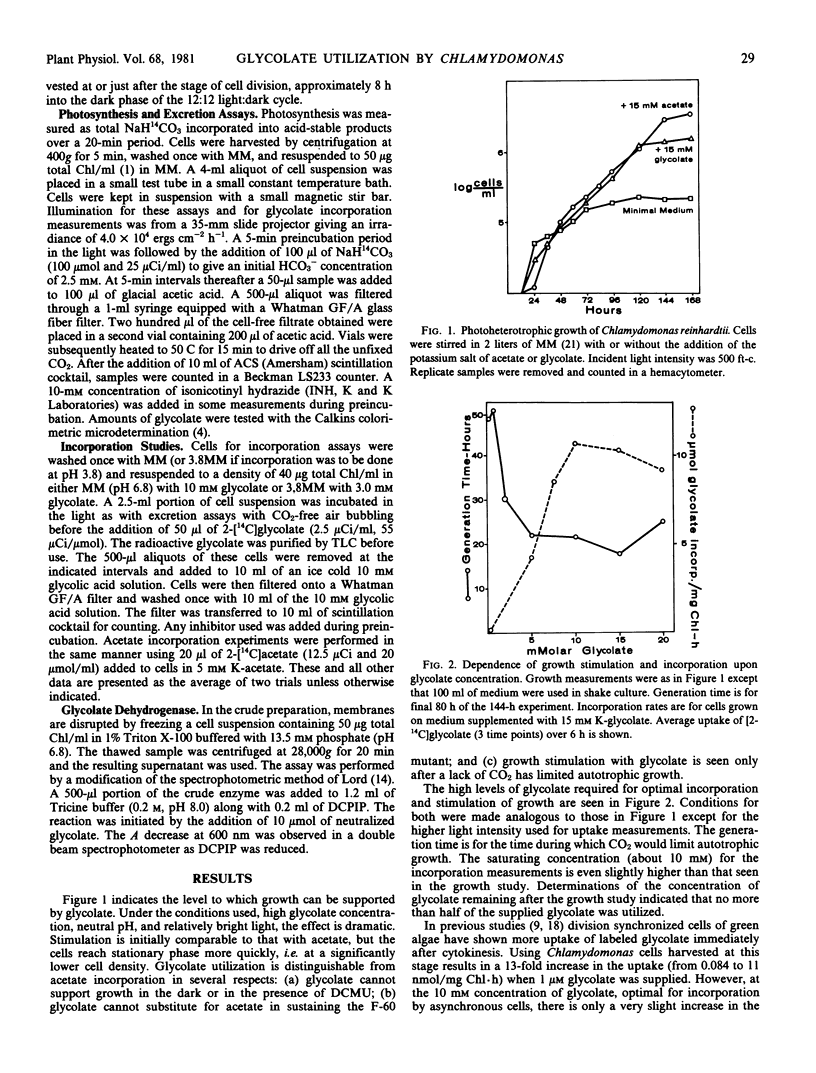
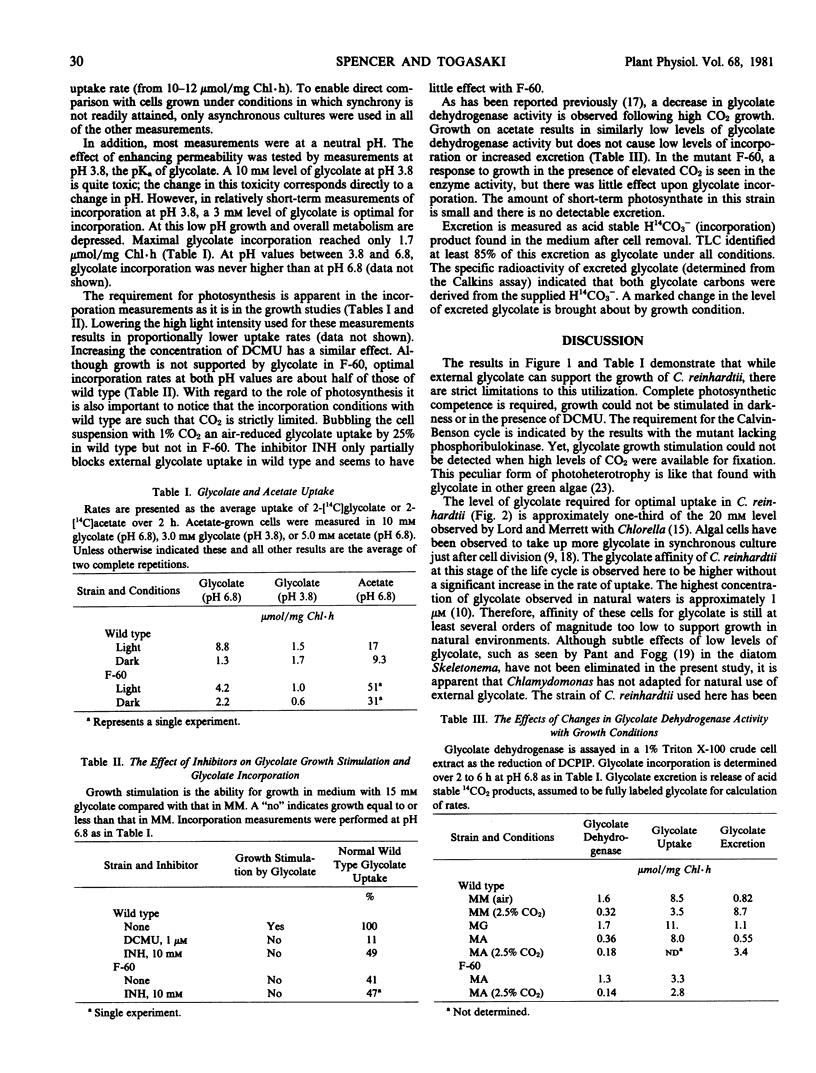
Growth and shorter term incorporation measurements with both wild type Chlamydomonas reinhardtii and a mutant (F-60, lacking phosphori-bulokinase activity) indicate that the rate of glycolate utilization is always relatively low. Growth support with external glycolate is restricted to cells with full photosynthetic capacity. A high concentration of glycolate is required for optimal growth support and incorporation of [14C]glycolate. Glycolate incorporation is low at pH 3.8 even with the relatively free permeability. The F-60 mutant can take up only small quantities of glycolate in spite of photosynthetic electron transport and photophosphorylation competencies. This requirement for photosynthetic carbon metabolism indicates a significant difference in the glycolate pathway of this alga. No growth condition significantly increases glycolate incorporation rates. There is no evidence that one of the primary enzymes, glycolate dehydrogenase, is limiting utilization; measurements of glycolate uptake and excretion do not always correlate with its activity. Since the maximal utilization rate of glycolate is low, control of glycolate formation is important in preventing the loss of this fixed carbon from the algal cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. The regulation of glycolate metabolism in division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):640–643. doi: 10.1104/pp.47.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman B., Miller A. G., Grodzinski B. A study of the control of glycolate excretion in chlorella. Plant Physiol. 1974 Mar;53(3):395–397. doi: 10.1104/pp.53.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M. Glycolate oxidoreductase in Escherichia coli. Biochim Biophys Acta. 1972 May 25;267(2):227–237. doi: 10.1016/0005-2728(72)90111-9. [DOI] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]


